Abstract
The risk of Hepatitis C Virus (HCV) acquisition among People Who Inject Drugs (PWID) remains high when injecting risk behavior within networks endures. Several psychosocial factors influence such behavior. Following a drive within Tayside, a geographic region in Scotland, to achieve World Health Organization HCV elimination targets, addressing HCV re-infection risk as a barrier to elimination is critically important. This cross-sectional study seeks to address this barrier to elimination by investigating associations between group identification (one’s subjective sense of belonging and connectedness to a social group coupled with a sense of shared goals, beliefs and values with the other members of the group) and injecting risk behavior among PWID on HCV treatment at needle and syringe provision sites in Tayside. Participants completed psychosocial questionnaires between treatment weeks zero and three of treatment. Correlation analyses were undertaken, and significant factors included in multiple linear regression models for injecting risk behavior. Injecting frequency, drug network identification, and family identification, were correlated with injecting risk behavior, and drug network identification had a positive predictive on injecting risk behavior. Identification with a social group, conventionally associated with improved health, may pose health risks in specific contexts. Healthcare providers should consider stratifying individuals with higher group identification with PWID networks for enhanced harm reduction engagement to mitigate transmissible infection risk among PWID. Additionally, psychological interventions to strengthen group identification with networks which impact positively on health behavior should be explored.
Introduction
Hepatitis C Virus (HCV) is a global public health threat—with an estimated 56.4 million infected people worldwide—which can lead to significant long-term negative health outcomes.Citation1 Recent estimates indicate that, despite a reduction in the global prevalence of chronic HCV, and the delivery of 9.4 (95% CI 7.5–11.7) million HCV treatments globally from 2015 to 19, World Health Organization (WHO) targets to eliminate it as a public health threat by 2030 are likely to be missed, with an attendant rise of up to 17% in rates of end-stage liver disease relative to 2020.Citation1–3 In high-income countries, a critical determinant of HCV transmission is injection drug use (IDU) among people who inject drugs (PWID). Globally, PWID account for an estimated 8.5% (95% CI 4.6–13.1) of all HCV cases, with close to 40% of individuals with recent IDU infected with HCV.Citation4 With the relatively recent availability of short courses of highly effective and well-tolerated Direct Acting Antivirals (DAAs) for HCV, cure rates among PWID, including those disclosing active IDU, have exceeded 95%.Citation5 Alongside contemporary diagnostics which can enable task-shifting to low-threshold non-clinical environments, the tools required to achieve HCV elimination—in the continuing absence of a vaccine—are broadly known to researchers and clinicians.Citation6,Citation7
Despite these therapeutic and diagnostic advances, and their associated high cure rates among PWID, HCV re-infection remains a critical barrier to sustained elimination of HCV.Citation8 Re-infection is associated with dynamism and social patterns within PWID networks, wherein sharing of injection-related equipment is common.Citation9,Citation10 Often persons supplying substances within a group will inject first, with the individuals supplying the equipment injecting next.Citation11 In the absence of sufficient global scale-up of harm reduction provision, including supply of approximately 300 clean needles and syringes per PWID per annum globally, exploring the dynamics which underlie risk behavior, such as sharing of injection equipment, among PWID will be important to understanding and reducing the likelihood re-infection.Citation2 Recent real-world data from Scotland has demonstrated high re-infection rates among PWID in carceral (14.3 per 100 person-years [PY]; 95% CI 11.1–18.5) and community (9.5 per 100PY; 95%CI 7.8–11.7) environments, as well as among those treated as part of Tayside’s regional treatment-as-prevention (TasP) program (15.20 per 100 PY; 95% CI 10.81–20.78).Citation12,Citation13 Importantly, recent figures suggest that from 2017 to 20 needle and syringe provision (NSP) per PWID per annum declined from 66 to 33 in Tayside and from 68 to 47 across Scotland.Citation14 Well below the WHO target. However, numerous re-infections were also identified as a proportion of new diagnoses (42/183; 23%) in the Icelandic TasP program, even in the context of high needle (404/PWID per year) and syringe (214/PWID per year) provision, so increased NSP provision is not necessarily a panacea for HCV re-infection.Citation15
Recommendations to address re-infection rates among PWID to date have included educational initiatives, behavioral interventions, improved linkage to care, counseling, and increased engagement with harm reduction services.Citation8 Reported initiatives have further been underpinned by peer-led or peer-informed approaches to NSP provision, home-based overdose education and naloxone distribution sites, and strategies to promote safer injecting practices.Citation16–18 However, despite these innovative approaches, public health educational initiatives targeting the risks of HCV acquisition have yet to be proven effective in reducing transmission, and recent global estimates have suggested that less than 1% of PWID live in countries with high coverage of both NSP and opioid agonist therapy (OAT).Citation1,Citation19 It is estimated that only 33 needles and syringes are distributed worldwide per PWID annually.Citation19 Even in a context of high NSP, some have suggested that harm reduction and counseling interventions may be erroneously targeting factors of lesser relevance, such as health motivation, and delay discounting (i.e., higher-valued, delayed outcomes are dismissed in favor of lower-valued, immediate outcomes, denoting decreased ability to control behavior).Citation20–22
The global inadequate access to harm reduction, high reported real-world rates of re-infection among PWID, and the evidence on the influence of social groups on injecting behavior,Citation23–30 demonstrate a need for further targets of behavior change to decrease risk of re-infection. This study aimed to explore psychosocial factors which may be associated at the individual level with self-reported risky injecting behavior among PWID receiving DAA treatment for HCV, in order to inform potential strategies to reduce the risk of re-infection among PWID. Specifically, we aimed to explore the role of group identification on injecting risk behavior. Group identification refers to one’s subjective sense of belonging and connectedness to a social group coupled with a sense of sharing goals, beliefs and values with the other members of the group.Citation31 Identifying with a social group therefore implies that the self is, to some extent at least, defined by the group. Research based on the social identity approach has demonstrated that a greater number of group identifications is associated with better mental and physical health.Citation31–33 Indeed, recent research has demonstrated that among PWID who do not share injecting paraphernalia, greater levels of community attachment (i.e., identification with a peer network) among PWID has significant positive impacts upon internalized stigma and well-being, though this was not the case among those who share injecting equipment.Citation34
To control for secondary influential factors which may be associated with group identification and that impact on injecting risk behavior, mental health variables and illness perception were investigated in the present study. High levels of depression and anxiety and experience of trauma have been evidenced to be associated with more sharing of injecting equipment practices in numerous studies. Citation23,Citation25–27,Citation29,Citation30,Citation35 Further to these, HCV risk perception (the perceived risk of acquiring HCV by undertaking a specific task, such as sharing equipment) has also been shown to be associated with injecting risk,Citation23,Citation28,Citation36,Citation37 coupled with illness perception (how an individual mentally frames living with an infection or disease, that can impact on one’s ability to cope with it), by influencing treatment coping and behavioral adjustments.Citation38–42 Therefore these parameters were also assessed in the present study to investigate any effects on injecting risk behavior and any associations with group identification.
To our knowledge, this is the first study to investigate the role of group identification on injecting risk behavior among PWID receiving HCV treatment who currently inject drugs.
Methods
Study design
This cross-sectional study was co-sponsored by National Health Service (NHS) Tayside and University of Dundee (ref: 2017PZ04) and received favorable ethical opinion from East of Scotland NHS Research Ethics Service (ref: 17/ES/0150). Individuals diagnosed with active HCV infection at two NSP sites were approached to participate in the research prior to initiating treatment with DAAs. The study was advertised through word-of-mouth by NSP nursing staff during routine NSP visits. Interested individuals were referred by nurses to a member of the research team (AM) to discuss the study and consent to participate. This same research team member completed the survey measures with participants. Participation was voluntary; there were no financial incentives, but participants could avail of nutrient drinks (Ensure nutritional shakes) which are routinely offered on site as part of standard care. The study visit lasted, on average, for 30 minutes, though this was not formally recorded.
Setting
NHS Tayside is Scotland’s fourth-largest health board and serves approximately 400,000 people in the East of Scotland, of whom approximately 2,800 are estimated to be PWID. HCV treatment for PWID is delivered by a multidisciplinary team through multiple community venues, including prisons, pharmacies, outreach clinics, NSP sites, and drug treatment services.Citation13 All healthcare provided by the NHS is free to patients at the point of delivery. This study was in a population of PWID diagnosed with HCV and receiving treatment with DAAs at two NSP sites in the region.
Participants
HCV RNA+ adults (≥18 years) prescribed DAA treatment for HCV at participating NSP sites, who self-reported active IDU, were eligible to participate. All participants provided written informed consent. Exclusion criteria included inability to communicate in English; inability to provide informed consent; or aggressive/violent behavior: no participants were excluded. All data collection occurred at the NSP sites between weeks zero and three of HCV treatment.
Measures
Basic demographic data was collected, and the following validated questionnaires were administered to participants by lead researcher (AM): Injecting Risk Questionnaire (IRQ),Citation43 Group Identification Scale (GIS)Citation44 adapted for Drug Network and for Family, Patient Health Questionnaire (PHQ-9),Citation45 General Anxiety Disorder (GAD-7),Citation46 Post Traumatic Stress Disorder (PTSD-5),Citation47 and Illness Perception Questionnaire (B-IPQ), adapted to HCV.Citation48 The primary outcome under investigation was injecting risk behavior (measured with the IRQ), defined as sharing injecting paraphernalia with other PWID. The IRQ was comprised of 15 items describing risky injection behaviors such as: 4) given or lent used needles/syringes to a friend or acquaintance; 6) injected with needles/syringes that had already been used by a sexual partner; Participants were asked to report how often they engaged in each behavior in the past 4 weeks on a 4-point Likert-type scale from “never” to “frequently.” The total score was taken as the mean of all items. Similarly, the GIS scales for family and drug injecting network were each comprised of 4 items, such as: 1) I feel a bond with my injecting/drug network; 3) I have a sense of belonging to my family; Participants were asked to report their level of identification on a 7-point Likert-type scale from “strongly agree” to “strongly disagree.” The total score was taken as the mean of all items. A score of 3 or more indicated a sense of identification with the group.
For each questionnaire, higher scale scores reflect greater levels of that measure; for example, higher IRQ scores reflect greater levels of injecting risk behavior; lower PTSD-5 scores reflect lower levels of PTSD, and so on across all instruments. Scoring systems and example scale items are shown in Supplementary file 1 (Supplementary Table S1). Including all measures, the survey took approximately 30 minutes to complete per participant.
Table 1. Descriptive characteristics of study participants (n = 50).
Recorded clinical parameters included HCV genotype; Sustained Virologic Response 12 weeks post treatment (SVR12), defined as undetectable HCV RNA (<10 IU/mL); or occurrence of re-infection, defined as quantifiable HCV RNA (>10IU/mL) following prior undetected RNA with a change in HCV genotype. Clinical outcomes were followed up to 03/01/2020. All testing was undertaken on whole blood obtained by conventional phlebotomy and analyzed by NHS laboratories at Ninewells Hospital, Dundee, using the Hologic Panther platform with HCV Quant Dx Assay or the Abbott Real-Time m2000sp and m2000rt Polymerase Chain Reaction (PCR) platform.Citation49,Citation50
Statistics
Descriptive analyses were undertaken to assess participant characteristics, scores of all measured variables, and median (interquartile range) days from pretreatment HCV RNA test to treatment initiation. Bivariate analysis was undertaken using Spearman’s rank correlation test and Pearson’s chi-squared test to explore factors associated with injecting risk behavior. Variables significantly correlated with injecting risk behavior were included in a multiple linear regression model to investigate possible predictors of injecting risk behavior. Consequent to minor regression assumption violations identified in post-hoc assessments, the model was re-fitted using the bootstrap option—a robust method in the presence of assumption violations—to verify parameter estimates (1,000 samples).Citation51 Analysis was undertaken using IBM SPSS Statistics 25 and p values of ≤0.05 were assumed to demonstrate statistical significance.
Results
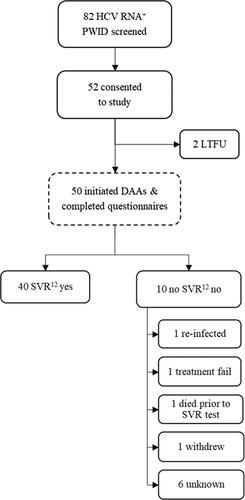
From 23 February 2018 to 09 August 2019, 82 HCV+ PWID were approached to participate in the study, of which 52 consented to take part and 50 participated (). Of those who declined to participate (n = 30), all were eligible but declined due to lack of interest.
Median time from pretreatment HCV RNA testing confirming infection to treatment initiation was 19 days (11–39). When followed-up for clinical outcomes, most participants obtained SVR12 (n = 40; 80%). Of those who did not obtain SVR12, one was confirmed re-infected at SVR12, one was a confirmed unsuccessful treatment, one died post treatment but prior to follow-up (cause unknown), one withdrew from treatment and was not followed-up for SVR12, with the reasons for the remaining six unknown to the researchers at the end of the study.
Participant characteristics are shown in . In summary, most were male, under 40 years of age, and reported injecting multiple times a week. The majority of individuals injected opioids, with some injecting both opioids and stimulants. A small proportion of participants had an intimate partner who also injected drugs, and most HCV infections were genotype 3, which is aligned with the known distribution of HCV infection in Tayside.
Questionnaire scores are reported in . As questionnaires were administered by the lead researcher (AM) no items were missing. The mean IRQ score (the primary outcome) was relatively low on the scale meaning that, on average, participants considered their injection behavior (e.g., sharing paraphernalia) to be very infrequent. However, the results () show that reported sharing of water to inject was disproportionately high relative to other items. On average, individuals had strong scores for identification with family networks and drug networks, as well as high scores for depression, anxiety, and post-traumatic stress disorder (PTSD). Interestingly, despite these high scores across several illness categories—as well as their diagnosed HCV infection—participants on average had middling illness perceptions scores. The higher the score on this scale the more threatening the perception of illness, with the results suggesting that participants did not perceive HCV as a particularly threatening illness.
Table 2. Average questionnaire scores at treatment initiation (n = 50).
Bivariate analyses () revealed several statistically significant associations between questionnaire items and self-reported injecting risk behavior. Injecting risk behavior was significantly positively correlated with injecting frequency (ρ = 0.57, p=<.001), implying that where injecting behavior (i.e., equipment sharing) was self-reported as more frequent by participants, injecting frequency was also higher; negatively correlated with identification with family (ρ = −0.31, p=.030), suggesting that where injecting behavior was self-reported as more frequent by participants, identification with family networks was lower; and positively correlated with identification with drug network (ρ = 0.46, p=.001), inferring that where injecting behavior was self-reported as more frequent by participants, identification with drug network(s) was higher. Chi-squared tests of association revealed no significant correlations between categorical variables (gender; HCV genotype; partner; SVR12; substances injected) and injecting risk behavior.
Table 3. Spearman’s correlation analysis for continuous variables (n = 50).
Additional correlations were observed between secondary variables: injecting frequency was positively correlated with group identification with drug network (ρ = 0.34, p =.014); and group identification with family was negatively correlated with anxiety (ρ = −0.29, p =.043). Mental health variables (depression, anxiety, and PTSD) were unsurprisingly mutually correlated, with coefficients ranging from ρ = 0.69 to ρ = 0.82. These variables were also positively correlated with illness perception, with all coefficients ranging between ρ = 0.39 to ρ = 0.48.
A multiple linear regression was undertaken with injecting frequency, identification with family, and identification with a drug network, as independent predictors of injecting risk behavior. Violations were observed relating to assumptions of homoscedasticity, linearity, and normality of residuals, and the model was therefore re-fitted with a bootstrapped sample.
Parameter estimates for both models are shown in . In the original model, injecting frequency and identification with a drug network were both positive predictors of injection risk behavior. However, in the re-sampled model, the effect for injecting frequency became non-significant. The re-sampled model was had a statistically significant fit with the data, F(3,46)=5.67; p=.002, and explained 27% (R2= 0.27) of the variance in injecting risk behavior.
Table 4. Multiple linear regression coefficients for original and bootstrap samples.
Discussion
In this study among actively injecting PWID on DAA treatment for HCV, participants reported, on average, infrequent injecting risk behavior, and high rates of identification with family and other PWID. Most individuals obtained SVR12 and there was one confirmed re-infection. Questionnaire data revealed high levels of anxiety, depression, and PTSD, and a perception of HCV infection as a non-threatening disease, which suggests that harm reduction provided within viral hepatitis services in Tayside may not sufficiently communicate the long-term risks associated with HCV (re)infection (e.g., hepatocellular carcinoma).
Despite low overall reported frequency of injecting risk behavior, sharing of water was disproportionate relative to other equipment pieces. Previous research has reported similar findings, suggesting spoons and water are more frequently shared than needles and syringes within PWID networks.Citation52,Citation53 In contrast, increased needle and syringe sharing has been shown to be associated more often with intimate partnerships, as it can be perceived as more personal and intrusive than spoon or water sharing.Citation51–54 The low rate of sharing of these items in our study aligns with the small proportion of individuals in intimate partnerships in our sample. The relationship between social factors, such as size or nature of one’s network, and injecting risk behavior has been widely documented,Citation55–58 but to our knowledge, this is the first report in the literature examining the relationship between the construct of drug network identification and injecting risk behavior among actively injecting HCV+ PWID. The findings of this study are consistent with prior research which suggests that social networks can negatively influence injection risk taking.Citation59–61 However, research by Brener and colleagues has shown the valuable protective role that strong community attachment to PWID networks can play—particularly among those who do not report sharing of injecting paraphernalia—in reducing internalized stigma.Citation34 Whilst our results suggest that stronger identification with PWID networks predicts higher injection equipment sharing and, by extension, risk of HCV re-infection, we acknowledge the potential positive effects of connectedness to a community of peers with similar lived experience can have. Multiple studies have demonstrated this through initiatives to promote harm reduction messaging; recruit peers into HCV testing and treatment; and drive engagement into established harm reduction services through providing HCV awareness, knowledge exchange and education sessions. Citation17,Citation18,Citation34,Citation62–64 We believe our work extends the existing literature by identifying associations between group identification, as a characteristic of psychological connectedness, and injecting risk behaviors.
The exploratory correlation analyses revealed that more frequent injecting risk behavior was significantly correlated with higher injecting frequency and greater identification with a drug network, whilst simultaneously being associated with lesser connection to family members. These results suggest that those who reported more frequent injecting risk practices felt a stronger sense of identification with peers who use drugs, tended to inject more often than participants who reported safer injecting practices, and felt a sense of disconnection from their family members. Some research has been carried out on the influence of group identification on substance use in adolescents and young adults, yielding conflicting results, whilst identification with family has been shown to promote healthy behavior in adults.Citation31,Citation65,Citation66 We would cautiously suggest, on the basis of this exploratory aspect of the analysis, that future research might consider developing and trialing interventions within PWID networks which foster a sense of connection to existing family members, or other supportive groups beyond the context of substance use, to investigate whether this would reduce the likelihood of risky injecting and the subsequent risk of HCV transmission. We purposefully suggest other supportive groups beyond the context of substance use, as PWID may purposefully be less connected to family members or disconnected for reasons beyond their control or remedy.
Indeed, the association between social factors and engagement in risk behaviors has potential to be a positive one. Some social associations, such as intimate partnerships, can become sources of social care and protection. They have the capacity to reduce risk behavior, such as injecting frequency, and increasing a sense of acceptance, belonging and self-worth.Citation67,Citation68 The exploratory associations further suggest that participants with higher scores for identification with family had lower scores for anxiety, which could, again, form the basis of further research to investigate a possible mediated relationship between connectedness to family members and injecting risk behavior, as anxiety has been shown to be associated with severity of substance use,Citation69 despite not being significantly associated with equipment sharing in our results. This may have been due to the limited sample size, as such associations have been reported in the literature previously.Citation25–27,Citation70
Group identification is characterized by the subjective dimension of an individual’s sense of communal experience and psychological connection with fellow group members.Citation44,Citation66, Citation67,Citation71 The re-sampled model suggested that group identification, particularly with an existing network of PWID, was a significant positive predictor of injecting risk behavior, revealing that the stronger the sense of identification with other PWID, the higher the likelihood that an individual would share injecting equipment. This suggests that risky injecting behavior is predicted and enhanced by strong social connectedness within PWID networks. In the absence of sufficient global coverage of harm reduction, which would increase the quantity of sterile injecting equipment within injecting networks at population levels, future work in a harm reduction context could focus on identifying those with higher scores of identification with PWID networks for enhanced harm reduction provision to mitigate individual risk. Thereby potentially decreasing the likelihood of non-sterile injecting equipment sharing within networks. The impact of such enhanced interventions could be critical to the long-term viability of achieving HCV elimination through TasP, which may be vulnerable to risks associated with re-infection among PWID, which is more likely to occur in the absence of sufficient harm reduction measures.Citation9,Citation10 Future work could explore this construct in a larger sample to validate our findings in this exploratory work.
The primary limitation of this study is the sample size which, being relatively small, may have been the root of assumption violations in the primary linear regression model. To overcome this, a bootstrap analysis was undertaken, which is a robust method that is appropriate when model assumptions are not met.Citation51 Further, as this was a cross-sectional study, it is possible that those who participated differed from those who did not, meaning the sample may not be wholly representative.Citation72 This was ameliorated somewhat by recruiting from NSP sites in two cities in Tayside, the high uptake of the offer to participate, and the purposefully broad inclusion criteria.
Conclusions
Injecting risk behavior is associated with multiple individual- and social-level factors. Demographic and clinical variables were explored and found to be unrelated to risk behavior. Mental health variables and HCV illness perception, although interrelated, showed no significant association with sharing behavior. On the whole, HCV was not perceived as a threatening illness. The findings of this study, underscore the importance of subjective identification, feelings of belonging, shared views, values, shared experiences and a sense of connectedness within PWID networks in affecting risk taking with injecting equipment. These findings have implications for HCV transmission as global provision of harm reduction and linkage to care is inadequate. The results may help healthcare providers to identify individuals at higher risk of risky injecting behavior and ensure enhanced harm reduction provision. Furthermore, future research may explore opportunities for individual behavioral/psychological interventions to strengthen group identification with networks that may confer positive influences on health behaviors, which may impact on reducing the likelihood of HCV transmission in this population.
Author contributions
AM conceived and designed the study, collected the data, analyzed, and interpreted the results, and contributed to the first, and subsequent drafts, of the manuscript. CJB interpreted the results and contributed to the first, and subsequent drafts, of the manuscript. AE, KP, FS, and JFD, provided senior supervision of the research. All authors critically reviewed and approved the submitted manuscript.
Supplemental Material
Download MS Word (17.9 KB)Data availability
The anonymized data will be held by the research team. Sharing of data will be considered for researchers who provide a methodologically sound proposal.
Disclosure statement
CJB has received honoraria from the International Network on Health and Hepatitis in Substance Users (INHSU), unrelated to the submitted work. AM, FS, AE and KP have no declarations. JFD has received honoraria for lectures and research grants from Janssen-Cilag, Roche, Merck Sharp & Dohme, AbbVie, Bristol-Myers Squibb and Gilead Sciences, outside the submitted work.
Additional information
Funding
References
- Blach S, Terrault N, Tacke F, et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7(5):396–415. doi:10.1016/S2468-1253(21)00472-6
- World Health Organization. 2016. Global Health Sector Strategy on Viral Hepatitis 2016–2021: Towards ending viral hepatitis. Geneva: WHO Document Production Services. Accessed February 22, 2022. https://apps.who.int/iris/handle/10665/246177.
- World Health Organization. 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Geneva: World Health Organization. Accessed February 22, 2022. https://www.who.int/publications/i/item/9789240027077.
- Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction. 2019;114(1):150–166. doi:10.1111/add.14393
- Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(11):754–767. doi:10.1016/S2468-1253(18)30304-2
- Lazarus J, Pericàs J, Picchio C, et al. We know DAAs work, so now what? Simplifying models of care to enhance the hepatitis C cascade. J Intern Med. 2019;286(5):503–525. doi:10.1111/joim.12972
- Oru E, Trickey A, Shirali R, Kanters S, Easterbrook P. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: a global systematic review and meta-analysis. Lancet Glob Health. 2021;9(4):e431–e445. doi:10.1016/S2214-109X(20)30505-2
- Falade-Nwulia O, Sulkowski M, Merkow A, Latkin C, Mehta S. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J Viral Hepat. 2018;25(3):220–227. doi:10.1111/jvh.12859
- Hellard M, Rolls D, Sacks Davis R ‐, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60(6):1861–1870. doi:10.1002/hep.27403
- Hellard M, McBryde E, Sacks Davis R, et al. Hepatitis C transmission and treatment as prevention—The role of the injecting network. Int J Drug Policy. 2015;26(10):958–962. doi:10.1016/j.drugpo.2015.05.006
- Lafferty L, Rance J, Treloar C. Who goes first? Understanding hepatitis C risk among injecting networks in the prison setting. Drug Alcohol Depend. 2018;183:96–101. doi:10.1016/j.drugalcdep.2017.10.030
- Yeung A, Palmateer N, Dillon J, et al. Population-level estimates of hepatitis C reinfection post scale-up of direct-acting antivirals among people who inject drugs. J Hepatol. 2022;76(3):549–557. doi:10.1016/j.jhep.2021.09.038
- Byrne C, Beer L, Inglis S, et al. Real‐world outcomes of rapid regional hepatitis C virus treatment scale‐up among people who inject drugs in Tayside, Scotland. Aliment Pharmacol Ther. 2022;55(5):568–579. doi:10.1111/apt.16728
- Public Health Scotland. Injecting Equipment Provision in Scotland: 2017/18, 2018/19 and 2019/20. Public Health Scotland; 2020. Table 3.2. Accessed February 23, 2022. https://publichealthscotland.scot/publications/injecting-equipment-provision-in-scotland/injecting-equipment-provision-in-scotland-2020-to-2021/.
- Olafsson S, Fridriksdottir R, Love T, et al. Cascade of care during the first 36 months of the treatment as prevention for hepatitis C (TraP HepC) programme in Iceland: a population-based study. Lancet Gastroenterol Hepatol. 2021;6(8):628–637. doi:10.1016/S2468-1253(21)00137-0
- Bryant J, Brener L, Pepolim L, Harrod M. Care, agency and criminality: Making sense of authorised extended distribution in the accounts of key stakeholders. Int J Drug Policy. 2019;71:56–61. doi:10.1016/j.drugpo.2019.06.008
- Kolla G, Strike C. ‘It’s too much, I’m getting really tired of it’: Overdose response and structural vulnerabilities among harm reduction workers in community settings. Int J Drug Policy. 2019;74:127–135. doi:10.1016/j.drugpo.2019.09.012
- Small W, Wood E, Tobin D, Rikley J, Lapushinsky D, Kerr T. The injection support team: a peer-driven program to address unsafe injecting in a Canadian setting. Subst Use Misuse. 2012;47(5):491–501. doi:10.3109/10826084.2012.644107
- Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health. 2017;5(12):e1208–e1220. doi:10.1016/S2214-109X(17)30373-X
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17(8):651–667. doi:10.1097/FBP.0b013e3280115f99
- Robles E, Huang B, Simpson P, McMillan D. Delay discounting, impulsiveness, and addiction severity in opioid-dependent patients. J Subst Abuse Treat. 2011;41(4):354–362. doi:10.1016/j.jsat.2011.05.003
- Ashe M, Newman M, Wilson S. Delay discounting and the use of mindful attention versus distraction in the treatment of drug addiction: A conceptual review. J Exp Anal Behav. 2015;103(1):234–248. doi:10.1002/jeab.122
- Bailey S, Ouellet L, Mackesy-Amiti M, et al. Perceived risk, peer influences, and injection partner type predict receptive syringe sharing among young adult injection drug users in five U.S. cities. Drug Alcohol Depend. 2007;91:S18–S29. doi:10.1016/j.drugalcdep.2007.02.014
- Bloor M, Robertson M, McKeganey N, Neale J. Theorising equipment-sharing in a cohort of Scottish drug users. Health Risk Soc. 2008;10(6):599–607. doi:10.1080/13698570802533697
- Broz D, Ouellet L. Prevalence and correlates of former injection drug use among young noninjecting heroin users in Chicago. Subst Use Misuse. 2010;45(12):2000–2025. doi:10.3109/10826081003682875
- Cepeda A, Kaplan C, Neaigus A, Cano M, Villarreal Y, Valdez A. Injecting transition risk and depression among Mexican American non-injecting heroin users. Drug Alcohol Depend. 2012;125:S12–S17. doi:10.1016/j.drugalcdep.2012.05.035
- Heimer R, Barbour R, Palacios W, Nichols L, Grau L. Associations between injection risk and community disadvantage among suburban injection drug users in Southwestern Connecticut, USA. AIDS Behav. 2014;18(3):452–463. doi:10.1007/s10461-013-0572-3
- McGowan C, Harris M, Rhodes T. Hepatitis C avoidance in injection drug users: a typology of possible protective practices. PLoS One. 2013;8(10):e77038. doi:10.1371/journal.pone.0077038
- Neaigus A, Gyarmathy V, Miller M, Frajzyngier V, Friedman S, Des Jarlais D. Transitions to injecting drug use among noninjecting heroin users. J Acquir Immune Defic Syndr. 2006;41(4):493–503. doi:10.1097/01.qai.0000186391.49205.3b
- Roux P, Lions C, Michel L, ANRS Methaville Study Group, et al. Factors associated with HCV risk practices in methadone-maintained patients: the importance of considering the couple in prevention interventions. Subst Abuse Treat Prev Policy. 2014;9(1):37. doi:10.1186/1747-597X-9-37
- Sani F, Madhok V, Norbury M, Dugard P, Wakefield J. Greater number of group identifications is associated with healthier behaviour: Evidence from a Scottish community sample. Br J Health Psychol. 2015;20(3):466–481. doi:10.1111/bjhp.12119
- Haslam C, Jetten J, Cruwys T, Dingle G, Haslam A. The New Psychology of Health: Unlocking the Social Cure. 1st ed. Abingdon: Routledge; 2018.
- Cruwys T, Haslam SA, Dingle GA, Haslam C, Jetten J. Depression and social identity. Pers Soc Psychol Rev. 2014;18(3):215–238. doi:10.1177/1088868314523839
- Brener L, Broady T, Cama E, Hopwood M, Byrne J, Treloar C. Positive effects of community attachment on internalised stigma and wellbeing among people who inject drugs. Int J Drug Policy. 2021;97:103323. doi:10.1016/j.drugpo.2021.103323
- Gossop M, Marsden J, Stewart D, Treacy S. Reduced injection risk and sexual risk behaviours after drug misuse treatment: results from the National Treatment Outcome Research Study. AIDS Care. 2002;14(1):77–93. doi:10.1080/09540120220097955
- Fairbairn N, Small W, van Borek N, Wood E, Kerr T. Social structural factors that shape assisted injecting practices among injection drug users in Vancouver, Canada: a qualitative study. Harm Reduct J. 2010;7:20–27. doi:10.1186/1477-7517-7-20
- Wagner K, Lankenau S, Palinkas L, Richardson J, Chou C, Unger J. The perceived consequences of safer injection: an exploration of qualitative findings and gender differences. Psychol Health Med. 2010;15(5):560–573. doi:10.1080/13548506.2010.498890
- Langston S, Edwards M, Lyvers M, Stapletone P. Illness perceptions and treatment outcomes in Hepatitis C. New Zealand J Psychol. 2016;45(2):22–28.
- Langston S, Edwards M, Lyvers M, Stapleton P. The self-regulatory model of illness and adjustment outcomes in hepatitis C. Profession Psychol Res Pract. 2017;48(5):317–326. doi:10.1037/pro0000136
- Langston S, Edwards M, Lyvers M. Illness perceptions, coping, benefit finding, and adjustment in individuals with Hepatitis C. Aust Psychol. 2018;53(1):87–96. doi:10.1111/ap.12255
- Zalai D, Sherman M, McShane K, Shapiro C, Carney C. The importance of fatigue cognitions in chronic hepatitis C infection. J Psychosom Res. 2015;78(2):193–198. doi:10.1016/j.jpsychores.2014.11.011
- Leventhal H, Meyer D, Nerenz D. Illness representations and coping with health threats. In Baum A, Taylor S, Singer J, edHandbook of Psychology and Health: Social Psychological Aspects of Health. 4th ed. New Jersey: Erlbaum; 1980. p. 219–252.
- Stimson G, Jones S, Sullivan D, Chalmers C. A short questionnaire (IRQ) to assess injecting risk behaviour. Addiction. 1998;93(3):337–347. doi:10.1046/j.1360-0443.1998.9333373.x
- Sani F, Madhok V, Norbury M, Dugard P, Wakefield J. Greater number of group identifications is associated with lower odds of being depressed: evidence from a Scottish community sample. Soc Psychiatry Psychiatr Epidemiol. 2015;50(9):1389–1397. doi:10.1007/s00127-015-1076-4
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ Primary Care Study. JAMA. 1999;282(18):1737–1744. doi:10.1001/jama.282.18.1737
- Spitzer R, Kroenke K, Williams J, Löwe B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166(10):1092–1097. doi:10.1001/archinte.166.10.1092
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206–1211. doi:10.1007/s11606-016-3703-5
- Broadbent E, Petrie K, Main J, Weinman J. The Brief Illness Perception Questionnaire. J Psychosom Res. 2006;60(6):631–637. doi:10.1016/j.jpsychores.2005.10.020
- Hologic. Aptima HCV Quant Dx Assay; 2018. https://www.hologic.com/sites/default/files/2019-03/AW-14498_002_01_0.pdf. Accessed November 3, 2021.
- Chevaliez S, Bouvier-Alias M, Pawlotsky J. Performance of the abbott real-time PCR assay using m 2000sp and m 2000rt for Hepatitis C Virus RNA quantification. J Clin Microbiol. 2009;47(6):1726–1732. doi:10.1128/JCM.01300-08
- Field A. Discovering Statistics Using IBM SPSS Statistics. 5th ed. London: Sage; 2018.
- Gaskin S, Brazil C, Pickering D. The sharing of injecting paraphernalia by intravenous drug users (IDUs) within a Worcestershire cohort, with specific reference to water and filters. Int J Drug Policy. 2000;11(6):423–435. doi:10.1016/S0955-3959(00)00069-4
- Gossop M, Griffiths P, Powis B, Williamson S, Fountain J, Strang J. Continuing drug risk behaviour: Shared use of injecting paraphernalia among London heroin injectors. AIDS Care. 1997;9(6):651–660. doi:10.1080/09540129750124687
- Unger J, Kipke M, De Rosa C, Hyde J, Ritt-Olson A, Montgomery S. Needle-sharing among young IV drug users and their social network members: The influence of the injection partner’s characteristics on HIV risk behavior. Addict Behav. 2006;31(9):1607–1618. doi:10.1016/j.addbeh.2005.12.007
- Day C, Ross J, Dietze P, Dolan K. Initiation to heroin injecting among heroin users in Sydney, Australia: Cross-sectional survey. Harm Reduct J. 2005;2(1):2. doi:10.1186/1477-7517-2-2
- Fraser S, Rance J, Treloar C. Hepatitis C prevention and convenience: why do people who inject drugs in sexual partnerships ‘run out’ of sterile equipment? Crit Public Health. 2016;26(3):294–306. doi:10.1080/09581596.2015.1036839
- Latkin C, Yang C, Srikrishnan AK, et al. The relationship between social network factors, HIV, and Hepatitis C among injection drug users in Chennai, India. Drug Alcohol Depend. 2011;117(1):50–54. doi:10.1016/j.drugalcdep.2011.01.005
- Nasir S, Rosenthal D. The social context of initiation into injecting drugs in the slums of Makassar, Indonesia. Int J Drug Policy. 2009;20(3):237–243. doi:10.1016/j.drugpo.2008.02.001
- De P, Cox J, Boivin J, Platt R, Jolly A. Social network-related risk factors for bloodborne virus infections among injection drug users receiving syringes through secondary exchange. J Urban Health. 2008;85(1):77–89. doi:10.1007/s11524-007-9225-z
- Dunn M, Degenhardt L, Bruno R. Transition to and from injecting drug use among regular ecstasy users. Addict Behav. 2010;35(10):909–912. doi:10.1016/j.addbeh.2010.06.007
- Shaw S, Shah L, Jolly A, Wylie J. Determinants of injection drug user (IDU) syringe sharing: the relationship between availability of syringes and risk network member characteristics in Winnipeg, Canada. Addiction. 2007;102(10):1626–1635. doi:10.1111/j.1360-0443.2007.01940.x
- Marshall Z, Dechman M, Minichiello A, Alcock L, Harris G. Peering into the literature: A systematic review of the roles of people who inject drugs in harm reduction initiatives. Drug Alcohol Depend. 2015;151:1–14. doi:10.1016/j.drugalcdep.2015.03.002
- Silano J, Treloar C, Leadbeatter K, Davidson S, Doidge J. Peer-facilitated treatment access for hepatitis C: the Live Hep C Free project. Harm Reduct J. 2022;19(1). doi:10.1186/s12954-022-00619-3
- INHSU, 2021. A peer-led approach to hepatitis C testing and treatment. [online] Available at: https://www.inhsu.org/infographic/a-peer-led-approach-to-hepatitis-c/. Accessed August 31, 2022.
- Savolainen I, Kaakinen M, Sirola A, Oksanen A. Addictive behaviors and psychological distress among adolescents and emerging adults: A mediating role of peer group identification. Addict Behav Rep. 2018;7:75–81. doi:10.1016/j.abrep.2018.03.002
- Sussman S, Dent C, McCullar W. Group self-identification as a prospective predictor of drug use and violence in high-risk youth. Psychol Addict Behav. 2000;14(2):192–196. doi:10.1037/0893-164X.14.2.192
- Malaguti A, Sani F, Stephens B, Ahmad F, Dugard P, Dillon J, ERADICATE-C Study Group. Change in injecting behaviour among people treated for hepatitis C virus: The role of intimate partnerships. J Viral Hepat. 2019;26(1):65–72. doi:10.1111/jvh.13009
- Rance J, Treloar C, Fraser S, Bryant J, Rhodes T. “Don’t think I’m going to leave you over it”: Accounts of changing hepatitis C status among couples who inject drugs. Drug Alcohol Depend. 2017;173:78–84. doi:10.1016/j.drugalcdep.2016.12.020
- Mohamed I, Ahmad H, Hassaan S, Hassan S. Assessment of anxiety and depression among substance use disorder patients: a case-control study. Middle East Curr Psychiatry. 2020;27(1). doi:10.1186/s43045-020-00029-w
- von Hippel C, Brener L, Horwitz R. Implicit and explicit internalized stigma: Relationship with risky behaviors, psychosocial functioning and healthcare access among people who inject drugs. Addict Behav. 2018;76:305–311. doi:10.1016/j.addbeh.2017.08.036
- Mackesy-Amiti M, Donenberg G, Ouellet L. Psychiatric correlates of injection risk behavior among young people who inject drugs. Psychol Addict Behav. 2014;28(4):1089–1095. doi:10.1037/a0036390
- Sedgwick P. Cross sectional studies: advantages and disadvantages. BMJ. 2014;348(mar26 2):g2276–g2276. doi:10.1136/bmj.g2276