Abstract
Debilitating fatigue is common in people living with kidney disease and often persists after a kidney transplant. Current understanding of fatigue is centered around pathophysiological processes. Little is known about the role of cognitive and behavioral factors. The aim of this study was to evaluate the contribution of these factors to fatigue among kidney transplant recipients (KTRs). A cross-sectional study of 174 adult KTRs who completed online measures of fatigue, distress, illness perceptions, and cognitive and behavioral responses to fatigue. Sociodemographic and illness-related information was also collected. 63.2% of KTRs experienced clinically significant fatigue. Sociodemographic and clinical factors explained 16.1% and 31.2% of the variance in the fatigue severity and fatigue impairment, respectively, increasing by 28% and 26.8% after adding distress. In adjusted models, all the cognitive and behavioral factors except for illness perceptions were positively associated with increased fatigue-related impairment, but not severity. Embarrassment avoidance emerged as a key cognition. In conclusion, fatigue is common following kidney transplantation and associated with distress and cognitive and behavioral responses to symptoms, particularly embarrassment avoidance. Given the commonality and impact of fatigue in KTRs, treatment is a clinical need. Psychological interventions targeting distress and specific beliefs and behaviors related to fatigue may be beneficial.
Introduction
Fatigue, which can be defined as “extreme and persistent tiredness, weakness or exhaustion—mental, physical or both,”Citation1,Citation2 is a common complication of advanced kidney disease yet is poorly understood within the context of kidney transplantation. Despite improvements in quality of life compared to dialysis modalities, persistent fatigue remains a challenging and debilitating symptom for kidney transplant recipients (KTRs). Although there is a scarcity of research investigating fatigue in KTRs, it is estimated that between 40 and 50% live with persistent fatigue.Citation3–5 Whilst fatigue has been found to be associated with increased risk of cardiovascular eventsCitation6 and mortalityCitation7–9 in dialysis patients, the relationship between fatigue and clinical outcomes in KTRs (such as graft loss or mortality) is not known. The mechanisms through which fatigue is associated with clinical outcomes and events are not well understoodCitation8 but may include abnormal lipid metabolism, lower heart rate variability and higher proinflammatory cytokines associated with fatigue,Citation10,Citation11 and also behavioral factors including self-management, non-adherence, and deconditioning.
The etiology of fatigue in advanced kidney disease is complex and yet to be fully defined, but is likely to involve interactions between biological, psychological, and social factors.Citation12–14 There is increasing evidence regarding the importance of cognitive, emotional, and behavioral factors that influence the perpetuation and maintenance of fatigue symptoms in long-term conditions (LTCs),Citation15–18 including evidence in the dialysis population.Citation12,Citation14,Citation19 Primary disease and treatment related factors, including kidney function, anemia, co-morbidity, inflammation, and medications are likely to be implicated in the onset of fatigue in KTRs, while thoughts, emotions, and behaviors in response to fatigue (and ongoing self-management) may maintain, exacerbate, and perpetuate fatigue over time. For example, research in Multiple Sclerosis (MS) has found that cognitive and behavioral responses to fatigue were associated with fatigue severity above and beyond disease-related factors.Citation18 Specifically, fatigue was associated with the tendency to attribute the majority of symptoms to MS, having embarrassment about fatigue symptoms, and engaging in unhelpful behaviors in response to fatigue like excessive resting (resting and avoidance) or overdoing things followed by long resting periods to recover (all-or-nothing behavior). Whilst there is emerging evidence to support the association between depression and anxiety with fatigue in KTRs,Citation3,Citation5 little is known about specific cognitive and behavioral factors.
A biopsychosocial cognitive-behavioral fatigue model suggests that fatigue is triggered by disease factors with a vicious cycle of possible cognitive, behavioral, emotional, and biological factors perpetuating it over time Citation13,Citation15,Citation19–22. Importantly, this biopsychosocial cognitive-behavioral model is not a generic biopsychosocial model, but rather one that has been originally proposed to understand the etiology of fatigue in MSCitation15 drawing on Leventhal’s Common-Sense Model of Self-RegulationCitation23,Citation24 important in the context of long-term physical conditions; and the cognitive-behavioral model of Chronic Fatigue SyndromeCitation25 that acknowledges the interaction between cognitions, emotions, behaviors, and biology in the experience of symptoms.
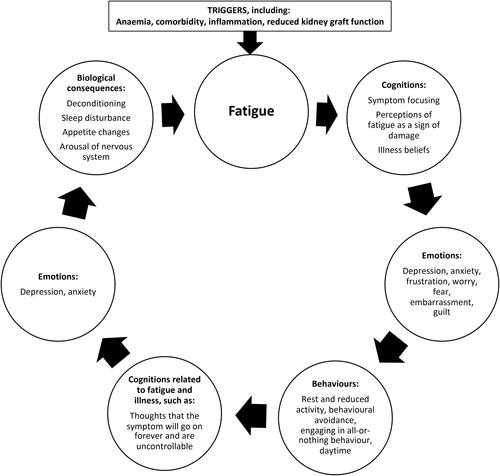
As applied to KTRs (), a biopsychosocial cognitive-behavioral fatigue model is important to increase our understanding of fatigue in this setting which might highlight possible interventional approaches designed to help and support the management of fatigue alongside medical and pharmacological approaches. To illustrate this model, fatigue among KTRs may be initially triggered by anemia in response to which thoughts such as “My fatigue means damage to my body” (likely particularly key given any uncertainty related to graft function) may manifest and drive behaviors such as reduction in activity and excessive rest, which in turn can lead to low mood, worry about not being able to do things, and over time a cascade of biological consequences such as deconditioning, further exacerbating, and maintaining fatigue.
Figure 1. Cognitive-behavioral model of fatigue in kidney transplant recipients (modelled on van Kessel and Moss-MorrisCitation15).
Accordingly, the overarching aim of this study was to evaluate the applicability of a biopsychosocial model of fatigue symptoms experienced by KTRs. Specifically, we were interested in understanding how illness beliefs and cognitive and behavioral responses to fatigue may contribute to fatigue severity and fatigue-related functional impairment in KTRs, above and beyond the role of sociodemographic and illness-related factors. Given the findings of past studies investigating fatigue in other LTCs, we tested the following hypotheses:
Higher levels of distress, measured by depression and anxiety levels will be associated with higher levels of fatigue severity and fatigue-related impairment, after controlling for age and sex.
More negative/unhelpful illness beliefs will be associated with higher levels of fatigue severity and fatigue-related functional impairment, after controlling for sociodemographic and disease-related factors and distress.
More unhelpful cognitive and behavioral responses to fatigue symptoms will be associated with higher levels of fatigue severity and fatigue-related functional impairment, after controlling for sociodemographic and disease-related factors and distress.
Methods
Design: An online cross-sectional survey study was used with data collected using Qualtrics on survey platform. Participants were recruited from online advertisements on social medial. The study received approval from the King’s College London Research Ethics Committee (REF: HR/DP-20/21-22970). All participants provided informed consent.
Participants: KTRs were recruited into this study between June and September 2021 via Twitter, Facebook, and outreach to kidney-related charities and organizations. Study adverts contained the electronic link to the survey held on Qualtrics. The survey took approximately 20 minutes to complete. KTRs were eligible if they were, (1) over 18 years old, (2) living in the UK, (3) received a living or deceased donor kidney transplant, (4) able to comprehend the questionnaires in English, and (5) willing to give informed consent. KTRs not fulfilling these criteria and those who had received a combined kidney pancreas transplant were excluded. To minimize missing data all questions required a response, however, in line with our ethical approval some responses include options “I don’t know,” “I don’t wish to disclose,” and not applicable.
Measures
Primary outcome: Fatigue severity was measured using the Chalder Fatigue Questionnaire.Citation26 This questionnaire has not been developed specifically for KTRs; therefore, it captures general fatigue. This scale consists of 11 items and scores are assigned for each response, using continuous scoring from 0 to 3. Higher scores represent greater fatigue severity. A cutoff of 4 or greater defines clinically meaningful fatigue using the questionnaires bimodal scoring.Citation26,Citation27 The measure has good psychometric properties in kidney patients.Citation28 Cronbach’s alpha in this sample was .91.
Secondary outcome: The Work and Social Adjustment Scale (WSAS)Citation29 is a valid and reliable self-report scale of functional impairment attributable to an identified problem (in this case fatigue). The scale consists of five items that correspond to impairment in work, home management, social activities, private leisure activities and relationships. Each item is rated on a 9-point scale ranging from 0 (“not at all a problem”) to 8 (“very seriously impaired”), with higher scores indicating greater impairment. Cronbach’s alpha in this sample was .93.
Psychosocial factors
Cognitive and behavioral responses to fatigue: Cognitive and Behavioral Responses to Symptoms Questionnaire (CBRQ)Citation30 was used to measure patients’ cognitive and behavioral responses to their fatigue symptoms. The short version questionnaire was used which includes 18 items measuring four cognitive subscales (fear avoidance, embarrassment avoidance, symptom damage, symptom focus) and two behavioral subscales (resting and avoidance of activity and, all-or-nothing behavior). Each item has five response options, scored from 0 “strongly disagree” to 4 “strongly agree,” with higher scores indicating greater use of the cognitive and behavioral responses. All subscales of the CBRQ had internal reliabilities above .75 (ranging from .75 to .94).
Depression symptoms: Depression symptoms over the last two weeks were evaluated using the eight-item Patient Health Questionnaire.Citation31–33 The PHQ-8 is a shortened version of the PHQ-9 which omits the ninth item, suicide ideation. The PHQ-8 was chosen over the PHQ-9 given the online nature of the study and therefore limited opportunity to provide appropriate safeguarding where suicidal risk present. A sum score ranging between 0 and 24 indicates severity, with higher scores representing more severe depression. A cutoff score of 10 or above maximizes combined sensitivity and specificity against diagnostic criteria for Major Depressive Disorder.Citation34 Cronbach’s alpha in this sample was .89.
Anxiety symptoms: The Generalized Anxiety Disorder-7 (GAD-7)Citation35 was used to evaluate symptoms of anxiety over the last two weeks. A sum score ranging between 0 and 21 indicates severity, with higher scores representing more severe anxiety. A cutoff score of 10 or above maximizes combined sensitivity and specificity against diagnostic criteria for Generalized Anxiety Disorder.Citation35 Cronbach’s alpha in this sample was .91.
Psychological distress: Given the high correlation between depression and anxiety (r = .78, p < .01), and for analytic purposes, the PHQ-8 and GAD-7 were combined to form a composite measure of distress; The Patient Health Questionnaire Anxiety and Depression Scale (PHQ-ADS).Citation36,Citation37 Scores can range from 0 to 45 (since PHQ item nine was removed), with higher scores indicating more distress. Cronbach’s alpha in this sample was .94.
Beliefs about kidney disease. The Brief Illness Perception Questionnaire (BIPQ)Citation38 was used to measure perceptions of kidney disease. The BIPQ is a seven-item scale which captured the following perceptions: (1) illness consequences, (2) illness timeline, (3) perceived personal control and treatment control, (4) emotional responses, and (5) illness understanding. The scoring is interpreted on a continuous scale with higher total scores indicating more unhelpful perceptions of their illness (e.g., more untreatable or difficult to understand). Cronbach’s alpha in this sample was .62.
Physical activity. International Physical Activity Questionnaire (IPAQ) short form collects information on time spent sitting, walking, doing moderate, and high intensity activity over the prior seven days.Citation39 The questionnaire was scored categorically according to developed cutoffs to classify individuals into low, moderate, or high physical activity groups. Due to the number of KTRs in each of the groups, high vs. low-moderate comparisons were made. Cronbach’s alpha in this sample was .71.
Demographic and illness-related information
Personal characteristics and illness-related information were self-reported, including, primary renal diagnosis, co-morbidity, kidney function (eGFR, mL/min per 1.73m2), dialysis history (duration and modality), immunosuppression regimen, and post-surgical readmission (see for full list of variables).
Table 1. Sample characteristics (N = 174).
Statistical analysis
Multivariate linear regression models examined the associations between illness-related factors and fatigue outcomes, adjusting for age and sex. For each of the cognitive and behaviors factors of interest, separate hierarchical regression models were computed which adjusted for age, sex, illness-related factors, and distress. These potential covariates were selected due to their univariate associations with the outcomes, or their potential as confounders. Since several models were evaluated to examine the contribution of these psychological factors, a more stringent p-value of <.01 was used to determine significance in these final hierarchical models.
Results
Missing data was minimal (<5%) hence no methods were used to impute missing values, with one exception. Twenty percent of KTRs selected “not applicable” for the first item of the WSAS fatigue-related functional impairment questionnaire (“because of my fatigue my ability to go to work is impaired”); to prevent exclusion of these participants in the WSAS total score, mean substitution was performed for missing values on this item only and then the total fatigue-related impairment score was computed.
Participants
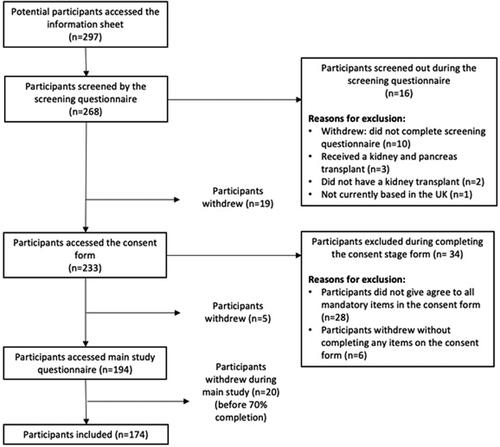
One hundred and seventy-four KTRs were included in this study. A total of 297 potential participants accessed the study information sheet, of which 194 (65.3%) were eligible, provided informed consent, and completed the survey. Twenty people were excluded prior to data analysis: 7 because they provided no data and 13 because they completed less than 70% of the survey (see for participant flow chart).
Demographic and clinical characteristics
A summary of the sample’s illness-related characteristics is presented in = 174). The mean age of recipients was 52.8 years (SD = 12.0, Range = 24 to 86). The majority were female (n = 120; 64.9%) and self-identified as White (n = 176; 94.3%). The most common primary renal diagnosis was polycystic kidney disease (43.7%). Mean time since (last) transplantation was 3,494 days (SD = 5,097 days). Self-reported mean eGFR was 49.5 mL/min per 1.73m2 (SD = 22.0).
Fatigue and psychological characteristics
Supplementary file 1 shows descriptive statistics for fatigue, depression and anxiety. 63.2% and 65.5% of KTRs had clinically significant levels of fatigue and impairment, respectively. Clinically significant levels of depression and anxiety severity were experienced in 63 (36.2%) and 50 (28.7%) of KTRs, respectively.
Demographic and clinical correlates of fatigue and functional impairment
Pearson’s correlation coefficients between the fatigue measures and continuous demographic and disease-related variables are shown in . There were no significant associations between age and time since transplantation with and either fatigue severity or impairment. Kidney function was negatively associated with fatigue-related impairment but not, severity. Hemoglobin was negatively associated with both fatigue severity and impairment. Both the number of comorbidities and BMI were positively associated with fatigue severity and impairment.
Table 2. Correlation matrix between demographic and clinical continuous factors and fatigue severity and fatigue-related functional impairment.
Further tests of association revealed no significant associations between, ethnicity (white vs. non-white), having a dialysis history, the number of past kidney transplants and donor type (data not shown). A significant difference by sex was found in relation to fatigue-related functional impairment, with women scoring on average 4.35 higher on the WSAS (p = .015). There were significant differences in fatigue by readmission to hospital after transplant, complications experiences after transplant and need for dialysis after transplant (Supplementary file 2). Fatigue severity and impairment were significantly higher in patients who were readmitted to hospital post-transplantation (compared with not hospitalised), and in those with delayed graft function requiring dialysis (compared with no dialysis post-transplantation). Furthermore, those who had high levels of physical activity had significantly lower fatigue severity (mean difference = 2.2, p = .024) and less fatigue related impairment (mean difference = 1.77, p = .03) compared to KTRs with low-moderate activity.
A multivariate linear regression model exploring the association between illness-related factors with fatigue severity and fatigue-related impairment, which also adjusted for age, sex, and physical activity status (high vs low-moderate), is shown in . 16.1% of the variance in fatigue severity was explained, with higher BMI and comorbidity, and low-moderate physical activity (compared to high) being significantly associated with greater fatigue severity. 31.2% of the variance in fatigue-related impairment was explained, with higher BMI and comorbidity, lower hemoglobin, and low-moderate physical activity (compared to high) being significantly associated with greater fatigue. Adding psychological distress to the model increased the explained variance in fatigue severity by 28% (p < .01), and fatigue-related impairment by 26.8% (p < .01). Holding all the covariates constant, a one-point increase in psychological distress was associated with a .36 increase in fatigue severity (p < .01) and .58 increase in fatigue-related impairment (p < .01).
Table 3. Disease-related associations with fatigue and fatigue-related impairment.
Psychological correlates of fatigue severity and fatigue-related functional impairment
Correlates between fatigue severity, fatigue-related functional impairment, and psychological factors are shown in Supplementary file 3. As expected, fatigue severity and fatigue-related impairment were correlated strongly (r = .69, p < .01). Both fatigue measures correlated either moderately or strongly with anxiety and depression, illness perceptions and all the cognitive and behavioral responses.
In multivariate models adjusted for demographic and illness-related factors and physical activity, and distress, embarrassment avoidance was significantly associated with fatigue severity (). Apart from illness perceptions, all the cognitive and behavioral factors were positively associated with increased fatigue-related impairment (). Effect sizes for the cognitive and behavior factors ranged from .20 to .41. Embarrassment avoidance appeared to be the most dominant cognition for both outcomes. Individual cognitive and behavioral factors explained between .3 and 8.7% in fatigue related outcomes above and beyond demographic and clinical factors and physical activity.
Table 4. Cognitive and behavioral factors associated with fatigue outcomes, adjusted for demographics, disease-related factors and distress.
Discussion
The overarching aim of the current study was to evaluate the utility of a biopsychosocial conceptualization of fatigue in KTRs, by evaluating the contribution of cognitive and behavioral factors to fatigue severity and fatigue-related functional impairment, whilst controlling for demographic and illness-related factors. A biopsychosocial approach to fatigue has been valuable to the understanding and management of fatigue in other long-term conditions,Citation15,Citation21 yet the role of cognitive and behavioral factors is largely unexplored in kidney disease, and even less so among KTRs.Citation40 A better understanding of the contribution of such modifiable factors to fatigue in this population might improve fatigue treatment.
Our findings confirm that fatigue is a common and impactful symptom in KTRs. 63.2% of the participants were experiencing significant levels of fatigue, an estimate higher than reported in previous studies.Citation3–5 The majority (65.5%) of participants were also experiencing moderate to severe fatigue-related impairment. No association was observed between age and ethnicity with the fatigue outcomes; however, females reported significantly higher fatigue impairment compared to males. Of the disease-related factors, there was indication of higher fatigue severity and impairment among patients with poorer outcomes following transplantation, based on markers such as re-admission to hospital, and greater number of comorbidities and higher BMI. On the other hand, higher kidney function and hemoglobin were associated with lower levels of fatigue. However, in the adjusted for age and sex model of fatigue severity, only physical activity, number of comorbidities, and BMI remained significant predictors. A similar pattern was observed with fatigue impairment, but hemoglobin also remained a significant predictor of lower fatigue impairment. An increase in distress was also significantly associated with both fatigue outcomes, above and beyond sociodemographic and disease-related factors. Although at the univariate level, significant positive correlations were evident between all cognitive and behavioral variables with both fatigue outcomes, in the adjusted model of fatigue severity, only embarrassment avoidance, fear of being embarrassed by their illness or symptoms in response to fatigue; remained a significant predictor. In contrast, all the cognitive and behavioral variables remained significant predictors of fatigue impairment, but embarrassment avoidance appeared to be a dominant predictor in this model, based on the standardized beta coefficient.
The findings of the study provide novel and valuable evidence on the role of cognitions and behaviors in fatigue among KTRs that have never been investigated to date. This is in line with previous evidence in other LTCsCitation15,Citation41–45 and the role of cognitive and behavioral factors also observed in hemodialysis,Citation14,Citation19 where distress, negative beliefs about fatigue, and unhelpful behaviors (all-or-nothing and avoidance behaviors) were associated with fatigue severity.Citation19 Similarly, distress, damage beliefs (interpretation of fatigue as a signal of damage happening to the body), avoidance behaviors, and fatigue severity were significant predictors of greater fatigue-related impairment.Citation19 Here, among KTRs, it is embarrassment avoidance beliefs that appeared to be most salient for fatigue severity and fatigue-related impairment. Consistent with the patterns observed in hemodialysis,Citation19 here as well, cognitive and behavioral factors explained a larger proportion of variance in fatigue-related impairment compared to fatigue severity. Interestingly, illness perceptions were not associated with fatigue severity and only at the .05 alpha level with fatigue-related functional impairment, which is in contrast to evidence in other LTCsCitation42,Citation43 and dialysis,Citation46 possibly because receiving a kidney transplant creates some distance to the kidney disease label.
The levels of clinically significant depression and anxiety were considerably high (36.5% and 26.9%, respectively) in this sample, which is consistent with previous research on the prevalence of distress in both dialysis and transplant patients.Citation47,Citation48 Prior research documents depression as a significant fatigue correlate,Citation3,Citation5,Citation49 as was also observed here. Nonetheless, even after controlling for distress, cognitive and behavioral factors had a unique and significant contribution to fatigue.
The findings here also revealed the importance of BMI, comorbidities, and physical activity to fatigue—these factors may need to be considered in conjunction as they are likely to be closely interconnected and these patterns closely reflect existing evidence emerging from other LTCs.Citation50 In particular, physical activity is key to fatigue trans-diagnostically.Citation50,Citation51 The findings of this study also complement existing evidence on the role of disease-related factors in fatigue. Across long-term conditions, understanding of the relationship between traditional biological markers of disease (severity and activity) and fatigue is generally poor or even non-existent. Inflammation and the autonomic nervous system are thought to be significant contributory factors across conditions, disease-specific pathophysiological processes underlying chronic fatigue and their relationship with psychological variables are yet to be defined.Citation52
There was little support for the role of sociodemographic factors in fatigue here. Research investigating the role of sociodemographic factors in KTR fatigue is essentially non-existent, however, evidence from dialysis patients is generally mixed and inconsistent.Citation12,Citation53 Unlike the aforementioned study of fatigue in hemodialysis,Citation14,Citation19 where white ethnicity was associated with higher fatigue severity cross-sectionally and longitudinally, this was not observed here. However, this is likely due to the lack of representation of different minority ethnic groups in this sample.
Considering the prevalence of fatigue observed here, greater recognition of fatigue as a debilitating symptom experienced by KTRs is required. However, a past study showed that only 13% of KTRs had fatigue documented in their medical records, despite 59% of patients reporting symptoms of fatigue.Citation5 The profound impact of fatigue on both patient-reported and clinical outcomes is well documented across LTCs.Citation54 Among KTRs, fatigue is associated with poorer quality of life and adherence to immunosuppressive medications.Citation4 A previous study found that only 27% of severely fatigued KTRs who also reported significant functional impairment were able to maintain paid employment, compared to 48% of non-fatigued participants.Citation3 Although there is currently no evidence on whether fatigue is a risk factor for adverse clinical outcomes following transplantation, a reduction in quality of life is indeed a risk factor for mortality and graft failure in KTRs.Citation4 In fact, according to the SONG initiative, fatigue is a critically important outcome for KTRs,Citation55 yet it remains currently largely underrecognized and untreated.Citation4
The findings of the study add to the growing evidence in support of the trans-diagnostic role of cognitive and behavioral factors to fatigue across LTCs.Citation50,Citation51 A biopsychosocial conceptualization of fatigue does not limit treatment to pathophysiological processes alone, but instead expands treatment possibilities to include non-pharmacological approaches that need to be underlined by theory and evidence targeting relevant to the population modifiable factors. A scoping review of fatigue interventions across LTCs found consistently promising evidence in support of cognitive behavioral therapy (CBT) and exercise for the treatment of fatigue.Citation17 Treatment of depression and anxiety alone is unlikely to be sufficient for the treatment of fatigue as has been observed in mediation analysis of trial data,Citation56,Citation57 which resonates with the unique contribution of cognitive and behavioral to fatigue outcomes evident here.
The study had several limitations including the design preventing any conclusions about causality. Recruitment was limited to online channels, subjecting the results to potential selection bias. KTRs without internet access were precluded from taking part. In addition to this, illness-related data were self-reported and therefore recall bias is likely at play. In fact, the highest proportion of missing data was observed for kidney function (eGFR) and hemoglobin. The sample was also almost exclusively white, limiting generalizability of the findings to other ethnic groups. Lastly, there may be potential non-response bias, with the most fatigued less likely to take part—an inherent limitation of fatigue research. However, the full range of possible scores on CFQ was captured in this sample (0–33), suggesting that the more severe end of fatigue was likely captured.
Conclusions
In conclusion, the present findings not only accentuate the common and debilitating nature of fatigue among KTRs, but also pave way to a biopsychosocial conceptualization of fatigue. In addition to distress, unhelpful cognitions in response to fatigue, particularly embarrassment avoidance beliefs, and behaviors play an important role in fatigue severity and impairment, above and beyond the role of sociodemographic and disease-related factors. Although promising, these findings need to be interpreted with caution particularly in light of the cross-sectional design, online recruitment, and almost exclusively white sample. It would therefore be valuable to evaluate the role of these factors in a more diverse sample over time, particularly capturing fatigue and biopsychosocial predictors prior to transplantation and onwards. Most importantly, routine screening and treatment of fatigue that is not limited to pharmacological agents are necessary, overcoming the assumption that fatigue resolves following kidney transplantation.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Supplemental Material
Download MS Word (19.7 KB)Supplemental Material
Download MS Word (18.3 KB)Supplemental Material
Download MS Word (17.4 KB)Acknowledgments
We wish to thank all the participants for agreeing to take part in the study and the kidney organisations for promoting the study.
Disclosure statement
The authors have no conflicting interests to report.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJ, Wessely SC. Population based study of fatigue and psychological distress. BMJ. 1994;308(6931):763–766. doi:10.1136/bmj.308.6931.763.
- David A, Pelosi A, McDonald E, et al. Tired, weak, or in need of rest: fatigue among general practice attenders. BMJ. 1990;301(6762):1199–1202. doi:10.1136/bmj.301.6762.1199.
- Goedendorp MM, Hoitsma AJ, Bloot L, Bleijenberg G, Knoop H. Severe fatigue after kidney transplantation: a highly prevalent, disabling and multifactorial symptom. Transpl Int. 2013;26(10):1007–1015. doi:10.1111/tri.12166.
- Bossola M, Arena M, Urciuolo F, et al. Fatigue in kidney transplantation: a systematic review and meta-analysis. Diagnostics (Basel). 2021;11(5):833. doi:10.3390/diagnostics11050833.
- Chan W, Bosch JA, Jones D, et al. Predictors and consequences of fatigue in prevalent kidney transplant recipients. Transplantation. 2013;96(11):987–994. doi:10.1097/TP.0b013e3182a2e88b.
- Koyama H, Fukuda S, Shoji T, et al. Fatigue is a predictor for cardiovascular outcomes in patients undergoing hemodialysis. Clin J Am Soc Nephrol. 2010;5(4):659–666. doi:10.2215/cjn.08151109.
- Picariello F, Norton S, Moss-Morris R, Macdougall IC, Chilcot J. Fatigue in prevalent haemodialysis patients predicts all-cause mortality and kidney transplantation. Ann Behav Med. 2019;53(6):501–514. doi:10.1093/abm/kay061.
- Jhamb M, Pike F, Ramer S, et al. Impact of fatigue on outcomes in the hemodialysis (HEMO) study. Am J Nephrol. 2011;33(6):515–523. doi:10.1159/000328004.
- Bossola M, Di Stasio E, Antocicco M, Panico L, Pepe G, Tazza L. Fatigue is associated with increased risk of mortality in patients on chronic hemodialysis. Nephron. 2015;130(2):113–118. doi:10.1159/000430827.
- Williams JE, Mosley TH, Jr., Kop WJ, Couper DJ, Welch VL, Rosamond WD. Vital exhaustion as a risk factor for adverse cardiac events (from the Atherosclerosis Risk In Communities [ARIC] study). Am J Cardiol. 2010;105(12):1661–1665. doi:10.1016/j.amjcard.2010.01.340.
- Jhamb M, Argyropoulos C, Steel JL, et al. Correlates and outcomes of fatigue among incident dialysis patients. Clin J Am Soc Nephrol. 2009;4(11):1779–1786. doi:10.2215/CJN.00190109.
- Artom M, Moss-Morris R, Caskey F, Chilcot J. Fatigue in advanced kidney disease. Kidney Int. 2014;86(3):497–505. doi:10.1038/ki.2014.86.
- Picariello F, Moss-Morris R, Macdougall IC, Chilcot J. The role of psychological factors in fatigue among end-stage kidney disease patients: a critical review. Clin Kidney J. 2017;10(1):79–88.
- Picariello F, Norton S, Moss-Morris R, Macdougall IC, Chilcot J. A prospective study of fatigue among in-centre haemodialysis patients. Br J Health Psychol. 2020;25(1):61–88. doi:10.1111/bjhp.12395.
- van Kessel K, Moss-Morris R. Understanding multiple sclerosis fatigue: a synthesis of biological and psychological factors. J Psychosom Res. 2006;61(5):583–585. doi:10.1016/j.jpsychores.2006.03.006.
- Hughes AM, Campbell L, Graham H, Post F, Chalder T. A Biopsychosocial approach to HIV fatigue: a cross-sectional and prospective analysis to identify key modifiable factors. Behav Med. 2021;47(3):205–213. doi:10.1080/08964289.2020.1712582.
- Hulme K, Safari R, Thomas S, et al. Fatigue interventions in long term, physical health conditions: a scoping review of systematic reviews. PLoS One. 2018;13(10):e0203367. doi:10.1371/journal.pone.0203367.
- Skerrett TN, Moss-Morris R. Fatigue and social impairment in multiple sclerosis: the role of patients’ cognitive and behavioral responses to their symptoms. J Psychosom Res. 2006;61(5):587–593. doi:10.1016/j.jpsychores.2006.04.018.
- Chilcot J, Moss-Morris R, Artom M, et al. Psychosocial and clinical correlates of fatigue in haemodialysis patients: the importance of patients’ illness cognitions and behaviours. Int J Behav Med. 2016;23(3):271–281. doi:10.1007/s12529-015-9525-8.
- Artom M, Czuber-Dochan W, Sturt J, Murrells T, Norton C. The contribution of clinical and psychosocial factors to fatigue in 182 patients with inflammatory bowel disease: a cross-sectional study. Aliment Pharmacol Ther. 2017;45(3):403–416. doi:10.1111/apt.13870.
- Hewlett S, Chalder T, Choy E, et al. Fatigue in rheumatoid arthritis: time for a conceptual model. Rheumatology (Oxford). 2011;50(6):1004–1006. doi:10.1093/rheumatology/keq282.
- Donovan KA, Small BJ, Andrykowski MA, Munster P, Jacobsen PB. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol. 2007;26(4):464–472. doi:10.1037/0278-6133.26.4.464.
- Leventhal H, Benyamini Y, Brownlee S, et al. Illness representations: theoretical foundations. In K. J. Petrie and J. Weinman, eds. Perceptions of Health and Illness. Vol. 2. Amsterdam: Harwood Academic Publishers; 1997:19–46.
- Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health, and behavior: a perceptual-cognitive approach. Psychol Health. 1998;13(4):717–733. doi:10.1080/08870449808407425.
- Wessely S, Sharpe M, Hotopf M. Chronic Fatigue and Its Syndromes. Oxford: Oxford University Press; 1998.
- Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–153. doi:10.1016/0022-3999(93)90081-P.
- White PD, Goldsmith KA, Johnson AL, et al. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet. 2011;377(9768):823–836. doi:10.1016/S0140-6736(11)60096-2.
- Picariello F, Moss-Morris R, Macdougall IC, Chilcot J. Measuring fatigue in haemodialysis patients: the factor structure of the Chalder Fatigue Questionnaire (CFQ). J Psychosom Res. 2016;84:81–83. doi:10.1016/j.jpsychores.2016.03.124.
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry. May 2002;180:461–464. doi:10.1192/bjp.180.5.461.
- Ryan EG, Vitoratou S, Goldsmith KA, Chalder T. Psychometric properties and factor structure of a long and shortened version of the cognitive and behavioural responses questionnaire. Psychosom Med. 2018;80(2):230–237. doi:10.1097/PSY.0000000000000536.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x.
- Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163–173. doi:10.1016/j.jad.2008.06.026.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi:10.1001/jama.282.18.1737.
- Levis B, Benedetti A, Thombs BD, Collaboration DESD, DEPRESsion Screening Data (DEPRESSD) Collaboration. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365:l1476. doi:10.1136/bmj.l1476.
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi:10.1001/archinte.166.10.1092.
- Kroenke K, Wu J, Yu Z, et al. Patient Health Questionnaire Anxiety and Depression Scale: initial validation in three clinical trials. Psychosom Med. 2016;78(6):716–727. doi:10.1097/PSY.0000000000000322.
- Chilcot J, Hudson JL, Moss-Morris R, et al. Screening for psychological distress using the Patient Health Questionnaire Anxiety and Depression Scale (PHQ-ADS): initial validation of structural validity in dialysis patients. Gen Hosp Psychiatry. 2018;50:15–19. doi:10.1016/j.genhosppsych.2017.09.007.
- Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception Questionnaire. J Psychosom Res. 2006;60(6):631–637. doi:10.1016/j.jpsychores.2005.10.020.
- Booth ML, Ainsworth BE, Pratt M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exer. 2003;195(9131/03):3508.
- Bossola M, Pepe G, Vulpio C. Fatigue in kidney transplant recipients. Clin Transplant. 2016;30(11):1387–1393. doi:10.1111/ctr.12846.
- Zalai D, Sherman M, McShane K, Shapiro CM, Carney CE. The importance of fatigue cognitions in chronic hepatitis C infection. J Psychosom Res. 2015;78(2):193–198. doi:10.1016/j.jpsychores.2014.11.011.
- Alsén P, Brink E, Persson L-O, Brändström Y, Karlson BW. Illness perceptions after myocardial infarction: relations to fatigue, emotional distress, and health-related quality of life. J Cardiovasc Nurs. 2010;25(2):E1–E10. doi:10.1097/JCN.0b013e3181c6dcfd.
- Grayson PC, Amudala NA, Mcalear CA, et al. Illness perceptions and fatigue in systemic vasculitis. Arthritis Care Res (Hoboken). 2013;65(11):1835–1843. doi:10.1002/acr.22069.
- Jopson NM, Moss-Morris R. The role of illness severity and illness representations in adjusting to multiple sclerosis. J Psychosom Res. 2003;54(6):503–511. doi:10.1016/S0022-3999(02)00455-5.
- Ali S, Matcham F, Irving K, Chalder T. Fatigue and psychosocial variables in autoimmune rheumatic disease and chronic fatigue syndrome: a cross-sectional comparison. J Psychosom Res. 2017;92:1–8. doi:10.1016/j.jpsychores.2016.11.002.
- Timmers L, Thong M, Dekker FW, et al. Illness perceptions in dialysis patients and their association with quality of life. Psychol Health. 2008;23(6):679–690. doi:10.1080/14768320701246535.
- Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–191. doi:10.1038/ki.2013.77.
- Veater NL, East L. Exploring depression amongst kidney transplant recipients: a literature review. J Ren Care. 2016;42(3):172–184. doi:10.1111/jorc.12162.
- Rodrigue JR, Mandelbrot DA, Hanto DW, Johnson SR, Karp SJ, Pavlakis M. A cross-sectional study of fatigue and sleep quality before and after kidney transplantation. Clin Transplant. 2011;25(1):E13–E21. doi:10.1111/j.1399-0012.2010.01326.x.
- Goërtz YMJ, Braamse AMJ, Spruit MA, et al. Fatigue in patients with chronic disease: results from the population-based Lifelines Cohort Study. Sci Rep. 2021;11(1):20977. doi:10.1038/s41598-021-00337-z.
- Menting J, Tack CJ, Bleijenberg G, et al. Is fatigue a disease-specific or generic symptom in chronic medical conditions? Health Psychol. 2018;37(6):530–543. doi:10.1037/hea0000598.
- Matura LA, Malone S, Jaime-Lara R, Riegel B. A systematic review of biological mechanisms of fatigue in chronic illness. Biol Res Nurs. 2018;20(4):410–421. doi:10.1177/1099800418764326.
- Bossola M, Vulpio C, Tazza L. Fatigue in chronic dialysis patients. Semin Dial. 2011;24(5):550–555. doi:10.1111/j.1525-139X.2011.00956.x.
- Knoop V, Cloots B, Costenoble A, et al. Fatigue and the prediction of negative health outcomes: a systematic review with meta-analysis. Ageing Res Rev. 2021;67:101261. doi:10.1016/j.arr.2021.101261.
- Sautenet B, Tong A, Manera KE, et al. Developing consensus-based priority outcome domains for trials in kidney transplantation: a multinational Delphi survey with patients, caregivers and health professionals. Transplantation. 2017;101(8):1875–1886. doi:10.1097/TP.0000000000001776.
- Knoop H, van Kessel K, Moss-Morris R. Which cognitions and behaviours mediate the positive effect of cognitive behavioural therapy on fatigue in patients with multiple sclerosis? Psychol Med. 2012;42(1):205–213. doi:10.1017/S0033291711000924.
- van den Akker L, Beckerman H, Collette E, et al. Cognitive behavioural therapy for MS-related fatigue explained: a longitudinal mediation analysis. J Psychosom Res. 2018;106:13–24. doi:10.1016/j.jpsychores.2017.12.014.