 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Occupational exposure to respirable crystalline silica (cSiO2) has been linked to lupus development. Previous studies in young lupus-prone mice revealed that intranasal cSiO2 exposure triggered autoimmunity, preventable with docosahexaenoic acid (DHA). This study explores cSiO2 and DHA effects in mature lupus-prone adult mice, more representative of cSiO2-exposed worker age.
Methods
Female NZBWF1 mice (14-week old) were fed control (CON) or DHA-supplemented diets. After two weeks, mice were intranasally instilled saline (VEH) or 1 mg cSiO2 weekly for four weeks. Cohorts were then analyzed 1- and 5-weeks postinstillation for lung inflammation, cell counts, chemokines, histopathology, B- and T-cell infiltration, autoantibodies, and gene signatures, with results correlated to autoimmune glomerulonephritis onset.
Results
VEH/CON mice showed no pathology. cSiO2/CON mice displayed significant ectopic lymphoid tissue formation in lungs at 1 week, increasing by 5 weeks. cSiO2/CON lungs exhibited elevated cellularity, chemokines, CD3+ T-cells, CD45R + B-cells, IgG + plasma cells, gene expression, IgG autoantibodies, and glomerular hypertrophy. DHA supplementation mitigated all these effects.
Discussion
The mature adult NZBWF1 mouse used here represents a life-stage coincident with immunological tolerance breach and one that more appropriately represents the age (20–30 yr) of cSiO2-exposed workers. cSiO2-induced robust pulmonary inflammation, autoantibody responses, and glomerulonephritis in mature adult mice, surpassing effects observed previously in young adults. DHA at a human-equivalent dosage effectively countered cSiO2-induced inflammation/autoimmunity in mature mice, mirroring protective effects in young mice.
Conclusion
These results highlight life-stage significance in this preclinical lupus model and underscore omega-3 fatty acids’ therapeutic potential against toxicant-triggered autoimmune responses.
Introduction
Systemic lupus erythematosus (lupus) is a devastating systemic autoimmune disease affecting multiple organ systems. Lupus is associated with unresolved inflammation, aberrant exposure to self-antigens leading to the breach of immunologic tolerance, autoreactive B- and T-cell expansion, and a robust pathogenic autoantibody (AAb) response (Tsokos Citation2011; Yu et al. Citation2021). Resulting AAbs bind their respective autoantigens (AAgs), forming immune complexes that are distributed via the systemic circulation and deposited in various organs such as the kidney. This process chronically reoccurs, manifesting in cyclical bouts of inflammation and autoimmunity known as lupus flares that impact the quality of life and increase morbidity and mortality in lupus patients (Adamichou and Bertsias Citation2017). Importantly, repeated flaring promotes the development of glomerulonephritis and irreparable damage to the kidney, eventually leading to end-stage renal failure (Yu et al. Citation2017).
Lupus susceptibility is strongly influenced by heredity (Ramos et al. Citation2010). However, environmental exposures to toxicants, infections, UV radiation, and certain medications are linked to lupus onset, flaring, and progression (Wu et al. Citation2020; Zucchi et al. Citation2022). Importantly, inhalation of respirable crystalline silica (cSiO2) exposure in the workplace has been implicated in the occurrence of autoimmune diseases such as lupus, rheumatoid arthritis, scleroderma, and ANCA-associated vasculitis (Parks et al. Citation1999, Citation2017; Hoy and Chambers Citation2020). The Occupational Health and Safety Administration (OSHA) estimated that 2.3 million workers practicing stonecutting, construction, ceramics, and mining are exposed to respirable cSiO2 dust(https://www.osha.gov/silica-crystalline). Significantly, lupus risk has been shown to increase with the duration of exposure for women of color in these occupations (Finckh et al. Citation2006).
Animal models are critical to understanding how the interplay between genes and the environment influences the onset of lupus in humans. The NZBWF1 mouse, which has a genetic predisposition to autoimmune disease, has been a widely utilized preclinical lupus model for over five decades to investigate mechanisms underlying disease development, the impact of environmental factors, and the effectiveness of pharmacological and immunotherapeutic treatments (Dubois et al. Citation1966; Crampton et al. Citation2014; Celhar and Fairhurst Citation2017). Unexposed female NZBWF1 mice spontaneously begin to lose immunologic tolerance and produce AAb at 16–20 weeks of age and develop glomerulonephritis as they age into adults at approximately 32–34 weeks of age (Richard and Gilkeson Citation2018), significantly earlier than their male counterparts. Female NZBWF1 mice rarely survive beyond 12 months (Gelfand et al. Citation1972), mirroring the gender-related patterns in human lupus.
Consistent with human epidemiological studies linking cSiO2 exposure to autoimmunity, our lab has demonstrated that short-term repeated cSiO2 instillations in young female NZBWF1 lupus-prone mice beginning at 8 weeks of age-accelerated disease onset by three months with glomerulonephritis being observed at 20–22 weeks of age (Bates et al. Citation2015). Before evident kidney disease, cSiO2 exposure triggers pulmonary ectopic lymphoid tissue (ELT) development, robust AAb production in bronchoalveolar lavage fluid (BALF) and plasma, and proinflammatory gene expression in lung and kidney tissues (Bates et al. Citation2015, Citation2019; Rajasinghe et al. Citation2020). Altogether, these studies show that inhalation of cSiO2 leads to extensive pulmonary inflammation, creating a nexus for a local autoimmune response leading to systemic autoimmunity and downstream renal pathology.
Diet is an additional potential environmental factor that can impact lupus onset and progression. It has been shown in clinical and preclinical studies that heightened consumption of fish oil containing ω-3 polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), can reduce disease symptoms in lupus and RA patients (Pestka Citation2010; Arriens et al. Citation2015; Akbar et al. Citation2017; Li et al. Citation2019; Djuricic and Calder 2021). These clinical findings are supported by preclinical studies demonstrating that dietary ω-3 PUFAs suppress spontaneous autoimmunity development and extend life in several lupus-prone mouse models (Prickett et al. Citation1982; Robinson et al. Citation1985; Godfrey et al. Citation1986; Alexander et al. Citation1987; Watson et al. Citation1988; Westberg et al. Citation1989; Chandrasekar et al. Citation1995; Bhattacharya et al. Citation2003; Halade et al. Citation2013; Pestka et al. Citation2014).
Increasing ω-3 PUFA intake might be an effective intervention against the triggering of autoimmunity by environmental agents such as cSiO2. In support of this contention, we have previously shown that female NZBWF1 mice exposed to cSiO2 weekly from ages 8- to 11-weeks and fed DHA-amended diets at a human equivalent dose (HED) of 2 to 5 g/d DHA exhibited a dramatic reduction in autoimmunity with significantly less inflammation, ELT, AAb, and glomerulonephritis compared to cSiO2-exposed mice fed non-DHA diets (Bates et al. Citation2016; Benninghoff et al. Citation2019; Gilley et al. Citation2020; Rajasinghe et al. Citation2020; Pestka et al. Citation2021).
A limitation of these investigations of cSiO2-induced lupus and ω-3 intervention to date was the exclusive employment of young adult mice (8–11 weeks of age) for cSiO2 exposures. This age range precedes the spontaneous onset of immunological tolerance breach, which could influence cSiO2-induced autoimmune response. Furthermore, this life-stage corresponds to a human age of 12 to 20 yr (Flurkey et al. Citation2007; Hagan Citation2017), which is an important consideration because occupational exposures to cSiO2 predominantly begin in adult workers during their third decade of life (Ranaan et al Citation2022). In this investigation, we addressed these limitations by employing mature adult mice (16–19 weeks of age)—a life-stage documented to coincide with the onset of immunological tolerance breach (Richard and Gilkeson Citation2018) and one that more appropriately represents the age (20-30 yr) of cSiO2-exposed workers (Flurkey et al. Citation2007; Hagan Citation2017). The goals of this study were twofold. The first was to characterize how mature adult female NZBWF1 lupus-prone mice responded to subchronic cSiO2 exposure. The second was to determine how DHA supplementation altered cSiO2-induced inflammation and autoimmunity in this mature adult disease model.
Materials and methods
Animals and diets
All experiments involving mice were sanctioned by the Institutional Animal Care and Use Committee (IACUC) of Michigan State University, under approval number AUF # 201800113. We acquired mature adult female NZBWF1 mice, 14-weeks old, from Jackson Laboratories (Bar Harbor, ME). The mice were housed in groups of three per cage and provided with continuous access to food and water throughout the experiment. The animal facility maintained a stable environment, with a consistent temperature range of 21–24 °C, humidity maintained between 40–55%, and a regular 12-h light/dark cycle.
The Control (CON) diet based on the American Institute of Nutrition (AIN)-93G guidelines (Reeves et al. Citation2009) and a DHA-enriched variant were prepared following previously described methods (Bates et al. Citation2016) (). The CON diet comprised 70 g/kg of fat, including 10 g/kg of food-grade corn oil and 60 g/kg of high-oleic safflower oil (LouAna, Brea, CA, USA), ensuring adequate essential fatty acids. In the DHA-supplemented diet, 25 g/kg of safflower oil was substituted with 25 g/kg of microalgal oil containing 40% DHA (DHASCO; DSM Nutritional Products, Columbia, MD, USA). This alteration resulted in a diet containing 10 g/kg of DHA, calorically equivalent to a human equivalent dose (HED) of 5 g/d (Bates et al. Citation2018). Diets were freshly prepared on a weekly basis to mitigate fatty acid oxidation and were stored at −20 °C. The mice received a daily supply of fresh food.
Table 1. Formulation of experimental diets.
Experimental design
depicts the overall experimental design. Specifically, we tested two hypotheses in the female NZBWF1 mouse model: (i) mature adult mice receiving the same instilled dose of cSiO2 used in prior studies would develop similar or more severe pulmonary and renal pathology compared to that previously observed in young mice, and (ii) DHA-amended diet (HED 5 g/d) administered to cSiO2-exposed mature adult mice would attenuate the pulmonary autoimmune response and glomerulonephritis like that previously observed in young mice. Briefly, 14-week-old female NZBWF1 mice were randomly sorted into experimental groups and fed either CON or DHA-supplemented diets. At 16 weeks of age, mice were intranasally instilled once weekly for four consecutive weeks with either 25 µL PBS vehicle (VEH) or 1.0 mg of cSiO2(Min-U-Sil-5, 1.5–2.0 µm average particle size, Pennsylvania Sand Glass Corporation, Pittsburgh, PA, USA) suspended in PBS. Therefore, there were a total of three experimental groups as follows: (i) VEH-treated mice fed CON diet (VEH/CON, n = 12), (ii) cSiO2-instilled mice administered CON diet (cSiO2/CON, n = 12), and (iii) cSiO2-instilled mice administered DHA-supplemented diet (cSiO2/DHA, n = 12). For instillations, mice were anesthetized with 4% isoflurane. Following the final cSiO2 instillation (19 weeks of age), urine was collected weekly until the time of necropsy and evaluated for proteinuria (≥300 mg/dL protein) using diagnostic dipsticks (Cortez Diagnostics, Calabasas, CA, USA). One cohort of mice (n = 6) from each experimental group was sacrificed one-week postinstillation last cSiO2 instillation (PI), while the remaining mice were sacrificed at 5 weeks PI.
Figure 1. Experimental design. Female NZBWF1 mice were obtained at 14 weeks of age and were initiated on either an AIN-93G (CON) diet or an isocaloric diet amended with 1% DHA (5 g/d human equivalent dose [HED]). At 16 weeks of age, mice were intranasally instilled with either saline (VEH) or 1 mg cSiO2 1/week for four consecutive week. Proteinuria was assessed weekly starting at 20 weeks of age to monitor disease progression. Six animals per exposure/treatment group were sacrificed at 20 week of age (1-week PI), and the remaining 6 mice per group/treatment were euthanized at 24 week of age (5 weeks PI). At both timepoints, BALF was collected for differential cell counts and AAb microarray; blood was collected for AAb microarray and fatty acid analysis; lung and kidney tissues were collected for histopathology, immunohistochemistry (IHC), morphometric analysis, NanoString, and cytokine multiplex array.
![Figure 1. Experimental design. Female NZBWF1 mice were obtained at 14 weeks of age and were initiated on either an AIN-93G (CON) diet or an isocaloric diet amended with 1% DHA (5 g/d human equivalent dose [HED]). At 16 weeks of age, mice were intranasally instilled with either saline (VEH) or 1 mg cSiO2 1/week for four consecutive week. Proteinuria was assessed weekly starting at 20 weeks of age to monitor disease progression. Six animals per exposure/treatment group were sacrificed at 20 week of age (1-week PI), and the remaining 6 mice per group/treatment were euthanized at 24 week of age (5 weeks PI). At both timepoints, BALF was collected for differential cell counts and AAb microarray; blood was collected for AAb microarray and fatty acid analysis; lung and kidney tissues were collected for histopathology, immunohistochemistry (IHC), morphometric analysis, NanoString, and cytokine multiplex array.](/cms/asset/a47ed3d2-318d-4dd0-9c5c-87c8cd1bc146/iiht_a_2318378_f0001_c.jpg)
Animal necropsy, lung lavage, and tissue selection
Prior to exsanguination via the abdominal aorta, mice were rendered unconscious through intraperitoneal administration of sodium pentobarbital (56 mg/kg). The abdominal vena cava was accessed to collect blood for subsequent analysis of plasma and red blood cells, following the procedure outlined below. Subsequent to the cessation of vital signs, the trachea was exposed and cannulated, and the heart and lungs were carefully removed as a unit from the carcass. A saline solution of 0.8 mL was introduced into the lung via intratracheal instillation and then withdrawn to recover bronchoalveolar lavage fluid (BALF). This process was repeated with a second saline lavage, and the resulting BALF was pooled with the initial sample for further analysis. The left lung lobe was inflation fixed via the trachea with 10% neutral buffered formalin (NBF) (Fischer Scientific, Pittsburgh, PA, USA) at a constant pressure of 30 cm H2O for a minimum of 1 hr. One whole kidney was also fixed in 10% NBF. Tissues fixed in NBF remained fixative for 72 hr before being processed for histology. Right cranial lung lobes were frozen at −80 C for chemokine analysis. In addition, the caudal lung lobe was stored in RNA later at (Thermo Fisher Scientific, Wilmington, DE, USA) at 4 °C for 16 hr, then moved to −80 °C. Collected venous blood was spun down by centrifuge at 3500 × g for 10 min at 4 °C to separate the plasma and RBCs. Both plasma and RBCs were stored at −80 °C for AAb microarray and fatty acid analysis.
BALF cytology
A hemocytometer was used to determine total BALF leukocyte cell counts. Centrifugal forces (40 × g for 10 min) were used to immobilize BALF cells onto glass slides (Shandon Cytospin 3) that were then air-dried and stained with Diff-Quick (Fisher Scientific). Unused BALF was centrifuged at 465 × g for 15 min, and the resultant supernatant fraction was stored at −80 °C. BALF differential inflammatory cell counts were performed based on morphological criteria to identify monocytes, neutrophils, lymphocytes, and eosinophils out of 200 cells per cytological slide.
Fatty acid analysis
The analysis of fatty acid composition in red blood cells (RBCs) was conducted through gas-liquid chromatography by OmegaQuant Analytics, LLC (Sioux Falls, SD). The RBC Omega-3 Index, recognized as a biomarker for tissue ω-3 content (Wierenga et al. Citation2020), was calculated by summing the percentages of EPA and DHA (%EPA + DHA) in relation to the total fatty acids.
Lung and kidney histopathology
The left lung lobes, fixed in formalin, were cut into approximately 2 mm thick transverse tissue blocks, totaling four blocks each. These blocks were then embedded in paraffin. Tissue sections of 5 µm thickness were prepared and subjected to deparaffinization, followed by staining with hematoxylin and eosin (H&E) for subsequent microscopic evaluation of histopathology. Unbiased semi-quantitative analyses were conducted by a board-certified veterinary pathologist (JRH) with expertise in mouse lung histopathology using a well-defined set of histopathologic criteria and without previous knowledge of individual animal exposure/treatment history (‘blinded’). Lung lesions scored for severity encompassed (a) perivascular and peribronchiolar ectopic lymphoid tissue (ELT), (b) alveolar proteinosis, (c) alveolitis, (d) alveolar type II epithelial cell hyperplasia and (e) mucous cell metaplasia in the bronchiolar epithelium. This overall approach kept intact the spatial aspects of these lymphoid interstitial cell/tissue lesions in the lung, which are lost with other analytical techniques like flow cytometry.
Similarly, the fixed kidney tissues were sectioned into three transverse sections and prepared for histopathological analysis. These sections were stained using Periodic acid-Schiff and hematoxylin (PASH) stain or subjected to immunohistochemical staining with IgG. The board-certified veterinary pathologist assessed the kidney tissue, evaluating tubular proteinosis, global proliferative and sclerosing glomerulonephritis using a modified International Society of Nephrology/Renal Pathology Lupus Nephritis Classification system (Weening et al. Citation2004), and glomerular IgG deposition.
Immunohistochemistry and morphometric analysis of lung and kidney
Immunohistochemistry (IHC) was carried out on lung and kidney tissue sections that had been formalin-fixed and embedded in paraffin using antibodies and employed in previous studies of cSiO2 in lupus-prone mice (Bates et al. Citation2015, Citation2016, Citation2018; Heine et al. Citation2022). Specifically, in the lung tissue, B-cells were identified using a 1:600 dilution of rat anti-CD45R monoclonal antibody, while T-cells were identified using a 1:250 dilution of rabbit anti-CD3 polyclonal antibody (refer to Supplementary Table 1). Additionally, IgG+ plasma cells in the lung and IgG deposition in the kidney were identified using a goat anti-IgG polyclonal antibody (refer to Supplementary Table 1).
The slides were scanned using the VS200 virtual slide system (Olympus, Hicksville, NY, USA) utilizing NewCast software (Visiopharm, Hoersholm, Denmark). Each glass slide was randomly subsampled to acquire a minimum of 100 digital microscopic images from lung tissue sections captured at 20X magnification. We utilized mathematically robust, well-proven/documented methods of image analysis and stereological assessment of digital images (STEPanizer 1.8 Stereology Tool (Tschanz et al. Citation2011)) to ensure unbiased digital image sampling and morphometric/stereological analysis. Briefly, pulmonary CD45R+, CD3+, and IgG+ lymphoid cells were enumerated by superimposing a point grid on the randomly sampled images. Total cell densities were computed by dividing the grid points overlaying positively stained cells by the reference tissue area, yielding a percentage of the overall lung area exhibiting positive staining.
Multiplex analysis of cytokines
Right cranial lung lobes were weighed and placed in appropriate amounts of 1X RIPA buffer (Thermo Fisher Scientific) and protease inhibitor (1:100 dilution; Thermo Fisher Scientific) to make 20% homogenates. Lung tissue was homogenized using the Qiagen TissueLyser bead mill, and protein concentrations were measured by BCA assay (Thermo Fisher Scientific). Protein concentrations were then normalized to 1000 ug/mL/sample. All samples were submitted to Eve Technologies Corp. (Calgary, Alberta) for quantification of chemokines using a multiplexed array that contained the target analytes MCP-5, IP-10, MIP-1α, MIP-1β, MIP-3β, MIG, MCP-1, and KC(MilliporeSigma, Burlington, Massachusetts, USA). Assay sensitivities for these chemokines ranged from 0.3 − 30.6 pg/mL.
NanoString autoimmune gene profiling
Lung tissue RNA (collected at 1- and 5-weeks post-infection) was extracted using RNeasy Mini Kits with DNase treatment (Qiagen, Valencia, CA). Subsequently, the extracted RNA was dissolved in nuclease-free water, its quantity determined using Qubit (Thermo Fisher Scientific), and its integrity validated using a TapeStation (Agilent Technologies). Samples with RNA integrity exceeding 8 were subjected to analysis using the NanoString Autoimmune Gene Expression assay (XT-CSO-MAIP1-12, NanoString Technologies, Seattle, WA, USA) at the MSU Genomics Core. The assays were conducted and reparated on the nCounter MAX system, sample reparationn station, and digital analyzer (NanoString Technologies), following the provided manufacturer’s guidelines.
Gene expression data were evaluated with NanoString’s software nSolver v3.0.22 with the Advanced Analysis Module v2.0as previously described (Bates et al. Citation2019; Benninghoff et al. Citation2019; Citation2020; Heine et al. Citation2022). For differential gene expression, a statistically significant difference in gene expression was specified as a 1.5-fold change in expression (log2 > 0.58 or <-0.58) with BH q < 0.05. Two pairwise comparisons within each timepoint (1- and 5-weeks PI) were determined a priori: cSiO2/CON vs VEH/CON and cSiO2/CON vs cSiO2/DHA.
We evaluated the influence of cSiO2 exposure and experimental diets on annotated gene sets by computing both global and directed significance scores for each pathway at various time points, following the approach outlined in a previous study (Benninghoff et al. Citation2020). The global score provides an estimation of cumulative evidence regarding differential gene expression within a pathway, serving as a complementary tool to compare pathways and distinguish between experimental groups. Pathway Z scores were computed as the Z-scaled first principal component of the normalized expression of genes associated with the pathway.
For unsupervised hierarchical cluster analyses (HCC) using log2 transcript count data for Differentially Expressed Genes (DEGs), ClustVis (Metsalu, 2015, #33) was employed. Additionally, Spearman rank correlations were conducted to investigate overall patterns in gene expression profiles by comparing the pathway Z score with other disease biomarkers in lung tissues at 1- and 5-weeks postinfection (PI). A significant correlation was considered when the Spearman correlation coefficient (ρ) was more significant than 0.5 or less than −0.5, and the p-value was less than 0.05.
AAb microarray
BALF and plasma samples underwent assessment for the presence of AAbs through GeneCopoeia (Rockville, Maryland). Diluted plasma and BALF samples were incubated on microscope slides, each containing 16 identical OmicsArray™ panels consisting of 120 known autoantigens affixed to nitrocellulose filters. One panel was designated for a PBS-negative sample control. After incubation, the slides underwent washing and subsequent incubation with Cy3-labeled anti-mouse IgG and Cy5-labeled anti-mouse IgM secondary antibodies. Following this, the slides were rewashed, and fluorescent signals (532 nm for Cy3/IgG, 635 nm for Cy5/IgM) were detected using a GenePix™ 4000B two-channel laser microarray scanner (Molecular Devices, San Jose, California). The fluorescent signal intensities were determined using GenePixPro™ 7.0 software (Molecular Devices, San Jose, California). To calculate antibody scores, normalized signal intensities (NSI) and signal-to-noise ratios (SNR) were utilized in the following equation:
The Ab-score values, normalized and scaled for unit variance, were presented visually using ClustVis software (Metsalu, 2015). The data were subjected to clustering using unsupervised hierarchical co-clustering (HCC). The rows were clustered using Euclidean distance and Ward linkage, with imputation applied for estimating missing values. Selected AAb-score values were presented as violin plots, generated using Prism 9 (GraphPad Prism v 9.2, San Diego, CA, USA).
Statistical analysis
All data underwent analysis and subsequent statistical tests utilizing Prism 9 (GraphPad Prism v 9.2, San Diego, CA, USA), with the exclusion of the NanoString gene expression data, as previously mentioned. Examination for outliers was conducted utilizing Grubb’s outlier test (with Q = 1%), and normality was assessed using the Shapiro–Wilk test (p < 0.01). Regarding the histopathological endpoints, a one-way ANOVA was employed to explore our hypothesis that dietary DHA would mitigate cSiO2-triggered responses at each time point. For non-normal and semi-quantitative data, analysis was performed using the nonparametric Kruskal–Wallis test. The presentation of data includes mean ± standard error of the mean (SEM), and a p-value of ≤ 0.05 was regarded as statistically significant.
Results
DHA consumption increases omega-3 index
cSiO2exposure and experimental diets did not influence body weight increases (Supplementary Figure 1). Consistent with previous studies (Bates et al. Citation2016; Gilley et al. Citation2020), the replacement of high-oleic sunflower oil with DHA-enriched algal oil in cSiO2/DHA diets corresponded with increased ω-3 PUFA content in RBCs from mice necropsied at both 1 and 5 weeks PI (; ). Concomitantly, cSiO2/DHA mice had significantly reduced percentage of total omega-6 PUFAs in their RBCs compared to cSiO2/CON-fed mice. DHA content in RBCs was significantly increased in DHA-fed mice compared to those on the CON diet at the expense of arachidonic acid. Moreover, the omega-3 index, which is a biomarker that describes the sum of the DHA + EPA in RBCs, was three times higher in cSiO2/DHA mice (17-19%) compared to either VEH/CON and cSiO2/CON mice (5-6%).
Figure 2. DHA supplementation skews long-chain PUFA profile in mature adult female NZBWF1 mice. In VEH/CON and cSiO2/CON mice, total omega-6 fatty acids were more abundant than ω-3 fatty acids in RBC cell membranes at 1-week and 5-weeks PI. In cSiO2/DHA mice, total omega-6 fatty acids were significantly reduced, whereas total ω-3 fatty acids were significantly increased at both time points. Letters: a, significantly different from VEH/CON for the specified endpoint (p < 0.05); b, significantly different from cSiO2/CON for the specified endpoint (p < 0.05).
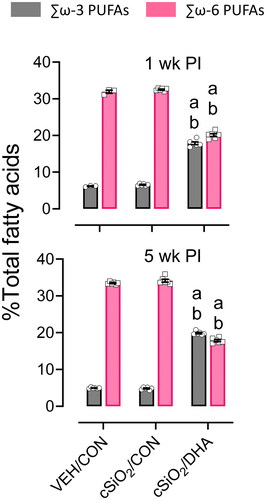
Table 2. Fatty acid content of RBCs at 1- and 5-weeks post-instillation.
DHA attenuates cSiO2-inducedpulmonary inflammatory cell infiltration and ELT formation
The most conspicuous lung lesions in mice instilled with cSiO2 and fed CON diet were lymphoplasmacytic infiltrations in perivascular and peribronchiolar interstitial (ectopic lymphoid tissue, ELT) (). cSiO2-instilled mice also had mild to moderate mixed inflammatory cell infiltrates in the alveolar parenchyma (alveolitis; composed of neutrophils, lymphocytes, and monocytes) that were associated with accumulations of extracellular proteinaceous material (proteinosis) and cSiO2 particles (extracellular and intracellular) throughout the alveolar airspaces. cSiO2 particle-laden alveolar macrophages with morphologic features of degeneration (vacuolation) and necrosis (cellular enlargement, cytoplasmic vacuolation, and nuclear pyknosis or karyorrhexis) was another frequent finding in these cSiO2-instilled lungs at both 1- and 5-weeks PI. Lungs of mice at 5 weeks, but not 1-week PI, also had widely scattered small granulomas, composed of macrophages, lymphocytes, and cSiO2 particles, in alveolar septa.
Figure 3. cSiO2-triggered pulmonary perivascular and peribronchiolar lymphoid cell infiltration and ELT neogenesis in mature female NZBWF1 mice are inhibited by DHA supplementation. (A) Light photomicrographs of hematoxylin and eosin-stained lung tissue sections from mice instilled with saline vehicle and fed CON diet (VEH/CON), mice instilled with cSiO2and fed CON diet (cSiO2/CON), and mice instilled with cSiO2 and fed DHA-enriched diet (cSiO2/DHA). cSiO2 exposure resulted in perivascular and peribronchiolar infiltration of lymphoid cells at 1-week PI, with even greater infiltration (ectopic lymphoid tissue, ELT, asterisks) at 5-weeks PI. DHA attenuated ELT formation at both time points. b, bronchioles; v, blood vessel; a, alveolar parenchyma; solid arrows, proteinaceous debris; stippled arrows, inflammatory cell influx in alveolar parenchyma (alveolitis).(B) Graphic representation of semi-quantitative severity scores following assessment criteria of (1) minimal (<10%); (2) slight (10-25%); (3) moderate (26-50%); (4) marked (51-75%); and (5) severe (>75%) amount of tissue affected. (C) cSiO2 instillation caused a significant increase in total leukocytes, monocytes, and neutrophils in the BALF. DHA significantly reduced total leukocyte and monocyte cellularity in the BALF but did not influence neutrophils. a, significantly different from VEH/CON group; b, significantly different from cSiO2/CON group; p < 0.05.
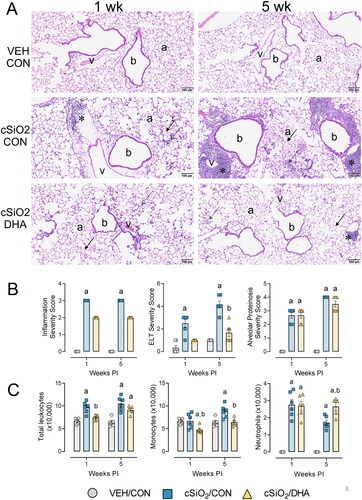
ELT onset was most severe in the lungs of mice 5 weeks after the last cSiO2 instillation (), with significantly lesser amounts in mice 1 week PI. At both time points, no or minimal perivascular/bronchiolar lymphoid cell infiltrates were present in VEH/CON mice. VEH/CON mice also lacked other lung histopathology (e.g. alveolitis). In contrast to the large amounts of ELT in cSiO2/CON mice, cSiO2-exposed DHA-fed mice had only small amounts of ELT in their lungs at 1 and 5 weeks PI. Dietary DHA supplementation, however, had minimal to no apparent attenuation of other cSiO2-triggered pulmonary pathology, including alveolar accumulation of amorphous protein, silica, macrophages, and inflammatory cells. Semi-quantitative severity scores of lung lesions supported histopathological observations that cSiO2 exposure significantly increased inflammation, ELT formation, and alveolar proteinosis at both timepoints (). Similarly, dietary supplementation with DHA markedly attenuated ELT formation but did not affect alveolitis and alveolar proteinosis.
cSiO2 exposure significantly elevated total leukocytes, monocytes, and neutrophils at 1 and 5 weeks PI in the BALF compared to VEH/CON mice. In contrast, DHA consumption reduced the numbers of total leukocytes and monocytes in the BALF but interestingly resulted in increased neutrophil numbers at 5 weeks PI compared to cSiO2/CON mice ().
Dietary DHA suppresses cSiO2-driven chemokine protein expression in the lung
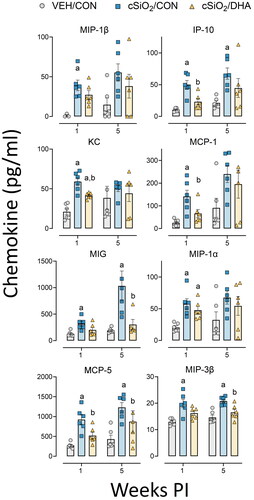
Consistent with increased lymphoplasmacytic infiltrations in cSiO2-exposed mice, there was augmented chemokine protein expression in lung tissue at 1- and 5-weeks PI (). These included monocyte chemoattractant proteins (MCP-5, MCP-1), macrophage inflammatory proteins (MIP-3β, MIP-1β, MIP-1α), IFN-induced protein (IP-10), keratinocyte-derived cytokine (KC), and monokine induced by gamma interferon (MIG). DHA supplementation attenuated cSiO2-triggered MCP-5, IP-10, MCP-1, and KC protein expression in lung tissue.
Figure 4. cSiO2-induced chemokine protein expression in lung tissue of mature adult female NZBWF1 mice is inhibited by DHA consumption. Proinflammatory cytokine production was measured in lung tissue homogenate using a multiplex cytokine discovery assay. Cytokine production significantly increased with cSiO2 exposure and was attenuated with the DHA-enriched diets at either 1-week or 5-weeks PI. Letters: a, significantly different from VEH/CON for the specified endpoint (p < 0.05); b, significantly different from cSiO2/CON for the specified endpoint (p < 0.05).
DHA supplementation suppresses cSiO2-induced B-, T-, and plasma cell accumulation in lung
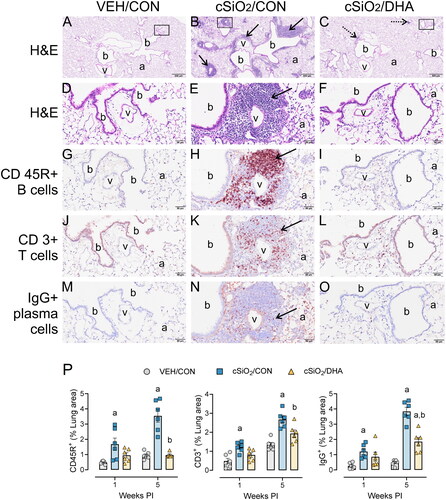
Lymphoid cell populations in ELT were identified using IHC () and quantified using standard morphometric techniques (). cSiO2-induced ELT was composed predominantly of CD3+ T-cells and CD45R+B-cells, with smaller numbers of IgG+ plasma cells located in the margins of ELT. cSiO2-triggered lymphoid cell infiltration was significant at 1 week PI and markedly increased at 5-weeks PI. Dietary supplementation with DHA significantly reduced CD45R+ B-cell, CD3+ T-cell, and IgG+ plasma cell infiltration at 5-weeks PI.
Figure 5. cSiO2-induced lung infiltration of perivascular and peribronchiolar CD45R+ B-cells, CD3+ T-cells, and IgG+ plasma cells in mature adult female NZBWF1 mice is suppressed by DHA feeding. (A-O) Light photomicrographs of lung tissue from VEH/CON, cSiO2/CON, and cSiO2/DHA mice at 5-weeks PI. Tissue sections were histochemically stained with hematoxylin and eosin (H&E; low power, A-C; high power, D-E) identifying perivascular and peribronchiolar ectopic lymphoid tissue (ELT, solid arrows in B and E) or scant lymphoid cell aggregates (stipple arrows in C). Other sections were immunohistochemically stained for CD45R+ B lymphoid cells (G-I), CD3+ T-cells (J-L), and IgG+ plasma cells (M-O) in ELT. In cSiO2/CON mice, silica instillation triggered conspicuous formation of ELT containing CD45R+ B-cells, CD3+ T-cells, and IgG+ plasma cells. Only scant amounts of lymphoid cells were present in the lungs of cSiO2/DHA mice (C, F, I, L,O). DHA treatment markedly attenuated lymphoid cell infiltration. b, bronchioles; v, blood vessels; a, alveolar parenchyma. (P) Graphical representation of morphometrically determined density of CD45R+ B-cells, CD3+ T-cells, and IgG+ plasma cells in lung tissue of mice at 1 week and 5 weeks after instillation. a, significantly different from VEH/CON group; b, significantly different from cSiO2/CON group; p < 0.05.
DHA quells cSiO2-triggered autoimmune-related gene expression in lung
NanoString global and directed significance scores revealed that immunological pathways were significantly altered in lung tissue by both cSiO2 exposure and diet (). cSiO2 exposure led to the activation of autoimmune-related pathways such as MHC Class I antigen presentation, Nod-like receptor (NLR) signaling, and Type I/II IFN signaling at 1 and 5 weeks PI. DHA-fed cSiO2-exposed mice had significantly reduced enrichment of these pathways at 1 week PI.
Figure 6. SiO2-induced inflammatory/autoimmune pathways in lung tissue 1- and 5-weeks post-cSiO2 instillation in mature adult female NZBWF1 mice are quelled by DHA supplementation. Z scores of selected inflammatory/autoimmune pathways are presented as Tukey box plots for select pathways of interest for lung tissue. Within a tissue type, different letters indicate that the treatment groups are significantly different (p < 0.05), as described in materials and methods. cSiO2 exposure significantly enriched MHC antigen presentation, NLR signaling, and Type I/II Interferon signaling pathways. DHA significantly reduced pathway Z scores for MHC antigen presentation and Type I/II Interferon signaling at 1-week PI and for NLR signaling at both 1- and 5-weeks PI. Letters: a, significantly different from VEH/CON for the specified endpoint (p < 0.05); b, significantly different from cSiO2/CON for the specified endpoint (p < 0.05).
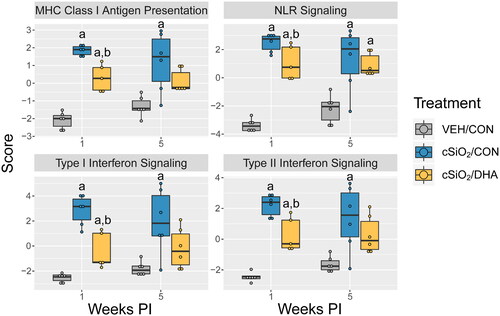
cSiO2 exposure resulted in the upregulation of genes associated with several significantly enriched pathways at 1 and 5 weeks PI compared to VEH/CON mice (Supplementary Figures 2 and 3). DHA significantly downregulated representative genes associated with NLR signaling (e.g. Cyld, Tlr4, Casp4) (Supplementary Figure 2 A), MHC Class I antigen presentation (e.g. Fcgr1, Herc6) (Supplementary Figure 2B,D), cytosolic DNA sensing (e.g. Ddx58, Zbp1) (Supplementary Figure 2C,D), and Type I/II IFN signaling (e.g. Irf7, Mx1, Isg15) (Supplementary Figure 3 A–C) at 1-week PI.
DHA suppresses diverse IgG AAb responses in BALF induced by cSiO2
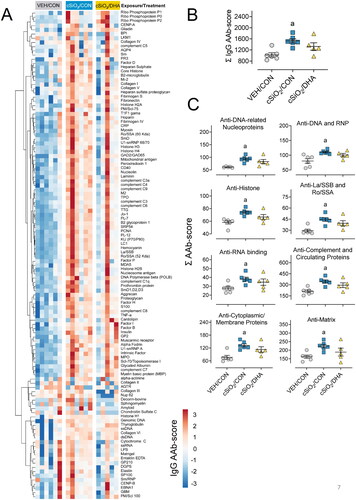
AAg microarray revealed that cSiO2induced a diverse range ofIgG AAbs () in the BALF of CON-fed that was less. These responses were markedly less robust in DHA-fed mice. cSiO2 also induced IgMAAbs in CON-fed mice, but these responses were not affected by DHA (Supplementary Figure 4). Significant differences were not detected among VEH/CON, cSiO2/CON, and cSiO2/DHA groups regarding IgG AAbs in the plasma (Supplementary Figure 5).
Figure 7. DHA feeding affects cSiO2-induced IgG AAb responses in the BALF at 5-weeks PI in mature adult female NZBWF1 mice. (A) AAb-score values at 5 weeks PI in BALF for the expression of 120 IgG AAbs are illustrated in a heatmap using unsupervised clustering (Euclidian distance method). Scale bar values reflect the range of variance stabilized AAb scores centered across rows. (B) cSiO2induced increase total IgG AAb the BALF in mice-fed control diet but not in mice fed DHA. (C) cSiO2 exposure significantly increased various classes of autoimmunity-related AAbs detected in the BALF. A downward trend was observed in the same classes of cSiO2-triggered AAbs when mice were fed DHA. Letter a indicates significantly different from VEH/CON for the specified endpoint (p < 0.05).
DHA suppresses cSiO2-induced hypertrophy and IgG deposition in the kidney
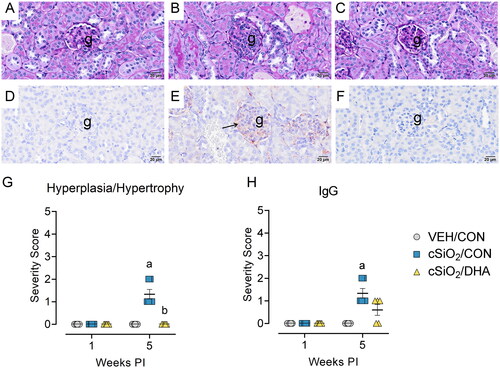
Mild histopathological changes were observed in renal tissuefromcSiO2/CONmice at 5-weeks PI (). These mice had modest glomerular hypertrophy () and mild renal IgG deposition () compared to VEH/CON mice (). Renal pathology and IgG immunohistochemical staining were absent in cSiO2/DHA mice (). Consistent with histopathological analysis, semi-quantitative severity scoring revealed mild but significant increases in glomerular hypertrophy and renal IgG deposition at 5-weeks PI (). These cSiO2-induced glomerular changes were attenuated in DHA-fed mice. Concordant with the mild nature of glomerular inflammation, urinalysis revealed no evidence of proteinuria in mice exposed to cSiO2 (Supplementary Figure 6).
Figure 8. cSiO2-triggered membranoproliferative glomerulonephritis and glomerular IgG deposition at 5-weeks PI in mature adult female NZBWF1 mice is attenuated by DHA feeding. Light photomicrographs of kidney tissue stained with periodic acid Schiff (PAS) and hematoxylin (A, B, C) and immunohistochemically stained for IgG with hematoxylin counterstain (D, E, F). VEH/CON (A, D), cSiO2/CON (B, E), and cSiO2/DHA mice (C, F). cSiO2-exposed mice (B, E) had modest increases in glomerular size, cellularity, size, and IgG deposition. No histopathology was observed in VEH/CON mice (A, D) and DHA-fed mice (C, F). Individual kidney sections were semi-quantitatively scored based on the modified International Society of nephrology/renal pathology lupus Nephritis Classification system described methods for lupus nephritis score for hyperplasia/hypertrophy (G) and IgG deposition (H). cSiO2/CON mice had significantly increased severity scores for hyperplasia/hypertrophy and IgG deposition at 5-weeks PI. DHA-enriched diets attenuated renal hyperplasia and hypertrophy. G, glomeruli; arrow, IgG proteinaceous material. Letters: a, significantly different from VEH/CON for the specified endpoint (p < 0.05); b, significantly different from cSiO2/CON for the specified endpoint (p < 0.05).
Discussion
Researchers often categorize preclinical mouse models into specific age groups to understand how human developmental stages influence disease pathogenesis (Flurkey et al. Citation2007; Hagan Citation2017). Radulescu and colleagues (Radulescu et al. Citation2021) have recently introduced a set of life-stage categories, which included juvenile (< 4 weeks), young adult (4-16 weeks), mature adult (16–70 weeks), and late adult (70–98 weeks). Since these life stages represent different human ages and phases of immunological tolerance breach, they are potentially helpful reference points for investigating toxicant-triggered disease modulation in preclinical lupus models. Here, we queried for the first time how cSiO2 treatment and DHA intervention impact early inflammatory and autoimmune responses in the lungs of mature adult female NZBWF1 mice that appropriately represent 20 to 30-year-old individuals occupationally exposed to cSiO2 (Raanan et al. Citation2022).
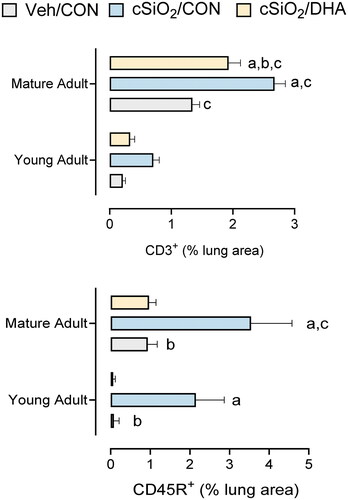
We have previously established that ELT neogenesis in the lung serves as a critical nexus for cSiO2-induced autoimmunity and a target for DHA intervention in the NZBWF1 mouse (Bates et al. Citation2016, Citation2018, Citation2019; Benninghoff et al. Citation2019; Wierenga et al. Citation2019, Citation2020). When ELT morphometric data from this study were compared to that previously reported in young adult mice (Bates et al. Citation2019) (), we found that (i) cSiO2 exposure induced significant infiltration of CD3+ T-cells at 5-weeks PI in mature adult mice but not in young adult mice, (ii) cSiO2-induced infiltration of CD45R+ B-cells at 5-weeks PI in mature adult mice was greater than that in young adult mice, and (iii) DHA significantly reduced CD3+ T-cell infiltration in mature adult cSiO2-exposed mice and CD45R+ B-cell infiltration in both mature adult and young adult mice.
Figure 9. cSiO2-triggered infiltration of T and B cells is greater in mature adult NZBWF1 mice compared to young adult NZBWF1 mice. Morphometric data from this study were compared to that previously reported in for young adult mice (Bates et al. Citation2019). (A) cSiO2 exposure induced significant infiltration of CD3+ T-cells at 5-wk PI in mature adult mice but not in young adult mice. (B) cSiO2-induced infiltration of CD45R+ B-cells at 5-weeks PI in mature adult mice was greater than that in young adult mice. DHA significantly reduced (A) CD3+ T-cell infiltration in marine adult cSiO2-exposed mice and (B) CD45R+ B-cell infiltration in both mature adult and young adult mice. Letters: a, significantly different from VEH/CON for the specified endpoint (p < 0.05); b, significantly different from cSiO2/CON for the specified endpoint (p < 0.05); c, significantly different from young adult mice within the same treatment group and specified endpoint (p < 0.05).
NanoString analysis of global and targeted significance scores revealed insights into potential mechanisms for profound histopathologic and immunologic changes induced by cSiO2 within lung tissue. Notably, there were notable alterations of autoimmune-related pathways including MHC Class I antigen presentation, NLR signaling, and Type I/II IFN signaling as early as 1-week PI. In prior studies using young mice, these effects were essentially discernable at 5-weeks PI (Benninghoff et al. Citation2019). Importantly, when DHA-fed mice were exposed to cSiO2, a substantial reduction in the enrichment of these autoimmune-related pathways was observed at 1-week PI. This finding implies that the inclusion of DHA in the diet may have a mitigating effect at the transcriptional level on the activation of immune pathways induced by cSiO2 exposure. The observed reduction in pathway enrichment here and previously highlights the intricate interplay between environmental exposures and dietary influences on immunological processes in the lung tissue.
Interestingly, while the cSiO2-triggered endpoints in the lungs of mature adult mice were relatively robust, we observed only mild pathology in the kidney at 5-weeks PI. These changes in renal pathology are consistent with minimally elevated IgG AAb responses in the plasma of cSiO2/CON mice. In previous studies focused on cSiO2-triggered glomerulonephritis in young NZBWF1 mice, proteinuria was not evident until at approximately 10-weeks after cSiO2-exposure with concomitant renal pathology was observed at 12-weeks PI (Bates et al. Citation2015). Considering the lung serves as the nexus for respirable cSiO2-mediated autoimmune responses in this model, likely, the threshold for recurring inflammation originating from the lung necessary for robust downstream damage to the kidney was not reached at 5-weeks PI in this experimental model. It is possible that if cSiO2-exposed mature adult mice were euthanized later (e.g. 9-weeks PI), we might observe more advanced glomerulonephritis with proteinuria.
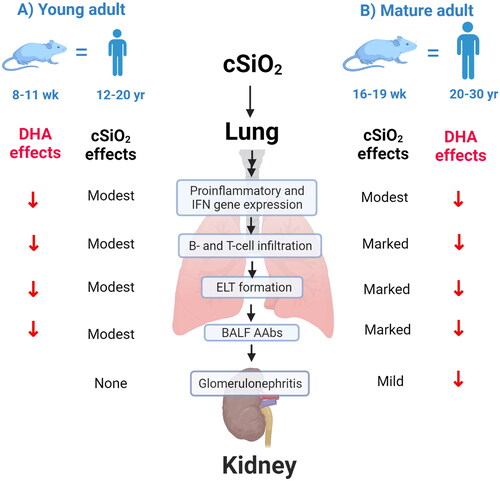
qualitatively compares the severity of cSiO2-triggered inflammatory/autoimmune endpoints and effects of DHA supplementation in young adult female NZBWF1 mice at 5-weeks PI as reported in prior studies (Bates et al. Citation2015, Citation2016, Citation2019; Benninghoff et al. Citation2019; Gilley et al. Citation2020; Rajasinghe et al. Citation2020; Pestka et al. Citation2021) and mature adult NZBWF1 mice at 5-weeks PI as determined in the present investigation. Weekly exposure of young adult mice to 1 mg cSiO2 for 4 weeks led to modest pathological changes and ELT development in the lung and no observable glomerulonephritis. In contrast, exposure of mature adult mice to the same cSiO2 treatment resulted in marked pulmonary pathology and with mild but significant glomerular hypertrophy and IgG deposition in the kidney consistent with glomerulonephritis demonstrating the age-dependent response to cSiO2 in this experimental model. Accordingly, our data suggest that mouse age influences cSiO2-triggered inflammatory and autoimmune responses but not preventive effects of DHA.
Figure 10. Mouse age influences cSiO2-triggered inflammatory and autoimmune responses but not preventive effects of DHA. Diagrammatical summary of the severity of cSiO2-triggered inflammatory/autoimmune endpoints and effects of DHA supplementation in (A) young adult female NZBWF1 mice at 5 weeks PI as reported in prior studies (Bates et al. Citation2015, Citation2016, Citation2019; Benninghoff et al. Citation2019; Gilley et al. Citation2020; Rajasinghe et al. Citation2020; Pestka et al. Citation2021) and (B) mature adult NZBWF1mice at 5-weeks PI as determined in the present investigation. (A) Weekly exposure of young adult mice to 1 mg cSiO2 for 4 weeks led to modest pathological changes and ELT development in the lung and no observable glomerulonephritis, whereas (B) exposure of mature adult mice to the same cSiO2 resulted in marked pulmonary pathology and with significant glomerular hypertrophy and IgG deposition in kidney consistent with glomerulonephritis demonstrating the age-dependent response to cSiO2 in this experimental model.
Currently, the most common drugs used for treating lupus are glucocorticoids (e.g. prednisone), antimalarials (e.g. hydroxychloroquine), immunosuppressants (e.g. cyclophosphamide), and monoclonal antibodies (Basta et al. Citation2020). Although treatment with these drugs effectively manages and slows disease progression, their chronic use is associated with significant adverse effects (Flammer and Rogatsky Citation2011; Porta et al. Citation2020). While newer biologic therapies have yet to demonstrate similar off-target toxic effects, they are expensive and remain unaffordable for many lupus patients (Lamore et al. Citation2012). Therefore, there is a critical need for nontoxic, cost-effective therapies to protect against environmental-triggered lupus. Toward that end, DHA was efficacious in attenuating cSiO2-induced inflammation, ELT formation, Type I/II IFN, innate and adaptive immune response gene signatures, and IgG AAb production in the lung at both 1- and 5-weeks PI with no evident toxicity. These findings and those in previous studies (Bates et al. Citation2016, Citation2018; Benninghoff et al. Citation2019; Gilley et al. Citation2020; Rajasinghe et al. Citation2020) collectively support the notion that ω-3 PUFA supplementation is a potential steroid-sparing approach to prevent/treat lupus onset and flaring triggered by environmental agents like airborne cSiO2.
There are multiple mechanisms by which ω-3s can interfere with the cSiO2-induced autoimmune phenotype. ω-3 PUFAs possess the capability to inhibit the metabolism of ω-6 PUFAs into proinflammatory eicosanoids, including thromboxanes, prostaglandins, and leukotrienes (Lands et al. Citation2018). The lipid byproducts from the arachidonic acid cascade predominantly exhibit inflammatory actions, especially during episodes of acute inflammation. Shifting the PUFA balance in favor of ω-3 PUFAs, as opposed to ω-6 PUFAs like arachidonic acid, has the potential to augment the pro-resolving characteristics promoted by lipid mediators derived from ω-3s. Recent research has demonstrated a robust correlation between ω-3 PUFA levels in both plasma and red blood cells and the subsequent production of downstream lipid mediators (Ostermann and Schebb Citation2017). Importantly, supplementation with DHA or EPA led to a reduction in ω-6 PUFAs, particularly arachidonic acid, along with a decrease in ω-6 PUFA-derived metabolites. In line with this mechanism, we have recently illustrated that DHA supplementation effectively suppresses the induction of various cSiO2-induced eicosanoids in alveolar macrophages (Favor et al. Citation2023).
In addition to their competition with ω-6 PUFAs, ω-3 PUFAs can directly impact inflammatory pathways (Calder Citation2017). For instance, DHA and EPA disrupt the activation of transmembrane receptors associated with inflammatory signaling by enhancing membrane fluidity and inhibiting the formation of lipid rafts (Wong et al. Citation2009). Additionally, both extracellular and intracellular phospholipases can cleave PUFAs from the cell membrane, and the resulting liberated DHA and EPA may stimulate transmembrane receptors or intracellular receptors known for their role in suppressing proinflammatory signaling (Li et al. Citation2013; Yan et al. Citation2013). Specifically, ω-3 PUFAs can counteract Toll-like receptor (TLR) activation (Weatherill et al. Citation2005; Hwang et al. Citation2016) and interfere with NF-kB-dependent transcription by activating peroxisome proliferator-activated receptor gamma (PPARγ) (Ricote and Glass Citation2007; Chang et al. Citation2015). Consistent with this mechanism, we have demonstrated that DHA suppresses cSiO2-induced inflammasome activation and IL-1 cytokine release in macrophages by acting at the level of TLR-mediated priming. Furthermore, both DHA and EPA undergo metabolism to generate specialized pro-resolving mediators (SPMs) like maresins, resolvins, protectins, and anti-inflammatory epoxide metabolites (Serhan et al. Citation2015; Ostermann and Schebb Citation2017). These SPMs effectively inhibit inflammatory signaling (Titos et al. Citation2016; Sham et al. Citation2018) and facilitate the process of efferocytosis, which is crucial for the removal of deceased cells, a critical step in thwarting the pathogenesis of autoimmune diseases (Chiang et al. Citation2015; Fredman et al. Citation2016).
It should be noted that both here and previously (Bates et al. Citation2015, Citation2016, Citation2018; Pestka et al. Citation2021), we chose to study the effects of cSiO2 exclusively female NZBWF1 mice because these have higher lupus disease penetrance than male NZBWF1 mice (Burnet and Holmes Citation1965; Hicks and Burnet Citation1966), thus mimicking lupus predilection in human females (Tsokos Citation2011). However, we cannot exclude the possibility that males and females differentially respond to cSiO2 relative to inflammation and autoimmunity. Consistent with this notion, an investigation of Chinese pottery workers reported that at the same average level of cSiO2 exposure, males exhibited a higher risk of developing silicosis than females (Poinen-Rughooputh et al. Citation2021). Even more germane to the present study, Parks and coworkers (Parks et al. Citation2002) reported that the risk of lupus was higher among men than women occupationally exposed to cSiO2. From a preclinical perspective, following four weekly exposures to 1 mg cSiO2, male C57Bl6 mice, which are not lupus-prone, had worse alveolitis and greater dendritic cell presence within the lungs than their similarly treated female counterparts (Ray and Holian Citation2019). A later study in C57Bl6 mice employing a single 10 mg cSiO2 dose reported a slight downregulation of classical fibrosis-related pathways in female mice compared with males; however, over the long term, there was no difference in the extent of fibrosis between the sexes (Jin et al. Citation2022). Finally, although in earlier studies by Holian and coworkers (Brown et al. Citation2003; Citation2004), it was observed that both male and female NZM2410 mice were susceptible to cSiO2-accelerated lupus, unlike NZBWF1 mice, both biological sexes in this strain have a similar predilection for autoimmunity (Rudofsky and Lawrence Citation1999). Given the lack of clarity on effects of biological sex on cSiO2-induced inflammation and autoimmunity and the preponderance of males occupationally exposed to cSiO2, it will be desirable in future studies to compare the effects of this particle in male and female NZBWF1 mice.
This investigation had several other limitations. Firstly, we limited our endpoints to 1 and 5 weeks after the final cSiO2 instillation, whereas our prior studies in young adult mice endpoints were determined in the lung at 1-, 5-, 9-, and 13-weeks PI. Subtle differences in autoimmune phenotype observed here between young and mature adult mice at the early time points might have been greatly magnified at 9- and 13-weeks PI, providing additional insight into the importance of the mature phenotype. Secondly, it would have been interesting to compare mature adult responses to cSiO2 in control mice that were not lupus-prone. In prior studies, we found that subchronic exposure to cSiO2 in young adult female C57Bl6 and NZB/LacJ, which are not lupus-prone, does not induce an autoimmune phenotype at 13 weeks PI (Bates et al. Citation2015, Citation2016). However, it cannot necessarily be assumed mature adult non-lupus mice would be similarly recalcitrant to autoimmune triggering by cSiO2. Finally, it would have been informative to include mice fed only DHA in our experimental design. In a prior study using female NZBWF1 mice, we compared the impact of feeding three different diets: a ω-3 PUFA-rich diet with docosahexaenoic acid-enriched fish oil, an ω-6 PUFA-rich diet with corn oil, and an ω-9 monounsaturated fatty acid (MUFA)-rich diet (Pestka et al. Citation2014). Mice consuming the ω-3 PUFA diet exhibited attenuated plasma autoantibodies, proteinuria, and glomerulonephritis at age 34-weeks compared to those on ω-6 PUFA or ω-9 MUFA diets. There was a generalized downregulation associated with inflammatory response, antigen presentation, T cell activation, B cell activation/differentiation, and leukocyte recruitment in mice fed the ω-3 PUFA diet. Thus, the inclusion of a DHA-only group in future studies could provide additional understanding of the subtle effects of the ω-3 PUFA diet alone on gene expression in lung and other autoimmune-related endpoints.
Conclusion
Subchronic IN administration of cSiO2to mature adult NZBW/F1 mice led to heightened pulmonary inflammatory and autoantibody responses, along with glomerulonephritis that were generally more pronounced at 5 weeks PI compared to previously documented effects in similarly treated young adult mice. Significantly, supplementation with DHA at a dosage relevant to human translation effectively alleviated cSiO2-induced inflammation and autoimmunity in mature adult mice, mirroring the protective outcomes observed in their younger counterparts. These results underscore the influence of life-stage as a contributing factor in this preclinical model and again emphasize the therapeutic potential of ω-3 fatty acids in mitigating autoimmune responses triggered by toxicants.
Author contributions
LH: study design, coordination, feeding study, necropsy, data curation, data analysis/interpretation, figure preparation, manuscript preparation, and submission; TS: morphometric analysis, data acquisition and interpretation; JW: study design, necropsy, lab analysis; RL: instillations, necropsy, lab analysis; AB: data procurement and statistical analysis; AS: morphometric analysis; AT: animal handling, urinalysis; JH: study design, oversight, lung/kidney histopathology, morphometry, data analysis, manuscript preparation; JP: study design, oversight, funding acquisition, data analysis, manuscript preparation.
Supplemental Material
Download MS Word (2 MB)Acknowledgments
The authors thank Amy Porter of the Michigan State University Laboratory for Investigative Histopathology for their assistance with histotechnology. The authors also thank Dr. Kevin Childs and the Research Technology Support Genomics Core Facility for processing samples using the NanoString nCounter Analysis System.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
Original NanoString normalized linear counts and statistical analyses from the NanoString autoimmune profiling panel, and a summary of statistical analyses are available at Dryad. https://doi.org/10.5061/dryad.2280gb5vx.
Additional information
Funding
References
- Adamichou C, Bertsias G. 2017. Flares in systemic lupus erythematosus: diagnosis, risk factors and preventive strategies. Mediterr J Rheumatol. 28(1):4–12. doi: 10.31138/mjr.28.1.4.
- Akbar U, Yang M, Kurian D, Mohan C. 2017. Omega-3 fatty acids in rheumatic diseases: a critical review. J Clin Rheumatol. 23(6):330–339. doi: 10.1097/RHU.0000000000000563.
- Alexander NJ, Smythe NL, Jokinen MP. 1987. The type of dietary fat affects the severity of autoimmune disease in NZB/NZW mice. Am J Pathol. 127(1):106–121.
- Arriens C, Hynan LS, Lerman RH, Karp DR, Mohan C. 2015. Placebo-controlled randomized clinical trial of fish oil’s impact on fatigue, quality of life, and disease activity in Systemic Lupus Erythematosus. Nutr J. 14(1):82. doi: 10.1186/s12937-015-0068-2.
- Basta F, Fasola F, Triantafyllias K, Schwarting A. 2020. Systemic lupus erythematosus (SLE) therapy: the old and the new. Rheumatol Ther. 7(3):433–446. doi: 10.1007/s40744-020-00212-9.
- Bates MA, Akbari P, Gilley KN, Wagner JG, Li N, Kopec AK, Wierenga KA, Jackson-Humbles D, Brandenberger C, Holian A, et al. 2018. Dietary docosahexaenoic acid prevents silica-induced development of pulmonary ectopic germinal centers and glomerulonephritis in the lupus-prone NZBWF1 mouse. Front Immunol. 9:2002. doi: 10.3389/fimmu.2018.02002.
- Bates MA, Benninghoff AD, Gilley KN, Holian A, Harkema JR, Pestka JJ. 2019. Mapping of dynamic transcriptome changes associated with silica-triggered autoimmune pathogenesis in the lupus-prone NZBWF1 mouse. Front Immunol. 10:632. doi: 10.3389/fimmu.2019.00632.
- Bates MA, Brandenberger C, Langohr I, Kumagai K, Harkema JR, Holian A, Pestka JJ. 2015. Silica triggers inflammation and ectopic lymphoid neogenesis in the lungs in parallel with accelerated onset of systemic autoimmunity and glomerulonephritis in the lupus-prone NZBWF1 mouse. PLoS One. 10(5):e0125481. doi: 10.1371/journal.pone.0125481.
- Bates MA, Brandenberger C, Langohr II, Kumagai K, Lock AL, Harkema JR, Holian A, Pestka JJ. 2016. Silica-triggered autoimmunity in lupus-prone mice blocked by docosahexaenoic acid consumption. PLoS One. 11(8):e0160622. doi: 10.1371/journal.pone.0160622.
- Benninghoff AD, Bates MA, Chauhan PS, Wierenga KA, Gilley KN, Holian A, Harkema JR, Pestka JJ. 2019. Docosahexaenoic acid consumption impedes early interferon- and chemokine-related gene expression while suppressing silica-triggered flaring of murine lupus. Front Immunol. 10:2851. doi: 10.3389/fimmu.2019.02851.
- Benninghoff AD, Hintze KJ, Monsanto SP, Rodriguez DM, Hunter AH, Phatak S, Pestka JJ, Wettere AJV, Ward RE. 2020. Consumption of the total western diet promotes colitis and inflammation-associated colorectal cancer in mice. Nutrients. 12(2):544. doi: 10.3390/nu12020544.
- Bhattacharya A, Lawrence RA, Krishnan A, Zaman K, Sun D, Fernandes G. 2003. Effect of dietary n-3 and n-6 oils with and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice. J Am Coll Nutr. 22(5):388–399. doi: 10.1080/07315724.2003.10719322.
- Brown JM, Archer AJ, Pfau JC, Holian A. 2003. Silica accelerated systemic autoimmune disease in lupus-prone New Zealand mixed mice. Clin Exp Immunol. 131(3):415–421. doi: 10.1046/j.1365-2249.2003.02094.x.
- Brown JM, Pfau JC, Holian A. 2004. Immunoglobulin and lymphocyte responses following silica exposure in New Zealand mixed mice. Inhal Toxicol. 16(3):133–139. doi: 10.1080/08958370490270936.
- Burnet FM, Holmes MC. 1965. The natural history of the NZB/NZW F1 hybrid mouse: a laboratory model of systemic lupus erythematosus. Australas Ann Med. 14(3):185–191. doi: 10.1111/imj.1965.14.3.185.
- Calder PC. 2017. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 45(5):1105–1115. doi: 10.1042/BST20160474.
- Celhar T, Fairhurst AM. 2017. Modelling clinical systemic lupus erythematosus: similarities, differences and success stories. Rheumatology (Oxford). 56(suppl_1):i88–i99.
- Chandrasekar B, Troyer DA, Venkatraman JT, Fernandes G. 1995. Dietary omega-3 lipids delay the onset and progression of autoimmune lupus nephritis by inhibiting transforming growth factor beta mRNA and protein expression. J Autoimmun. 8(3):381–393. doi: 10.1006/jaut.1995.0030.
- Chang HY, Lee HN, Kim W, Surh YJ. 2015. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor γ activation. Life Sci. 120:39–47. doi: 10.1016/j.lfs.2014.10.014.
- Chiang N, Dalli J, Colas RA, Serhan CN. 2015. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 212(8):1203–1217. doi: 10.1084/jem.20150225.
- Crampton SP, Morawski PA, Bolland S. 2014. Linking susceptibility genes and pathogenesis mechanisms using mouse models of systemic lupus erythematosus. Dis Model Mech. 7(9):1033–1046. doi: 10.1242/dmm.016451.
- Djuricic I, Calder PC. 2021. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients. 2021. 13:2421. doi:10.3390/nu13072421.
- Dubois EL, Horowitz RE, Demopoulos HB, Teplitz R. 1966. NZB/NZW mice as a model of systemic lupus erythematosus. JAMA. 195(4):285–289. doi: 10.1001/jama.1966.03100040091025.
- Favor OK, Rajasinghe LD, Wierenga KA, Maddipati KR, Lee KSS, Olive AJ, Pestka JJ. 2023. Crystalline silica-induced proinflammatory eicosanoid storm in novel alveolar macrophage model quelled by docosahexaenoic acid supplementation [Original Research. Front Immunol. 14:1274147. doi: 10.3389/fimmu.2023.1274147.
- Finckh A, Cooper GS, Chibnik LB, Costenbader KH, Watts J, Pankey H, Fraser PA, Karlson EW. 2006. Occupational silica and solvent exposures and risk of systemic lupus erythematosus in urban women. Arthritis Rheum. 54(11):3648–3654. doi: 10.1002/art.22210.
- Flammer JR, Rogatsky I. 2011. Minireview: glucocorticoids in autoimmunity: unexpected targets and mechanisms. Mol Endocrinol. 25(7):1075–1086. doi: 10.1210/me.2011-0068.
- Flurkey KM, Currer J, Harrison DE, et al. 2007. Chapter 20 - Mouse models in aging research. In: Fox JG, Davisson MT, Quimby FW, editors. The mouse in biomedical research 2nd ed. Burlington: Academic Press; p. 637–672.
- Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, et al. 2016. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 7(1):12859. doi: 10.1038/ncomms12859.
- Gelfand MC, Steinberg AD, Nagle R, Knepshield JH. 1972. Therapeutic studies in NZB-W mice. I. Synergy of azathioprine, cyclophosphamide and methylprednisolone in combination. Arthritis Rheum. 15(3):239–246. doi: 10.1002/art.1780150304.
- Gilley KN, Wierenga KA, Chauhuan PS, Wagner JG, Lewandowski RP, Ross EA, Lock AL, Harkema JR, Benninghoff AD, Pestka JJ, et al. 2020. Influence of total western diet on docosahexaenoic acid suppression of silica-triggered lupus flaring in NZBWF1 mice. PLoS One. 15(5):e0233183. doi: 10.1371/journal.pone.0233183.
- Godfrey DG, Stimson WH, Watson J, Belch JF, Sturrock RD. 1986. Effects of dietary supplementation on autoimmunity in the MRL/lpr mouse: a preliminary investigation. Ann Rheum Dis. 45(12):1019–1024. doi: 10.1136/ard.45.12.1019.
- Hagan C. 2017. When are mice considered old? [cited. https://www.jax.org/news-and-insights/jax-blog/2017/November/when-are-mice-considered-old.
- Halade GV, Williams PJ, Veigas JM, Barnes JL, Fernandes G. 2013. Concentrated fish oil (Lovaza(R)) extends lifespan and attenuates kidney disease in lupus-prone short-lived (NZBxNZW)F1 mice. Exp Biol Med (Maywood). 238(6):610–622. doi: 10.1177/1535370213489485.
- Heine LK, Benninghoff AD, Ross EA, Rajasinghe LD, Wagner JG, Lewandowski RP, Richardson AL, Li QZ, Buchweitz JP, Zyskowski J, et al. 2022. Comparative effects of human-equivalent low, moderate, and high dose oral prednisone intake on autoimmunity and glucocorticoid-related toxicity in a murine model of environmental-triggered lupus. Front Immunol. 13:972108. doi: 10.3389/fimmu.2022.972108.
- Hicks JD, Burnet FM. 1966. Apr Renal lesions in the "auto-immune" mouse strains NZB and F1 NZBxNZW. J Pathol Bacteriol. 91(2):467–476. doi: 10.1002/path.1700910221.
- Hoy RF, Chambers DC. 2020. Silica‐related diseases in the modern world. Allergy. 75(11):2805–2817. doi: 10.1111/all.14202.
- Hwang DH, Kim JA, Lee JY. 2016. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur J Pharmacol. 785:24–35. doi: 10.1016/j.ejphar.2016.04.024.
- Jin F, Li Y, Wang X, Yang X, Li T, Xu H, Wei Z, Liu H et al. 2022. Effect of sex differences in silicotic mice. Int J Mol Sci. 23(22):14203. doi: 10.3390/ijms232214203.
- Lamore R, Parmar S, Patel K, Hilas O. 2012. Belimumab (Benlysta): a breakthrough therapy for systemic lupus erythematosus. P and T. 37(4):212–226.
- Lands B, Bibus D, Stark KD. 2018. Sep Dynamic interactions of n-3 and n-6 fatty acid nutrients. Prostaglandins Leukot Essent Fatty Acids. 136:15–21. doi: 10.1016/j.plefa.2017.01.012.
- Li X, Bi X, Wang S, Zhang Z, Li F, Zhao AZ. 2019. Therapeutic potential of ω-3 polyunsaturated fatty acids in human autoimmune diseases. front Immunol. 10:2241. doi: 10.3389/fimmu.2019.02241.
- Li X, Yu Y, Funk CD. 2013. Dec Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). Faseb J. 27(12):4987–4997. doi: 10.1096/fj.13-235333.
- Ostermann AI, Schebb NH. 2017. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food Funct. 8(7):2355–2367. Jul 06. doi: 10.1039/c7fo00403f.
- Parks CG, Conrad K, Cooper GS. 1999. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 107 (Suppl 5):793–802. doi: 10.1289/ehp.99107s5793.
- Parks CG, Cooper GS, Nylander-French LA, Sanderson WT, Dement JM, Cohen PL, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS, et al. 2002. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case-control study in the southeastern United States. Arthritis Rheum. 46(7):1840–1850. doi: 10.1002/art.10368.
- Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH. 2017. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 31(3):306–320. doi: 10.1016/j.berh.2017.09.005.
- Pestka JJ, Akbari P, Wierenga KA, Bates MA, Gilley KN, Wagner JG, Lewandowski RP, Rajasinghe LD, Chauhan PS, Lock AL, et al. 2021. Omega-3 polyunsaturated fatty acid intervention against established autoimmunity in a murine model of toxicant-triggered lupus. Front Immunol. 12:653464. doi: 10.3389/fimmu.2021.653464.
- Pestka JJ, Vines LL, Bates MA, He K, Langohr I. 2014. Comparative effects of n-3, n-6 and n-9 unsaturated fatty acid-rich diet consumption on lupus nephritis, autoantibody production and CD4+ T cell-related gene responses in the autoimmune NZBWF1 mouse [Comparative Study Research Support, N.I.H., Extramural]. PLoS One. 9(6):e100255. doi: 10.1371/journal.pone.0100255.
- Pestka JJ. 2010. n-3 Polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis. Prostaglandins Leukot Essent Fatty Acids. 82(4-6):251–258. doi: 10.1016/j.plefa.2010.02.013.
- Poinen-Rughooputh S, Rughooputh MS, Guo Y, Lai H, Sun W, Chen W. 2021. Sex-related differences in the risk of silicosis among chinese pottery workers: a cohort study. J Occup Environ Med. 63(1):74–79. doi: 10.1097/JOM.0000000000002068.
- Porta S, Danza A, Arias Saavedra M, Carlomagno A, Goizueta MC, Vivero F, Ruiz-Irastorza G. 2020. Glucocorticoids in systemic lupus erythematosus. ten questions and some issues. J Clin Med. 9(9):2709–2709. doi: 10.3390/jcm9092709.
- Prickett JD, Robinson DR, Steinberg AD. 1982. A diet enriched with eicosapentaenoic acid suppresses autoimmune nephritis in female (NZB x NZW) F1 mice. Trans Assoc Am Physicians. 95:145–154.
- Raanan R, Zack O, Ruben M, Perluk I, Moshe S. 2022. Occupational silica exposure and dose; response for related disorders; silicosis, pulmonary TB, AIDs and renal diseases: results of a 15-year Israeli surveillance. Int J Environ Res Public Health. 19(22):15010. doi: 10.3390/ijerph192215010.
- Radulescu CI, Cerar V, Haslehurst P, Kopanitsa M, Barnes SJ. 2021. The aging mouse brain: cognition, connectivity and calcium. Cell Calcium. 94:102358. 2021/03/01/doi: 10.1016/j.ceca.2021.102358.
- Rajasinghe LD, Li Q-Z, Zhu C, Yan M, Chauhan PS, Wierenga KA, Bates MA, Harkema JR, Benninghoff AD, Pestka JJ, et al. 2020. Omega-3 fatty acid intake suppresses induction of diverse autoantibody repertoire by crystalline silica in lupus-prone mice. Autoimmunity. 53(7):415–433. doi: 10.1080/08916934.2020.1801651.
- Ramos PS, Brown EE, Kimberly RP, Langefeld CD. 2010. Genetic factors predisposing to systemic lupus erythematosus and lupus nephritis. Semin Nephrol. 30(2):164–176. doi: 10.1016/j.semnephrol.2010.01.007.
- Ray JL, Holian A. 2019. Sex differences in the inflammatory immune response to multi-walled carbon nanotubes and crystalline silica. Inhal Toxicol. 31(7):285–297. doi: 10.1080/08958378.2019.1669743.
- Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. 2009. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 30(9):455–464. doi: 10.1016/j.it.2009.06.003.
- Richard ML, Gilkeson G. 2018. Mouse models of lupus: what they tell us and what they don’t. Lupus Sci Med. 5(1):e000199. doi: 10.1136/lupus-2016-000199.
- Ricote M, Glass CK. 2007. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 1771(8):926–935. doi: 10.1016/j.bbalip.2007.02.013.
- Robinson DR, Prickett JD, Polisson R, Steinberg AD, Levine L. 1985. The protective effect of dietary fish oil on murine lupus. Prostaglandins. 30(1):51–75. doi: 10.1016/s0090-6980(85)80010-1.
- Rudofsky UH, Lawrence DA. 1999. New Zealand mixed mice: a genetic systemic lupus erythematosus model for assessing environmental effects. Environ Health Perspect. 107 (Suppl 5):713–721. doi: 10.1289/ehp.99107s5713.
- Serhan CN, Chiang N, Dalli J. 2015. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. 27(3):200–215. doi: 10.1016/j.smim.2015.03.004.
- Sham HP, Walker KH, Abdulnour R-EE, Krishnamoorthy N, Douda DN, Norris PC, Barkas I, Benito-Figueroa S, Colby JK, Serhan CN, et al. 2018. Apr 15 15-epi-lipoxin A(4), resolvin D2, and resolvin D3 induce NF-kappa B regulators in bacterial pneumonia. J Immunol. 200(8):2757–2766. doi: 10.4049/jimmunol.1602090.
- Titos E, Rius B, López-Vicario C, Alcaraz-Quiles J, García-Alonso V, Lopategi A, Dalli J, Lozano JJ, Arroyo V, Delgado S, et al. 2016. Signaling and immunoresolving actions of resolvin D1 in inflamed human visceral adipose tissue. J Immunol. 197(8):3360–3370. doi: 10.4049/jimmunol.1502522.
- Tschanz SA, Burri PH, Weibel ER. 2011. A simple tool for stereological assessment of digital images: the STEPanizer. J Microsc. 243(1):47–59. doi: 10.1111/j.1365-2818.2010.03481.x.
- Tsokos GC. 2011. Systemic lupus erythematosus. N Engl J Med. 365(22):2110–2121. doi: 10.1056/NEJMra1100359.
- Watson J, Godfrey D, Stimson WH, Belch JJ, Sturrock RD. 1988. The therapeutic effects of dietary fatty acid supplementation in the autoimmune disease of the MRL-mp-lpr/lpr mouse. Int J Immunopharmacol. 10(4):467–471. doi: 10.1016/0192-0561(88)90135-x.
- Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. 2005. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 174(9):5390–5397. doi: 10.4049/jimmunol.174.9.5390.
- Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, et al. 2004. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 15(2):241–250. doi: 10.1097/01.asn.0000108969.21691.5d.
- Westberg G, Tarkowski A, Svalander C. 1989. Effect of eicosapentaenoic acid rich menhaden oil and MaxEPA on the autoimmune disease of Mrl/l mice. Int Arch Allergy Appl Immunol. 88(4):454–461. doi: 10.1159/000234732.
- Wierenga KA, Harkema JR, Pestka JJ. 2019. Lupus, silica, and dietary omega-3 fatty acid interventions. Toxicol Pathol. 47(8):1004–1011. doi: 10.1177/0192623319878398.
- Wierenga KA, Strakovsky RS, Benninghoff AD, Rajasinghe LD, Lock AL, Harkema JR, Pestka JJ. 2020. Requisite omega-3 HUFA biomarker thresholds for preventing murine lupus flaring. Front Immunol. 11:1796. doi: 10.3389/fimmu.2020.01796.
- Wong SW, Kwon M-J, Choi AMK, Kim HP, Nakahira K, Hwang DH. 2009. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 284(40):27384–27392. doi: 10.1074/jbc.M109.044065.
- Wu H, Chang C, Lu Q. 2020. The Epigenetics of Lupus Erythematosus. Springer Singapore; p. 185–207.
- Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R, et al. 2013. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 38(6):1154–1163. doi: 10.1016/j.immuni.2013.05.015.
- Yu F, Haas M, Glassock R, Zhao MH. 2017. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol. 13(8):483–495. doi: 10.1038/nrneph.2017.85.
- Yu H, Nagafuchi Y, Fujio K. 2021. Clinical and Immunological Biomarkers for Systemic Lupus Erythematosus. Biomolecules. 11(7):928. doi: 10.3390/biom11070928.
- Zucchi D, Elefante E, Schilirò D, Signorini V, Trentin F, Bortoluzzi A, Tani C. 2022. One year in review 2022: systemic lupus erythematosus. Clin Exp Rheumatol. 40(1):4–14. doi: 10.55563/clinexprheumatol/nolysy.

