Abstract
AmBisome (liposomal amphotericin B) is among the earliest approved liposomal therapeutics, and has been in commercial use since the early 1990s. This review provides examples of non-clinical, regulatory, clinical label expansion, adverse event management, and supply chain control reflecting the real world challenges of a commercial liposomal therapeutic. We review examples of post-approval clinical development in severe lung infections, development of US and European guidance documents around liposomal therapeutics, the creation of a suitable placebo for blinded clinical trials, response to findings of a possible new category of adverse event (what turned out to be pseudohyperphosphatemia), challenges in handling the finished product in a setting with high risk of exposure of the product to temperatures outside of the established label storage conditions, and elements of continuingly increased aseptic processing requirements for manufacturing.
Introduction
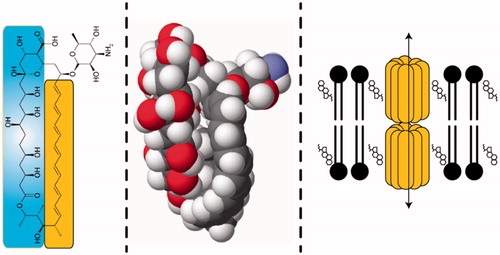
AmBisome (liposomal amphotericin B) was formulated and brought to clinical trials by Vestar Inc., a company specializing in the development of highly stable so-called conventional liposomes built around small unilamellar vesicles (SUVs) formulated using a 2:1 DSPC:cholesterol backbone formulation (Jensen and Bunch Citation2007). This paradigm was primarily driven towards tumour applications, while the AmBisome formulation (Adler-Moore and Proffitt Citation1993) derivatized the basic formulation to include an anionic phospholipid component (final lipid formula 2:1:0.8 HSPC:cholesterol:DSPG) and the pore forming drug amphotericin B, present as intact membrane spanning channels in what is a biomimetic, natural phospholipid bilayer. Amphotericin B () preferentially and stably binds to cholesterol in this bilayer, substantially reducing the toxicity of the formulation on injection by preventing the transfer of the drug to natural membranes in the host (Jensen et al. Citation1999, Adler-Moore et al. Citation2006). The drug has a higher avidity for the fungal sterol ergosterol, and this affords rapid transfer of the drug to fungal membranes and thence preserving efficacy. The precise mechanism of transfer, in various settings, is not, however, fully understood (see e.g. Lestner et al. Citation2010). AmBisome was commercialized by the successor company NeXstar (acquired by Gilead Sciences in 1999) in Europe in the early 1990s. Because of the substantial attenuation of toxicity, dose escalation is possible relative to the detergent suspension (‘free drug’) formulation. AmBisome leverages this dosing flexibility, an intrinsic broad spectrum of activity, and practical imperviousness to drug resistance to remain a critical drug for the treatment of systemic fungal infections of many varieties and manifestations. Launch in the United States of America was accompanied by an indication for empirical therapy for presumed fungal infection in febrile, neutropenic patients (‘fever of unknown origin’), and other specific fungal maladies. Clinical data supporting this approval included empiric therapy vs. the free drug formulation and a direct head to head study against a competing lipid based formulation (Abelcet, lacking cholesterol) (Prentice et al. Citation1997, Walsh et al. Citation1999, Wingard et al. Citation2000) (See the US package insert at www.ambisome.com). Pharmacokinetics and pharmacodynamics have been recently reviewed (Adler-Moore et al. Citation2017).
Figure 1. Three structures of amphotericin B: a chemical drawing showing the hydrophobic (lipophilic) polyene (yellow), and the hydrophilic poly-hydroxyl side (blue); a space filling model; and a model of the putative structure of a 16 membered double barrel channel entrapped in the phospholipid bilayer associated with cholesterol.
Continued clinical development
Post-approval, and under Gilead, additional clinical development has continued. Use of AmBisome was hampered in one specific indication – the highly lethal lung infection Aspergillosis – because of uncertainty as to lung tissue levels of drug (see e.g. Garey et al. Citation2001), local tissue MICs, and the relatively low efficacy exhibited by the ‘free drug’ formulation (∼20%). Impetus to examine AmBisome in this setting came from non-clinical studies (Olson et al. Citation2006) comparing AmBisome to Abelcet. Chemically immunosuppressed DBA/2 female mice were infected by intra-nasal challenge with Aspergillus fumigatus, and then given IV therapy. Creatinine and blood urea nitrogen (BUN) values were measured, along with evaluation of kidney and lung tissue samples for drug level, fungal burden, and tissue toxicity. Tissue levels were on the order AmBisome > Abelcet for the kidney, yet nephrotoxicity was in the reverse order. Lung tissue drug levels were Abelcet > AmBisome – up to 2 or 3 fold – but a fall-off in efficacy with higher dose was evidenced in Abelcet, athwart the higher drug levels, owing to local tissue toxicity. Abelcet toxicity appeared to be exacerbated in infected vs. healthy animals. In the end, a complex interplay between efficacy, toxicity, and tissue levels is at play in this system.
A 300 + patient study, principally carried out in Australia and Europe (Cornely et al. Citation2007), examined treatment at two dose levels and, at a 3 mg/kg/day dose, revealed a 50% response rate. This rate is comparable to competing azole therapies and has the advantage of an active that has the broadest spectrum of activity against potential lung pathogens – beyond A. fumigatus. Use for mucormycosis and azole resistant pathogens is suggested. This, along with experience in the treatment of HIV related cryptococcal meningitis, also factored into the usage of AmBisome as part of a treatment regimen in 2013 when more than 700 patients exhibited meningitis from Exoserohilum rostratum resulting from spinal or paraspinal infections. These infections were a consequence of contamination of methylprednisolone dosage forms prepared at a compounding pharmacy. As of May 31 2013 (Ritter et al. Citation2013), 55 out of 741 cases resulted in death.
Manufacturing
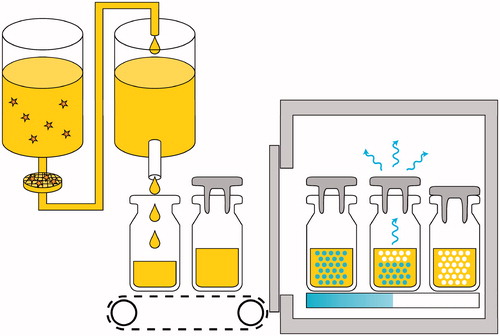
AmBisome is presented as a sterile, lyophilized small volume parenteral vial (). From the time of launch and through expanded product usage, consistent supply of uniform quality and consistency has been critical. Aspects of manufacturing and quality control have been discussed elsewhere (Jensen et al. Citation2006). What has become clear is that liposomal therapeutics, including AmBisome, are as much a function of production process and process control as of formulation (Jensen et al. Citation2006). This has been revealed also in analysis of a number of ostensible copy products, where an identical formulation is proffered, and yet where substantially degraded toxicity and efficacy are evidenced (Olson et al. Citation2008, Jensen and Soo Hoo Citation2014, Olson et al. Citation2015, Adler-Moore et al. Citation2016). Directly reflecting this, the EMA/CHMP has adopted as guidance a ‘Reflection Paper on the Data Requirements for Intravenous Liposomal Products Developed with Reference to an Innovator Liposomal Product;’ this document emphasizes aspects of potential variation in efficacy and/or toxicity that cannot be adduced by conventional bioequivalence approaches, and that many of these systems are too complex to avoid the generation of significant chemistry, physical, non-clinical, and clinical data. Additional emphasis is placed on risks of dose escalation, emphasis on pharmacodynamics, and action in the tissues, to include both entrapment and consequential efficacy and local toxicity. Aspects of infusion related hypersensitivity are also included (see additional discussion in Jensen and Soo Hoo Citation2014). A related concern was raised when a new clinical trial required a placebo to match AmBisome – as lyocake, reconstituted vial, and as diluted infusion preparation in dextrose (). Use of a placebo is not common for a drug like AmBisome, which treats life threatening disease, but this was for a prophylaxis application. The formulation chosen was SoyPC, rather than a drug free mimic of the AmBisome formulation, because it was not known how the drug free preparation might behave on injection relative to complement activation; the SoyPC formulation would rapidly come apart and clear on injection. Colour was from the SoyPC itself and riboflavin-5′-phosphate, both encapsulated and in the buffer, at a low enough doses to avoid observation in the urine. To develop the appropriate opalescence of the ∼80 nm median diameter AmBisome particle extrusion was used to process the SoyPC liposomes, as high sheer processing typically results in much smaller liposomes for this formulation (Jensen et al. Citation2008). The adverse events profile of the placebo in a recently published clinical trial reflects no evidence of complement activation for this formula (Cornely et al. Citation2017).
Managing the label: adverse events
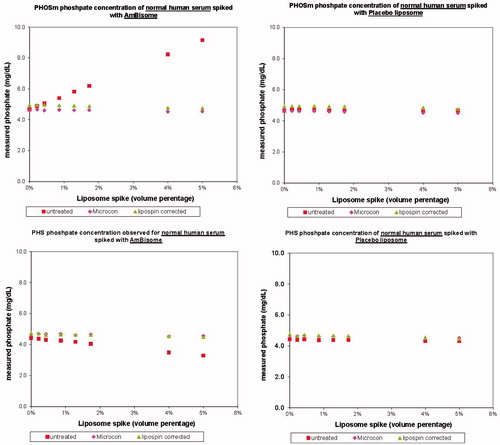
Many drugs begin to show previously unknown or unconnected adverse events – side effects – once they are in wider market use outside of the better controlled but patient number and profile limited clinical trial setting. This is especially true of liposomal therapeutics, which have complex pharmacodynamics profiles, and which can in addition remain present in meaningful quantities in blood/serum samples owing to slower clearance. An example of the latter emerged in reports of hyperphosphatemia – approximately 20 cases – usually with no other symptoms outside of measured serum phosphate levels. All cases were revealed to use of a specific clinical analyser – the Beckman-Coulter PHOSm on their Synchron LX20 (measuring as phosphate using a reaction product of ammonium molybdate at low pH). In a few cases, cross check with another assay system showed no hyperphosphatemia. If indeed these cases were a pseudohyperphosphatemia, it would be very undesirable to treat it, alter diet, or alter anti-fungal therapy. In collaboration with Beckman scientists (Jensen et al. Citation2010) we carried out studies using the PHOSm kit, an alternate kit PHS, and titrations with both AmBisome and placebo in serum samples (in this case, the placebo followed the AmBisome formulation absent drug). Titrations were also carried out with two methods to attempt to remove liposomes from the sample: the reagent LipoClear Plus (Iris International, Los Angeles, CA) – often used for lipemic samples – and microcentrifugation using a Microcon 30 (Millipore, Billerica, MA). Results () show that AmBisome, not placebo, causes psuedohyperphosphatemia in the PHOSm chemistry; placebo data prove that the result is not from phosphorous from liposomes (the placebo data), and contrary to an initial hypothesis is not from light scattering or optical interference. It turned out that the AmBisome spiked samples exhibited interference with the function of a carbohydrate agent used to control reaction kinetics in the assay. This mechanism explains both pseudohyperphosphatemia (PHOSm) and a mild pseudohypophosphatemia seen in the PHS kits. The effect was effectively ‘cured’ by use of LipoClear or Microcon-30 treatments. In addition to publication, the following language was added to the Patient Information Leaflet in the EU: ‘False elevations of serum phosphate may occur when samples from patients receiving AmBisome are analyzed using the PHOSm assay (e.g. used in Beckman Coulter analyzers including the Synchron LX20). This assay is intended for the quantitative determination of inorganic phosphorus in human serum, plasma or urine samples.’
Chemical and physical thermal stability and implications for storage, transport, and use
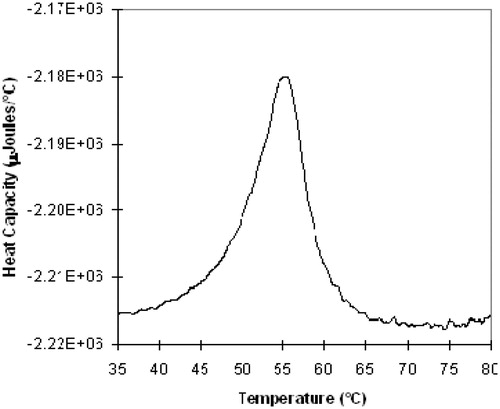
With a backbone distearoyl fatty acid and cholesterol formulation, the AmBisome liposomes exhibits a broad gel to liquid crystal transition centered at ∼55 °C (). One consequence of this is the novel temperature stability profile of the product. AmBisome has an approved storage condition in the United States of America ‘up to 25 °C.’ This storage condition is largely harmonized globally, and evolved from an earlier more restrictive refrigerated storage condition (2–8 °C). As part of commercialization, many studies of the chemical and physical stability of the unopened, un-reconstituted vial have been carried out. Gilead has no regulatory authorization to store AmBisome outside of the labelled storage condition. Nevertheless, chemical and physical stability information has been adduced and can be utilized, under appropriate regulatory guidelines, to assess excursions in temperature experienced by the product.
Stability at 2–25 °C. AmBisome is a lyophilized product which is shipped and stored at temperatures up to 25 °C. Stability of AmBisome is supported by data submitted in global regulatory filings. The data supports stability over the range 2–25 °C over a period of 48 months. The chemical and physical aspects of the lyophilized product conform to approve AmBisome stability specifications over the entire 48 months.
Stability at −40–2 °C. Gilead has studied the colloidal quality and drug entrapment stability over a > 10 year period for material stored at −40 °C. Thus, physical stability is established in this temperature range, and there is no expectation or evidence of any chemical change at these temperatures.
The container/closure components are Type I glass and a halobutyl elastomer lyophilization stopper. The glass maintains its properties to extremely low temperatures. However, the halobutyl elastomer can exhibit compromised visco-elastic properties at or below ∼−55 °C. Therefore, temperatures below −50 °C will begin to compromise the container closure integrity and thence potentially sterility assurance. It is therefore very important to avoid a situation where cooling – e.g. with dry ice – is utilized, and the stopper may be below −50 °C due to contact with the dry ice even though the container itself may on average be above this temperature.
Stability at 25–35 °C. Stability studies of AmBisome stored at 35 °C have been carried out. No trends are evident in this data.
Stability at >35 °C. At temperatures just below 37 °C, thermal melting of the liposomal structure and/or the structure of the lyophilized cake causes catastrophic and rapid failure of the colloidal dispersion that results from vial reconstitution. This owes in part to the nature of the liquid crystal to gel transition in the liposome structure itself noted above. The product so damaged should not be used for any purpose. For this reason, caution should be taken in evaluating excursions above 25 °C to assure they did not rise above 35 °C.
In summary, the AmBisome unopened and un-reconstituted vial is chemically and physically stable over the temperature range −40–35 °C for at least 6 months. Lower temperatures indicate excellent long-term stability as long as the temperature remains at or above −40 °C. Temperatures lower than −50 °C may compromise container closure integrity. Above 35 °C there is risk of significant and rapid damage to the product. As we will see below, this profile has consequence for use in hot climates with challenging pharmaceutical transport, storage, and use logistics.
Visceral leishmaniasis/kala azar
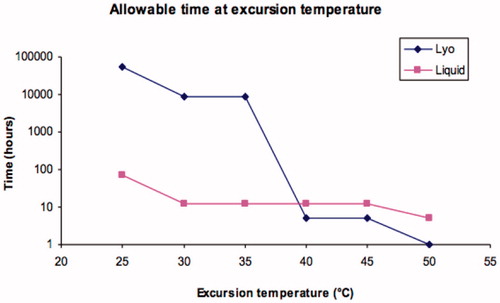
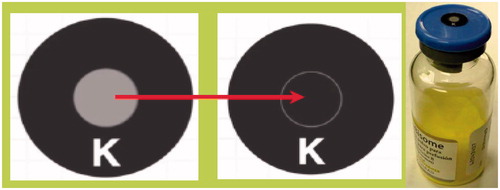
The parasite (leishmania, transmitted by the bite of sandflies) that causes the disease kala azar (visceral leishmaniasis) contains ergosterol as a membrane sterol. Thus, amphotericin B based regimens are part of the armamentarium in treatment. The disease is prevalent in Central and South America, Central Africa, portions of the Middle East, India and South/Southeast Asia. The disease is nearly always fatal if untreated, but a report from a study of patients in the Indian state of Bihar by Sundar et al. (Citation2010) revealed that a single large dose of AmBisome, a dose impossible with the ‘free drug’ formulation without a lengthy period of lower, daily doses, can be curative in the vast majority of cases. Kala azar is the second deadliest parasitic infection and at any given time, more than 300 000 cases may be active. The regions of highest disease burden are also those served by NGOs such as the World Health Organization (WHO) or Doctors Without Borders (MSF). In many of the areas of treatment, temperatures above 35 °C (often well above) are not uncommon, and the risk of product exposure is highest near or at the point of use, where logistical sophistication is likely to be at a nadir. To protect patients from heat damaged product, in these circumstances outside of the controls of the regulated label information, two recommendations are made. First, we note that while the liquid product is less stable overall, owing to slow hydrolysis mainly of the phospholipid components, the elements of patient risk – drug entrapment, for example, – are better for the liquid than the lyocake above 35 °C. This crossover is illustrated in . This in turn leads to the recommendation that when product is taken into the open air or other hot environment, e.g. at the point of treatment, the product be reconstituted in a location where a safe temperature can still be maintained. The second recommendation is the application of a safety indicator. The NGOs are steeped in usage of visual thermal stress indicators to assure the efficacy of vaccine products. Three types are utilized: threshold over time indicators, which display an estimate of time above a threshold temperature; integrated thermal exposure systems, the most common for vaccines, where a central indicator darkens with increased temperature and/or time exposure, with cessation of usage when the centre colour darkens to the border level; and lastly instant threshold systems, where an instantaneous (1–5 s) rise above a threshold temperature will indicate a failure. The latter is appropriate for the AmBisome lyocake thermal profile, and a temperature of 40 °C has been adduced as the ideal threshold to protect patients and limit loss of good product. This application is illustrated in . The TEMPTiME ‘K’ marker, made by Nichiyu Giken Kogyo Co. Ltd (Tokyo, Japan) has a threshold value of 40 ± 1 °C and an irreversible colour change from light coloured to black in a few seconds. It can be applied via an adhesive to the vial or carton.
Future directions
Despite extended presence in the marketplace, the AmBisome product continues to afford opportunities for exploration of enhanced usage. These include enhanced usage in the kala azar application by improved logistics and enhanced collaboration with vector control programs; the use of AmBisome, mainly in Africa, to treat HIV related cryptococcal meningitis; continued exploration of prophylaxis usage; evaluation of emerging fungal or yeast pathogens to confirm that appropriate MIC values obtain; the use of AmBisome in inhalation systems; treatment of biofilms, e.g. in catheters; and continued understanding of pharmacokinetics/pharmacodynamics, of pediatric usage, and of mechanism of action. See Stone et al. (Citation2016).
Aseptic quality
Though not explicitly liposome specific, the last word in this review is given to the concept of aseptic quality. There are ever rising standards of quality from the point of view of sterility assurance, bioburden, contamination, container integrity, and other aspects. Handling of material prior to, during, and after 0.2 micron filtration – the most common form of sterilization (), is being continually enhanced by industry in partnership with regulatory agencies. Examples of these requirements include the progression of filler systems of active barrier or isolator type; for the testing of sterile filter integrity before sterilization of the filter, after sterilization but prior to product exposure, and after use; enhanced quality (uniformity, velocity at multiple working heights, and the absence of turbulence) of laminar flow air in the Class A rooms utilized for production; active vial conveyance in closed areas; closed stopper addition systems; comprehensive line clearance for any occurrences of broken vials; and enhanced vial/closure integrity analysis including demonstration of gas tight seals before and after capping and full investigation of the origin of any visible particulate matter. For AmBisome, these sterility assurance aspects are particularly critical, given the immunocompromised nature of many patients. The time scale of changing standards is on the order of 1–2 years, so constant vigilance and upgrade is necessary, and must work in concert with the ongoing technical oversight necessitated by the complex dosage form and manufacturing process.
Acknowledgements
The author thanks the Technical Services group and other members of the AmBisome team. The author is an employee of Gilead Sciences.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Adler-Moore, J.P. and Proffitt, R.T., 1993. Development, characterization, efficacy and mode of action of Ambisome, a unilamellar liposomal formulation of amphotericin B. Journal of liposome research, 3, 429–450.
- Adler-Moore, J., Jensen, G. M., and Proffitt, R., 2006. Liposomal therapeutics: from animal to man. In: G. Gregoriadis, ed. Liposome technology. 3rd ed. Boca Raton, FL: CRC Press, Volume 3, 405–416.
- Adler-Moore, J.P., Gangneux, J.P., and Pappas, P.G., 2016. Comparison between liposomal formulations of amphotericin B. Medical mycology, 54, 223–231.
- Adler-Moore, J.P., et al., 2017. Tissue pharmacokinetics and pharmacodynamics of Ambisome (L-AmBis) in uninfected and infected animals and their effects on dosing regimens. Journal of liposome research. doi:10.1080/08982104.2017.1327543
- Cornely, O.A., et al., 2007. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clinical infectious disease, 44, 1289–1297.
- Cornely, O.A., et al., 2017. Randomized comparison of liposomal amphotericin B versus placebo to prevent invasive mycoses in acute lymphoblastic leukaemia. Journal of antimicrobial chemotherapy, 72, 2359–2367.
- Garey, K.W., et al., 2001. Cunninghamella bertholletiae infection in a bone marrow transplant patient: amphotericin lung penetration, MIC determinations, and review of the literature. Pharmacotherapy, 21 (7), 855–860.
- Jensen, G.M., et al., 1999. Determination of the relative toxicity of amphotericin B formulations: a red blood cell potassium release assay. Drug Delivery, 6, 81–88.
- Jensen, G. M., et al., 2006. Process development and quality control of injectable liposome therapeutics, In: G. Gregoriadis, ed. Liposome technology. 3rd ed. Boca Raton, FL: CRC Press, Volume 1, 297–310.
- Jensen, G.M., et al., 2008. A liposomal dispersion formulation of propofol: formulation, pharmacokinetics, stability, and identification of an oxidative degradant. Theoretical chemistry accounts, 119 (1–3), 291–296.
- Jensen, G.M., and Bunch, T., 2007. Conventional liposome performance and evaluation: lessons from the development of vescan. Journal of liposome research, 17, 121–137.
- Jensen, G.M., et al., 2010. Erroneous determination of hyperphosphatemia (‘Pseudohyperphosphatemia’) in sera of patients that have been treated with liposomal amphotericin B (AmBisome). Clinica chimica acta, 411, 1900–1905.
- Jensen, G. M., and Soo Hoo, L., 2014. Regulatory aspects of pharmaceutical nanopreparations. In: V.. Torchilin, ed. Frontiers of nanobiomedical research – A WSPC reference on the state-of-the-art: fundamentals, main applications, and vision for the future. Singapore: World Scientific, 119–143.
- Lestner, J.M. et al., 2010. Pharmacokinetics and pharmacodynamics of amphotericin B deoxycholate, liposomal amphotericin B, and amphotericin B lipid complex in an in vitro model of invasive pulmonary aspergillosis. Antimicrobial agents chemotherapy, 54, 3432–3441.
- Olson, J.A., et al., 2006. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrobial agents chemotherapy, 50, 2122–2131.
- Olson, J.A., et al., 2008. Comparision of the physicochemical, antifungal, and toxic properties of two liposomal amphotericin B products. Antimicrobial agents chemotherapy, 52, 259–268.
- Olson, J.A., et al., 2015. Toxicity and efficacy differences between liposomal Amphotericin B formulations in uninfected and Aspergillus fumigatus infected mice. Medical mycology, 53 (2), 107–118.
- Prentice, H.G., et al., 1997. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. British journal of haematology, 98, 711–718.
- Ritter, J.M., Exserohilum Infections Working Group., et al., 2013. Exserohilum infections associated with contaminated steroid injections: a clinicopathologic review of 40 cases. American journal of pathology, 183 (3), 881–892.
- Stone, N.R.H., et al., 2016. Liposomal amphotericin B (AmBisome): a review of pharmacokinetics, pharmacodynamics, clinical experience, and future directions. Drugs, 76 (4), 485–500.
- Sundar, S., et al., 2010. Single-dose liposomal amphotericin b for visceral leishmaniasis in India. The new England journal of medicine, 362, 504–512.
- Walsh, T.J., et al., 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. new England journal of medicine, 340, 764–771.
- Wingard, J.R., et al., 2000. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clinical infectious diseases: an official publication of the infectious diseases society of America, 31, 1155–1163.