Abstract
Light chain (AL) amyloidosis is a disease associated with significant morbidity and mortality arising from multi-organ injury induced by amyloidogenic light chain proteins (LC). There is no available treatment to reverse the toxicity of LC. We previously showed that chaperone glycoprotein clusterin (CLU) and nanoliposomes (NL), separately, restore human microvascular endothelial function impaired by LC. In this work, we aim to prepare PEGylated-nanoliposomal clusterin (NL-CLU) formulations that could allow combined benefit against LC while potentially enabling efficient delivery to microvascular tissue, and test efficacy on human arteriole endothelial function. NL-CLU was prepared by a conjugation reaction between the carboxylated surface of NL and the primary amines of the CLU protein. NL were made of phosphatidylcholine (PC), cholesterol (Chol) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)-2000] (DSPE-PEG 2000 carboxylic acid) at 70:25:5 mol%. The protective effect of NL-CLU was tested by measuring the dilation response to acetylcholine and papaverine in human adipose arterioles exposed to LC. LC treatment significantly reduced the dilation response to acetylcholine and papaverine; co-treatment of LC with PEGylated-nanoliposomal CLU or free CLU restored the dilator response. NL-CLU is a feasible and promising approach to reverse LC-induced endothelial damage.
Introduction
Light chain (AL) amyloidosis is a protein misfolding disease characterized by an overproduction of amyloidogenic light-chain proteins (LC) and tissue deposition of amyloid fibrils that lead to multiple organ failure (Migrino et al., Citation2014; Sanchorawala, Citation2006). Currently, the therapeutic approach is focused on reducing the production of LC by eliminating the clonal plasma cells that produce them by chemotherapy with or without autologous stem cell transplantation (Eisele et al., Citation2015). However, this approach can be associated with significant morbidity and mortality especially in patients with advanced disease (Dispenzieri et al., Citation2004b). There is currently no treatment that reverses the tissue toxicity of circulating LC.
Liposomal formulations have been used as delivery agents for therapeutic cargo to treat amyloid diseases. Lipid-based formulations have been successfully used in a Phase I clinical trial for the treatment of patients with transthyretin (TTR) amyloidosis, another disease characterized by the formation of amyloid TTR deposits in the heart and peripheral nerves (Coelho et al., Citation2013). These lipid formulations were comprised of the cationic amino lipid 2,2-dilinoleyl-4-dimethylaminoethyl-[1,3]-dioxolane (DLin-KC2-DMA), distearoylphosphatidylcholine (DSPC), cholesterol and (R)-2,3-bis(octadecyloxy)propyl-1-[methoxy poly(ethylene glycol)2000]propylcarbamate (PEG-lipid) and contained antiTTR siRNA. These loaded cationic liposomes resulted in the knockdown of TTR protein expression in 32 patients (Coelho et al., Citation2013; Jayaraman et al., Citation2012).
Unlike the approach just described, we previously showed that nanoliposome formulations alone, without therapeutic cargo, reversed the endothelial dysfunction in human arterioles induced by LC (Franco et al., Citation2016; Truran et al., Citation2014) and β-amyloid (Truran et al., Citation2016). In a separate study, we showed similar protective effects by clusterin (CLU) against LC in preserving endothelial function and viability (Franco et al., Citation2012). CLU is a constitutively expressed glycoprotein; although CLU does not refold proteins, it binds a variety of partly unfolded, stressed proteins, thereby preventing their precipitation through the formation of high molecular weight complexes, which in turn stabilize stressed proteins. (Humphreys et al., Citation1999; Lakins et al., Citation2002; Poon et al., Citation2002). Furthermore, CLU reduced the aggregation of β-amyloid peptides (Oda et al., Citation1995). Therefore, CLU seems to play a crucial role in the overall process of stabilizing damaged proteins and in preventing the formation of amyloid deposits. There are potential challenges in administering CLU intravenously to treat amyloid diseases. Because CLU is an apolipoprotein, like other lipoproteins, it could be diverted while in circulation to perform lipid transport functions prior to reaching the microvasculature, which reduces its ability to act as a chaperone protein on amyloidogenic protein species in relevant microvascular beds such as in coronary circulation. In addition, CLU is prone to degradation with rapid elimination from circulation and a reported half-life of <2 h (Rizzi et al., Citation2009). Functionalizing CLU into PEGylated nanoliposomes would allow the design of biologics that could persist in circulation and reach desired tissue targets for optimal efficacy. In this article, we describe the preparation of nanoliposomes functionalized with CLU and perform initial proof-of-concept functional assays to determine whether the formulation protects human arterioles against LC-induced endothelial dysfunction.
Methods
Lipids and chemicals
l-α-phosphatidylcholine (egg, chicken, PC), cholesterol (Chol) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)-2000] (DSPE-PEG-COOH 2000 carboxylic acid) were purchased from Avanti Polar Lipids (Alabaster, AL). 2-(N-Morpholino)ethanesulfonic acid hydrate (MES hydrate), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide sodium salt (sulfo-NHS) and Ficoll PM 400 were purchased from Sigma Aldrich (St. Louis, MO). Purified recombinant CLU, transcript variant 1 was purchased from Origene (Rockville, MD). Human Clusterin Quantikine ELISA was purchased from R&D Systems (Minneapolis, MN).
Preparation of PEG-functionalized liposomes
Small unilamellar vesicles (SUVs) consisting of PC, Chol, DSPE-PEG-COOH (2000) (70:25:5 mol%) were prepared by ultrasonication (Klibanov et al., Citation2003; Lasch et al., Citation2003). The corresponding lipid mixture dissolved in chloroform was added to a 10-mL pear-shaped flask followed by removal of the solvent by a rotary evaporator. The dried lipid film obtained was then hydrated with 0.1 M MES buffer, pH 4.7 and sonicated using an ultrasonic probe for 45 min at a power output of 5 Watts in an ice bath to avoid overheating. After sonication, the liposomes were centrifuged at 3000 rpm for 15 min at 4 °C in order to remove the shed titanium particles from the sonicator tip.
PEGylated-nanoliposomal CLU preparation
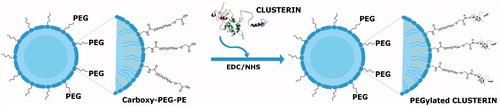
Nanoliposomes consisting of PC:Chol:DSPE-PEG-COOH with surface-linked CLU () in 0.1 M MES buffer pH 4.7 were prepared as previously described by Klibanov et al. (Citation2003). Briefly, pre-formed nanoliposomes were mixed with a 0.25 M solution of EDC and a 0.25 M solution of sulfo-NHS, previously dissolved in water. After 10 min incubation at room temperature, the reaction was neutralized to pH 7.4 with 2 M NaOH, followed by the addition of the CLU protein and incubation overnight at 4 °C with gentle stirring. The PEGylated CLU nanoliposomes (PC:Chol:DSPE-PEG-CLU) at a final lipid concentration of 50 μg/mL with CLU at 500 ng/mL were collected and stored at 4 °C for further analysis.
CLU-PEGylated nanoliposome purification
CLU-conjugated nanoliposomes (PC:Chol:DSPE-PEG-CLU) were separated from unbound protein by isopycnic centrifugation. A 0.5 mL of nanoliposomes were mixed with 1 mL of 30% Ficoll 400 to make a final concentration of 20%. Three milliliter of 10% Ficoll 400 were layered on top of the mixture, followed by the addition of 0.5 mL of 0.1 M MES buffer, pH 7.4. The tube was centrifuged at 100,000 × g for 30 min at 4 °C using a swinging rotor. After centrifugation, the separated CLU-conjugated liposomes and free CLU protein (unbound CLU) fractions were collected.
Particle size and zeta potential measurements
Particle size, polydispersity index (PdI) and zeta potential of PC:Chol:DSPE-PEG-COOH and CLU-conjugated nanoliposomes (PC:Chol:DSPE-PEG-CLU) were determined by dynamic light scattering (DLS) and laser Doppler electrophoresis using the instrument Zetasizer Nano (Malvern Instruments, Westborough, MA). All measurements were carried out in triplicate in 0.1 M MES buffer, pH 7.4.
Atomic force microscopy of nanoliposomes
Nanoliposomes were imaged by atomic force microscopy (AFM). Briefly, nanoliposomes were deposited on fresh-cleaved mica surfaces. After 15 min, the solution was removed and the surface was rinsed three times with imaging buffer (20 mM Tris, 150 mM KCl, 25 mM MgCl2, pH 7.8) to remove the unbound liposome molecules. CLU (1 ng/uL) was immobilized on APS-modified mica surface as previously described (Senapati et al., Citation2013). All AFM imaging was carried out in contact mode using Veeco Multimode AFM (Veeco Instruments Inc, Town of Oyster Bay, NY). Silicon nitride probes with spring constant 0.06 N/m were used for imaging. All the images were analyzed using Nanoscope (Bruker Corporation, Santa Barbara, CA) and Gwyddion (Czech Metrology Institute, Brno, Czech Republic) software.
CLU-to-liposome coupling reaction efficiency
The efficiency of the coupling reaction between CLU and PEG-functionalized nanoliposomes (PC:Chol:DSPE-PEG-COOH) was indirectly evaluated using the free CLU protein fraction (unbound protein) collected after isopycnic separation and compared with the initial CLU concentration. Protein quantification was determined by enzyme-linked immunosorbent assay (ELISA), using the Quantikine ELISA kit (R&D Systems, Minneapolis, MN) kit. Standards and samples were prepared according to the manufacturer’s instructions. The optical density was registered at 450 nm with a wavelength correction set at 570 nm using a microplate reader.
Nanoliposome stability assay
After liposomes preparation, the effect of temperature on the liposome size distribution was investigated. PEG-functionalized nanoliposomes (PC:Chol:DSPE-PEG-COOH) were incubated at 37 °C or room temperature and at different time points the particle size distribution of nanoliposomes was determined by DLS. All measurements were performed in triplicates.
Human LC purification
LC from the urine of two biopsy-proven AL subjects with cardiac amyloidosis (51 ± 8 years old, both males, both lambda type) were purified as per previous protocol (Migrino et al., Citation2010,Citation2011) using dialysis, size exclusion filtration and Affigel blue filtration. LC protein was verified by both Western blot (WB) and ELISA (human antiserum to lambda and kappa; Sigma-Aldrich, St. Louis MO). The sources of LC and adipose arterioles provided informed consent for collection and the study was approved by and under the supervision of the Institutional Review Boards of the Phenix Veterans Affairs Health Care System and the Medical College of Wisconsin.
Human arteriole vasoreactivity
Subcutaneous abdominal adipose tissues were collected by surgeons following informed consent from eight male volunteers (50.5 ± 6.1 years old) who were undergoing routine planned elective abdominal surgeries for clinical indications. These volunteers are not known to have AL, cardiovascular disease or diabetes. Arterioles were isolated from adipose tissue (∼80–300 μM pressurized diameter) and then cannulated and pressurized to 30 and then 60 mmHg (approximate physiologic pressure of similar sized vessels in vivo) similar to our previous methods (Migrino et al., Citation2010,Citation2011). The arterioles were pre-constricted with endothelin-1 at successive doses (10−9–10−4 M) to achieve ∼60% of maximum diameter. Thereafter, baseline (control) dilator responses to acetylcholine (10−9–10−4 M) and then papaverine (10−4 M) were measured by video microscopy to assess endothelium-dependent (acetylcholine) and smooth muscle-dependent (papaverine) responses. Following washout, arterioles were then exposed for 1 h to 20 μg/mL of LC alone or together with 1) free CLU (300 ng/mL) and 2) the corresponding volume of PEGylated-nanoliposomal CLU to 300 ng/mL. A second (post-treatment) dilator response to acetylcholine and papaverine was again measured. About 20 μg/mL LC was chosen as it is close to physiologic concentrations of LC in patients (Palladini et al., Citation2006) and the dose of NL was chosen as it was the dose used in our prior study that showed vascular protection against LC injury (Truran et al., Citation2016).
Data and statistical analyses
Data are expressed as mean ± standard error of means with significant p values set at p < 0.05 (two-sided). Maximum dilator response to acetylcholine (10−4 M) and papaverine (10−4 M) were compared between treatment response and baseline control response using paired t-test. Dilator responses between treatments were compared using unpaired t-test. Acetylcholine effective concentration 50% (EC50) is the dose of acetylcholine that produced 50% maximum dilation. EC50 was calculated using nonlinear regression using variable slope (four parameters) and least-squares ordinary fit similar to our previous method (Migrino et al., Citation2011) using GraphPad Prism 5.0 (GraphPad Software, San Diego CA). EC50 (expressed as LogM) was compared between treatment responses and baseline control responses as well as between treatment responses using paired and unpaired t-test, respectively. Statistical analyzes were also performed using Sigmastat 3.5 (Richmond, CA).
Results
Liposomes consisting of PC, Chol and DSPE-PEG-COOH (70:25:5 mol%) were prepared by ultrasonication followed by their surface functionalization with CLU to obtain PEGylated-nanoliposomal CLU (PC:Chol:DSPE-PEG-CLU). The presence of the carboxy group at the distal end of DSPE-PEG-COOH residue anchored in the liposomes, allowed the covalent attachment of CLU via a carboxy-to-amine crosslinking reaction in the presence of EDC and sulfo-NHS (). The conjugation reaction took place after the carboxy groups on the liposomes were activated and incubated with CLU in 0.1 M MES buffer overnight (Veronese, Citation2001).
CLU-conjugated liposomes (PC:Chol:DSPE-PEG-CLU) were separated from unbound CLU protein by isopycnic centrifugation using Ficoll 400 gradients. After centrifugation, the liposomes formed a milky layer in the interface between 10% Ficoll 400 and 0.1 M MES buffer, pH 7.4, while the free protein remained at the bottom of the tube. Both CLU-conjugated liposomes and unbound CLU fractions were collected.
The particle size distribution and zeta potential analysis of nanoliposome formulations measured by DLS and laser Doppler electrophoresis are shown in . The nanoliposomes containing only PC and cholesterol (PC:Chol) showed an average particle size of 121 nm, while those that incorporated the DSPE-PEG-COOH residue in the nanoliposome membrane (PC:Chol:DSPE-PEG-COOH), slightly increased their size to ∼131 nm. When analyzed, statistically significant differences between these two formulations were found (), which suggest the incorporation of DSPE-PEG-COOH in the liposome membrane. Additionally, after CLU was conjugated to the DSPE-PEG-COOH residue in the nanoliposome surface no modifications in the size were observed. On the other hand, all the nanoliposome formulations resulted in a negative zeta potential as observed in and .
Figure 2. Determination of particle size distribution (A) and zeta potential (B) of nanoliposomes before and after CLU conjugation. Statistically significant differences were found between the non-PEGylated (PC:Chol) and PEGylated formulations. *p < 0.05 versus PC:Chol, ***p < 0.001 versus PC:Chol, respectively.
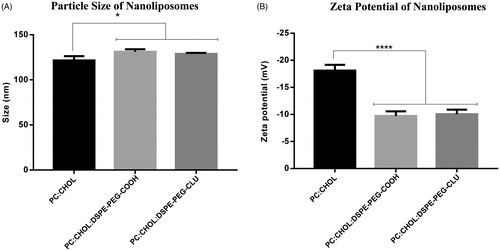
Table 1. Liposomal formulations and their characterization.
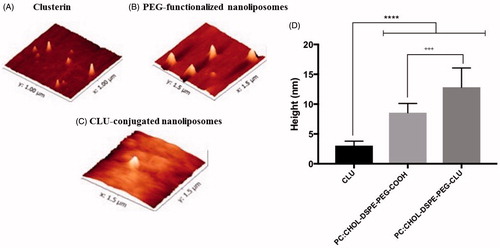
Often, the force applied by the AFM tip during imaging affect the morphology of the nanoliposomes, making them look wider (convolution effect) (Canet-Ferrer et al., Citation2014; Rouzi et al., Citation2007,Citation2011). Therefore, the height rather than the diameter of the nanoliposomes was recorded for AFM analysis. As observed in , our AFM data showed that CLU protein had an average height of ∼3.05 ± 0.7 nm (), while PEG-functionalized liposomes were 8.5 ± 1.5 nm high (). After the conjugation reaction took place and the unbound CLU protein was removed, CLU-conjugated liposomes (PC;Chol:DSPE-PEG-CLU) showed an average height of 12.8 ± 3.2 nm (), which was higher than either CLU or PEG-functionalized liposomes alone (), confirming that the conjugation reaction occurred.
Figure 3. AFM imaging of nanoliposomes. (A) 2D images of CLU protein, (B) PEG-functionalized liposomes, (C) purified CLU-PEGylated nanoliposomes and (D) their height analysis. ***p < 0.001 versus CLU, +++p < 0.001 versus CLU-PEGylated nanoliposome.
The coupling reaction efficiency between CLU and PEG-functionalized nanoliposomes (PC:Chol:DSPE-PEG-COOH) was determined indirectly by quantifying the amount of unbound protein by ELISA. The unbound CLU protein was first separated by isopycnic centrifugation from the CLU-conjugated nanoliposomes and subsequently quantified. The results showed that the coupling reaction of CLU to PEGylated nanoliposomes was very efficient as 99.4% of the CLU protein was covalently linked to the PEG-functionalized nanoliposomes.
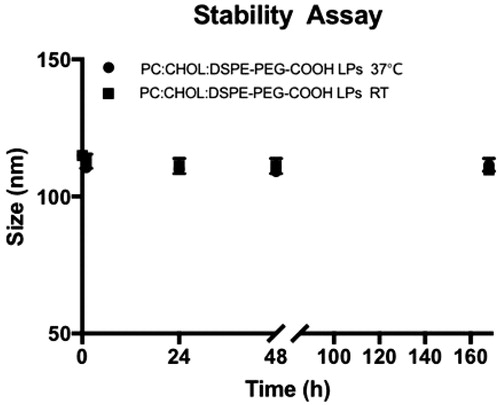
The stability of PEG-functionalized liposomes (PC:Chol:DSPE-PEG-COOH) was also evaluated by monitoring the changes in the particle size distribution of the liposomes after incubation at room temperature and 37 °C for 1, 24, 48 h and 1 week in 0.1 M MES buffer, pH 7.4. After the established time points the particle size distribution of liposomes was measured by DLS. shows that no significant differences in the average particle size of PEG-functionalized occurred at room temperature after a week. Differences between the particle size of nanoliposomes (PC:Chol:DSPE-PEG-COOH) incubated 37 °C and room temperature were also not found.
Figure 4. Stability assay of PEG-functionalized nanoliposomes. The integrity of PEG-functionalized liposomes at room temperature and 37 °C was monitored by DLS after 1, 24, 48 and 168 h.
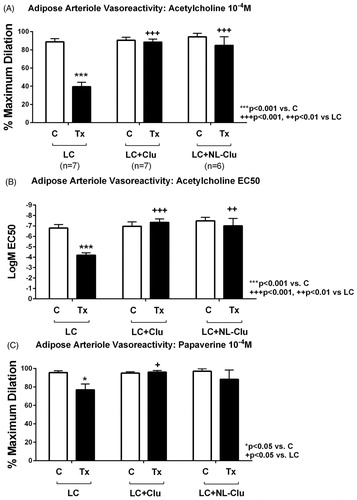
We investigated the effect of PEGylated-nanoliposomal CLU (PC:Chol:DSPE-PEG-CLU) on LC-induced human endothelial dysfunction using our previously reported method, which measures the arteriole vasoreactivity response to acetylcholine and papaverine after exposure to LC. As seen in , after exposure to LC arterioles had a significant reduction in dilation response to acetylcholine () and papaverine () compared to the baseline control. Treatment of arterioles with free CLU as well as with PEGylated-nanoliposomal CLU restored the dilation response to both acetylcholine and papaverine, which indicated that CLU conjugation to the nanoliposomal surface did not alter its protective activity. In addition, when compared to free CLU, PEGylated-nanoliposomal CLU did not show statistically significant differences in response.
Figure 5. Human arteriole dilation response. A and B show response to acetylcholine with A showing maximum dilation response (10−4 M dose) and B showing EC50 values. Light chain treatment reduced dilator response to acetylcholine compared to baseline control. Co-treatment of LC with free CLU and PEGylated-nanoliposomal CLU (NL-CLU) restored dilator response to acetylcholine. Note that the y-axis of B is in inverse order. C shows response to papaverine. There was a modest reduction in dilator response in LC treated arterioles; LC co-treatment with free CLU or NL-CLU showed no significant difference with baseline control response. There was no significant difference in dilator response to acetylcholine or papaverine in arterioles treated with LC and NL-CLU versus LC and free CLU.
Discussion
The novel aspects of our study include the design of a formulation of CLU-containing nanoliposomes by PEGylation and the initial proof-of-concept that the formulation protects against LC-induced human microvascular endothelial dysfunction. This is potentially relevant in efforts to develop novel therapeutic agents that directly reverse tissue toxicity of amyloid proteins.
AL amyloidosis remains a disease associated with significant mortality and morbidity. We showed that soluble LC-induced endothelial dysfunction in human adipose and coronary arterioles (Migrino et al., Citation2011), which is consistent with reports by other investigators that the prefibrillar protein species confer tissue toxicity (Shi et al., Citation2010). Currently, there is no available treatment that directly reverses LC toxicity. Contemporary treatment relies on use of chemotherapeutic drugs with or without stem cell transplantation to remove neoplastic plasma cells that produce the LC, an approach that often cannot be offered or has to be modulated to milder regimens/doses in patients with advanced cases in light of high treatment-related mortality (Dispenzieri et al., Citation2004a; Skinner et al., Citation2004).
CLU, also known as apolipoprotein J, is a constitutively expressed chaperone glycoprotein displaying cytoprotective properties, including the stabilization of damaged unfolded proteins, which subsequently prevents the formation and accumulation of amyloid fibrils. Several studies have shown that the protective effect of CLU on stressed proteins is associated with the formation of high molecular weight complexes via hydrophobic interactions which later favors their refolding by the heat-shock protein Hsp70 (Humphreys et al., Citation1999; Poon et al., Citation2002).
We reported that human endothelial cells exposed to LC showed reduced viability that was associated with reduction of CLU expression and secretion (Franco et al., Citation2012). Importantly, co-treatment with exogenous CLU restored endothelial cell viability and microvascular endothelial function. In-vivo, CLU was reported to have a short half-life (<2 h) (Rizzi et al., Citation2009), limiting its potential utility when administered intravenously on its own. CLU also acts as an apolipoprotein that could potentially be diverted for lipid transport while in circulation before it can interact with LC at the target microvascular tissue. One way of transporting CLU to vascular tissue is through the utilization of a carrier system. We chose nanoliposomes as they are well-established biodegradable, biocompatible, and nontoxic drug delivery systems (Lian & Ho, Citation2001). Additionally, we showed that nanoliposomes composed of 70 mol% phosphatidylcholine, 25 mol% cholesterol and 5 mol% of either phosphatidic acid (Truran et al., Citation2014) or monosialoganglioside (Franco et al., Citation2016) also restored endothelial function of human arterioles exposed to LC via increased NO bioavailability and reduced oxidative stress. We also showed that nanoliposomes may have chaperone protein-like properties when we demonstrated alteration in β-sheet properties of LC (Truran et al., Citation2014) and prevention of amyloid fibril formation of Aβ peptide (Truran et al., Citation2016) and LC (unpublished data). Because of these complementary protective effects on LC injury by CLU and nanoliposomes, we decided to pursue the design of a formulation of PEGylated-nanoliposomal CLU to maintain its protective effects while potentially creating a carrier system that would allow CLU to reach target tissue when used in-vivo.
We prepared PEGylated-nanoliposomal CLU composed of PC (70 mol%), Chol (25 mol%) and DSPE-PEG-COOH (2000) (5 mol%) at a final protein concentration of 500 ng/mL. The conjugation of CLU to the surface of the PEG-functionalized liposomes (PC:Chol:DSPE-PEG-COOH) was carried out by using a carboxy-to-amine reaction, where we first activated the carboxy groups present at the distal end of the PEG residue with the crosslinker carbodiimide to produce the intermediate O-acylisourea, which was reactive towards amines in an aqueous solution containing CLU. To further increase the efficiency of this reaction we also added sulfo-NHS so that the isourea was converted into a succinimide ester, which also reacted with the CLU amines (Dent, Citation2005). PEGylation of proteins, i.e. this covalent attachment of PEG to proteins is a widely used method for protein modification in order to alter their PK in vivo, as this polymer is neither toxic nor immunogenic and it has been approved by the FDA as safe (Jevsevar et al., Citation2010). In addition, a major advantage of using this method is the reduction of risk of protein denaturation or loss of activity as no ligand modifications are required prior to the conjugation reaction (Nobs et al., Citation2004).
Due to the high sensitivity and selectivity to detect very small quantities of proteins (Gan & Patel, Citation2013), ELISA was used to determine the CLU protein coupling efficiency to PEG-functionalized nanoliposomes. The attachment of CLU to PEG-functionalized nanoliposomes was indirectly evaluated by measuring the amount unbound protein fraction collected after isopycnic separation and compared with the initial CLU concentration. As described in the results section, the conjugation reaction resulted in a highly efficient coupling of CLU protein to PEGylated nanoliposomes. We attributed this efficiency to the high number of PEG activated groups on the surface of the liposomes (1/785 CLU to DSPE-PEG-COOH molar ratio), and the presence of both EDC and sulfo-NHS in the reaction, which in combination have shown to significantly increase the stability of the amine-reactive intermediates leading to highly efficient coupling reactions (Hermanson, Citation2013; Mercadal et al., Citation1999). Additionally, the conjugation reaction between the PEGylated nanoliposomes and CLU protein was facilitated by the excess of EDC/sulfo-NHS, which ultimately activated the carboxy groups in the surface of the nanoliposomes (PC:Chol:DSPE-PEG-COOH) to react with CLU. The conjugation of CLU to the PEG-functionalized liposomes was also confirmed by AFM imaging, showing that the height of the CLU-PEG-nanoliposome conjugate resulted in the sum of the individual heights of CLU and PEG-functionalized liposomes. This high coupling efficiency is consistent with other results published elsewhere. For example, Mercadal et al. (Citation1999) attached proteins to the distal end of the PEG-terminus in liposomes composed of 5 mol% total PEG derivatives and with an average particle size of 125 nm. After the reaction, Mercadal obtained coupling efficiencies ranging from 89 to 100%. The group also described that the amount of protein bound to liposomes was dependent on the number of reactive groups on the liposome surface as well as the reactive groups-to-protein molar ratio in the reaction.
In addition to the high coupling efficiency, PEG-functionalized nanoliposomes (PC:Chol:DSPE-PEG-COOH) appear to be stable under room temperature and 37 °C. Although analysis of nanoliposomes in the presence of serum remains to be tested, PEGylated nanoliposomes are expected to be stabilized not only by the presence of the PEG, but also by cholesterolat. Guo et al. (Citation1980), for example, showed that cholesterol prevented the disruption of liposomes in the presence of whole serum compared to PC only-containing liposomes by inhibiting the biding of the serum proteins to liposomes. Similar findings were described by Kirby et al. (Citation1980), who also reported that increasing amounts of cholesterol inhibited the phospholipid exchange of liposomes with serum proteins as well as their association, thus reducing their clearance by the reticuloendothelial system. This effect has been attributed to the ability of cholesterol to increase the phase transition temperature of phospholipids and to reduce their permeability (Gregoriadis et al., Citation1986).
By modifying the surface of liposomes with the hydrophilic and flexible polymer PEG the nanoliposomes could also potentially protect CLU from being recognized by the reticuloendothelial system and being rapidly removed from circulation, as PEG creates a steric effect that reduces nonspecific interactions with blood components (Klibanov et al., Citation1990; Mu et al., Citation2013). As a result, the circulation lifetime of liposomes is increased. In addition, the prolonged persistence in the circulation could increase the time and opportunity by which CLU residing on the surface of the liposome could interact with its endocytic receptors glycoprotein 330/megalin (Kounnas et al., Citation1995; Zlokovic et al., Citation1996) leading to increased intracellular delivery of CLU.
Our main goal for this article was to assess the impact of PEGylation on the activity of CLU using our experimental model of arteriole vasoreactivity and compare the functional activity of PEGylated-nanoliposomal CLU with free CLU. Our results clearly show that the protective effect of CLU against LC-induced endothelial damage was not diminished upon conjugation as both PEGylated CLU nanoliposomes and free CLU restored endothelial function. Our finding is supported by Mori (Mori et al., Citation1991) and Tanifum (Tanifum et al., Citation2012) who reported that the presence of DSPE-PEG (2000) on the surface of liposomes did not interfere with the binding of ligands to their receptors and suggested DSPE-PEG (2000) as the optimal polymer for target binding (Mori et al., Citation1991).
Although our PEGylated-nanoliposomal CLU (PC:Chol:DSPE-PEG-CLU) showed protective effect on isolated ex-vivo human arterioles, our study is limited in that we have not yet demonstrated whether it would also be effective on in-vivo animal models. By demonstrating this proof-of-concept, however, testing efficacy, bioavailability, safety, pharmacodynamics and pharmacokinetics of the formulation in-vivo will be the focus of future investigations. Additionally, the specific mechanisms by which PEGylated-nanoliposomal CLU confer protection need to be investigated especially in relation to known protective mechanisms by CLU and nanoliposomes alone.
Conclusion
PEGylated-nanoliposomal CLU provided a cellular delivery platform for CLU and maintained its protective effect against LC-induced human arteriole endothelial dysfunction, thus demonstrating its high potential to directly reverse the vascular toxicity of AL amyloid light chain proteins at the target organ level.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. R.Q.M. was funded with the National Institutes of Health (NIA R21AG044723), Veterans Affairs Merit (I01BX007080) and the Amyloidosis Foundation.
Acknowledgements
The authors would like to thank the College of Pharmacy-Glendale, Midwestern University, for the financial support provided for Diana Guzman-Villanueva, the surgeons and staff of the Phoenix VA Surgery Service, John Hatfield, the Carl T. Hayden Medical Research Foundation and the Phoenix VA Office of Research. The contents of the article do not represent the views of the Department of Veterans Affairs or the United States government.
References
- Canet-Ferrer J, Coronado E, Forment-Aliaga A, Pinilla-Cienfuegos E. (2014). Correction of the tip convolution effects in the imaging of nanostructures studies though scanning force microscopy. Nanotechnology 25:395703
- Coelho T, Adams D, Silva A, et al. (2013). Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 369:819–29
- Dent DM. 2005. Conjugation methods. In: Wild D, ed. The immunoassay handbook. UK: Elsevier Ltd, 213–223
- Dispenzieri A, Gertz MA, Kyle RA, et al. (2004a). Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol Off J Am Soc Clin Oncol 22:3751–7
- Dispenzieri A, Gertz MA, Kyle RA, et al. (2004b). Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood 104:1881–7
- Eisele YS, Monteiro C, Fearns C, et al. (2015). Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discov 14:759–80
- Franco DA, Truran S, Burciu C, et al. (2012). Protective role of clusterin in preserving endothelial function in AL amyloidosis. Atherosclerosis 225:220–3
- Franco DA, Truran S, Weissig V, et al. (2016). Monosialoganglioside‐containing nanoliposomes restore endothelial function impaired by AL amyloidosis light chain proteins. J Am Heart Assoc 5:e003318
- Gan SD, Patel K. (2013). Enzyme immunoassay and enzyme-linked immunosorbent assay. J Invest Dermatol 133:e12
- Gregoriadis G, Senior J, Wolff B. 1986. Targeting of liposomes: optimization of vesicle behavior in vivo. In: Yagi K, ed. Medical application of liposomes. Basel, Switzerland: Karger, 67–79
- Guo LSS, Hamilton RL, Goerke J, et al. (1980). Interaction of unilamellar liposomes with serum lipoproteins and apolipoproteins. J Lipid Res 21:993–1003
- Hermanson GT. 2013. Zero-length crosslinkers. Bioconjugate techniques. UK: Elsevier Inc., 259–63
- Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. (1999). Clusterin has chaperone-like activity similar to that of small heat shock proteins. J Biol Chem 274:6875–81
- Jayaraman M, Ansell SM, Mui BL, et al. (2012). Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl 51:8529–33
- Jevsevar S, Kunstelj M, Porekar VG. (2010). PEGylation of therapeutic proteins. Biotechnol J 5:113–28
- Kirby CJ, Clarke J, Gregoriadis G. (1980). Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem J 186:591–8
- Klibanov AL, Maruyama K, Torchilin VP, Huang L. (1990). Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 268:235–7
- Klibanov AL, Torchilin VP, Zaipsky S. 2003. Long-circulating sterically protected liposomes. In: Torchilin VP, Weissig V, eds. Liposomes: a practical approach. Oxford, UK: Oxford University Press, 231–65
- Kounnas MZ, Loukinova EB, Stefansson S, et al. (1995). Identification of glycoprotein 330 as an endocytic receptor for apolipoprotein J/clusterin. J Biol Chem 270:13070–5
- Lakins JN, Poon S, Easterbrook-Smith SB, et al. (2002). Evidence that clusterin has discrete chaperone and ligand binding sites. Biochemistry (Mosc.) 41:282–91
- Lasch J, Weissig V, BrandI M. (2003). Preparation of liposomes. In: Torchilini VP, Weissig V, eds. Liposomes: a practical approach. Oxford, UK: Oxford University Press, 3–27
- Lian T, Ho RJY. (2001). Trends and developments in liposome drug delivery systems. J Pharm Sci 90:667–80
- Mercadal M, Domingo JC, Petriz J, et al. (1999). A novel strategy affords high-yield coupling of antibody to extremities of liposomal Surface-grafted PEG chains. Biochim Biophys Acta 1418:232–8
- Migrino RQ, Hari P, Gutterman DD, et al. (2010). Systemic and microvascular oxidative stress induced by light chain amyloidosis. Int J Cardiol 145:67–8
- Migrino RQ, Harmann L, Christenson R, Hari P. (2014). Clinical and imaging predictors of 1-year and long-term mortality in light chain (AL) amyloidosis: a 5-year follow-up study. Heart Vessels 29:793–800
- Migrino RQ, Truran S, Gutterman DD, et al. (2011). Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins. Am J Physiol Heart Circ Physiol 301:H2305–12
- Mori A, Klibanov AL, Torchilin VP, Huang L. (1991). Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside GM1 on the circulation time of liposomes and on the target binding of immunoliposomes in vivo. FEBS Lett 284:263–6
- Mu Q, Hu T, Yu J. (2013). Molecular insight into the steric shielding effect of PEG on the conjugated staphylokinase: biochemical characterization and molecular dynamics simulation. PLOS One 8:e68559
- Nobs L, Buchegger F, Gurny R, Allémann E. (2004). Current methods for attaching targeting ligands to liposomes and nanoparticles. J Pharm Sci 93:1980–92
- Oda T, Wals P, Osterburg HH, et al. (1995). Clusterin (apoJ) alters the aggregation of amyloid β-peptide (Aβ1-42) and forms slowly sedimenting Aβ complexes that cause oxidative stress. Exp Neurol 136:22–31
- Palladini G, Lavatelli F, Russo P, et al. (2006). Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood 107:3854–8
- Poon S, Treweek TM, Wilson MR, et al. (2002). Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off-folding pathway. FEBS Lett 513:259–66
- Rizzi F, Caccamo AE, Belloni L, Bettuzzi S. (2009). Clusterin is a short half-life, poly-ubiquitinated protein, which controls the fate of prostate cancer cells. J Cell Physiol 219:314–23
- Rouzi B, Belletti D, Tombesi A, et al. (2011). AFM, ESEM, TEM, and CLSM in liposomal characterization: a comparative study. Int J Nanomedicine 6:557–63
- Rouzi B, Tosi G, Leo E, Vandelli MA. (2007). Application of atomic force microscopy to characterize liposomes as drug and gene carriers. Talanta 73:12–22
- Sanchorawala V. (2006). Light chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol 1:1331–41
- Shi J, Guan J, Jiang B, et al. (2010). Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38α MAPK pathway. Proc Natl Acad Sci USA 107:4188–93
- Senapati S, Manna S, Lindsay S, Zhang P. (2013). Application of catalyst-free click reactions in attaching affinity molecules to tips of atomic force microscopy for detection of protein biomarkers. Langmuir 29:14622–30
- Skinner M, Sanchorawala V, Seldin DC, et al. (2004). High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med 140:85–93
- Tanifum EA, Dasgupta I, Srivastava M, et al. (2012). Intravenous delivery of targeted liposomes to amyloid-β pathology in APP/PSEN1 transgenic mice. PLOS One 7:e48515
- Truran S, Weissig V, Madine J, et al. (2016). Nanoliposomes protect against human arteriole endothelial dysfunction induced by β-amyloid peptide. J Cereb Blood Flow Metab 36:405–12
- Truran S, Weissig V, Ramirez-Alvarado M, et al. (2014). Nanoliposomes protect against AL amyloid light chain protein-induced endothelial injury. J Liposome Res 24:69–73
- Veronese FM. (2001). Peptide and protein PEGylation: a review of problems and solutions. Biomaterials 22:405–17
- Zlokovic BV, Martel CL, Matsubara E, et al. (1996). Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci USA 93:4229–34