Abstract
Effective healing and regeneration of various bone defects is still a major challenge and concern in modern medicine. Calcium phosphates have emerged as extensively studied bone substitute materials due to their structural and chemical resemblance to the mineral phase of bone, along with their versatile properties. Calcium phosphates present promising biological characteristics that make them suitable for bone substitution, but a critical limitation lies in their low osteoinductivity. To supplement these materials with properties that promote bone regeneration, prevent infections, and cure bone diseases locally, calcium phosphates can be biologically and therapeutically modified. A promising approach involves combining calcium phosphates with drug-containing liposomes, renowned for their high biocompatibility and ability to provide controlled and sustained drug delivery. Surprisingly, there is a lack of research focused on liposome-calcium phosphate composites, where liposomes are dispersed within a calcium phosphate matrix. This raises the question of why such studies are limited. In order to provide a comprehensive overview of existing liposome and calcium phosphate composites as bioactive substance delivery systems, the authors review the literature exploring the interactions between calcium phosphates and liposomes. Additionally, it seeks to identify potential interactions between calcium ions and liposomes, which may impact the feasibility of developing liposome-containing calcium phosphate composite materials. Liposome capacity to protect bioactive compounds and facilitate localized treatment can be particularly valuable in scenarios involving bone regeneration, infection prevention, and the management of bone diseases. This review explores the implications of liposomes and calcium phosphate material containing liposomes on drug delivery, bioavailability, and stability, offering insights into their advantages.
1. Introduction
There are two fundamentally different ways a person can get a bone defect. One is due to various injuries, and the other is due to diseases, for example, osteoporosis, periodontitis, periimplantitis, and osteomyelitis. However, it should be noted that bone defects can also occur during the treatment of oncological diseases or orthopedic surgical treatment. Healing of bone defects is a complex physiological process during which the formation and growth of new bone tissue takes place. The size of a bone defect has a direct effect on the duration of bone regeneration, so in the case of large bone defects, it is necessary to use materials that promote the process of bone healing and recovery [Citation1].
Successful treatment of the critical size bone defects and the regeneration of damaged bone tissue is best promoted with the help of materials, which are structurally and chemically similar to natural bones. Research on calcium phosphate biomaterials and the identification of medical applications of these materials increased rapidly when calcium phosphates were discovered to be the major component of natural bones. Calcium phosphate mineral makes up about 70% of the bone mass, ensuring its stability, hardness, and mechanical strength.
However, a critical shortcoming of calcium phosphate materials is potentially low osteoinductive properties. Therefore, currently, a very important area of research is related to the therapeutical or biological modification of calcium phosphates, obtaining materials with specific properties [Citation2–4].
Calcium phosphates have a wide range of medical applications, as they are used not only as bone graft materials in orthopedics, dentistry, and maxillofacial surgeries but also as a part of composite materials in combination with other biomaterials. Examples of such composites are functionalized metal implant surfaces with calcium phosphate material coatings to improve the metal bioactivity [Citation5] and incorporated calcium phosphate nanoparticles in layered polymer (collagen) matrices to mimic the structure of a natural bone [Citation6,Citation7].
Promotion of bone tissue regeneration can be fostered by the supply of various biologically active substances, for example, bone morphogenetic proteins, growth factors (platelet-derived growth factor, insulin-like growth factor, fibroblast growth factor, transforming growth factor-beta and vascular endothelial growth factor) and hormones. These biologically active substances promote osteoinductive properties, angiogenesis, cell recruitment and differentiation, as well as ensure an analgesic effect [Citation8,Citation9]. Biologically active compounds such as anabolic agents, growth factors, genes, hormones, recombinant therapeutic proteins, somatic cells, blood, blood components, and osteoconductive matrices, as well as pharmacological agents (such as non-steroidal anti-inflammatory agents, anti-rheumatic drugs, strontium ranelate, bisphosphonates, abaloparatide, estradiol, and teriparatide) are under clinical trials or clinically available to improve bone regeneration and heal various bone diseases [Citation10–12].
Serious complications after implantation are caused by surgical site infections [Citation13]. Statistics show that in 2010, 2.5 million people in the United States needed a total hip replacement surgery and 4.7 million needed a total knee replacement surgery [Citation14]. Researchers predict that the need of total hip and knee arthroplasty will tend to increase [Citation15]. Total knee and hip replacement surgeries are associated with a 0.5–2% risk of infection, but in the case of revision arthroplasty, the risk of infection is more than double. On condition of surgical fracture fixation of the lower extremities, the risk of infection reaches 5%, but in the case of open fractures the risk of infections exceeds 10%. Although the probability of infection is statistically low, it causes a high risk of morbidity and mortality for patients. Implant-related infections lead to revision operations that involve a prolonged treatment process and high overall costs [Citation13], therefore, after surgery it is necessary to ensure the administration of antibiotics at the operation site to prevent the risk of postoperative infections [Citation16,Citation17], as well as, other drugs which treat bone diseases (e.g. osteoporosis and cancerous tumors) and prevent post-implantation pathologies [Citation4].
Calcium phosphates can be improved with either polymers which would improve calcium phosphate similarity to bone by mimicking the organic phase or enhance the phosphates with active substances [Citation18,Citation19]. To have a controlled and local active substance delivery the compounds need to be inside a drug delivery system, which ensures specific release dosage locally. A few examples of these delivery systems are – microparticles, microcapsules, micelles and liposomes [Citation20]. By combining both components, a new composite which can provide both – healing and regeneration can be achieved.
Advantages of drug delivery systems over systemic drug administration include local and controlled release of drugs and biologically active molecules at the required therapeutic concentration and for a predetermined duration. Since local drug delivery systems provide sustained drug release in the required area of the body [Citation4,Citation8,Citation21,Citation22] they reduce the risk of secondary effects, drug-induced side effects or general toxicity due to lower drug concentrations in plasma [Citation8,Citation22]. The most common side effects are pain, nausea, fatigue, gastric or intestinal ulcers, mucosal damage, thrombotic events, myocardial infarctions, strokes, renal dysfunctions, liver injuries, hypersensitivity reactions, allergic reactions, infections, skin reactions and necrosis [Citation23,Citation24]. Moreover, drug delivery systems improve drug efficacy and stability [Citation8,Citation22].
Various drug delivery systems have been extensively studied in the literature to adjust the rate, concentration, and duration of drug release [Citation25,Citation26]. Studies on drug carriers emphasize their need to be biocompatible and biodegradable within the body. To achieve this, efforts are being made to study the use of natural materials or synthetic analogs of natural materials in the development of drug carriers. Liposomes are drug delivery systems that exhibit structural similarity to cell membranes. Liposomes are formed by spherically organized amphiphilic phospholipid molecules arranged in two layers occurring during the phospholipid hydration process in an aqueous medium. The benefits of liposomes for drug delivery are related to the improved transport and efficacy of drugs or biologically active substances. Liposome encapsulated substances are protected from metabolism, are more stable in the body environment, are less toxic to surrounding tissues and the entire body due to controlled release, and have a longer exposure time. Liposomes provide more efficient absorption of the drugs into tissues, as well as the ability to control the distribution of the drug in the body by targeting the drug delivery to the desired injury site [Citation27,Citation28]. There are various studies in the scientific literature where drug-containing liposomes are studied for the treatment of bone diseases such as bone tumors, osteoporosis, and osteomyelitis. Liposome formulations such as doxorubicin-containing liposomes for cancer treatment have been clinically studied, approved, and commercially available [Citation29].
This paper presents a review on the most popular calcium phosphates used in bone regeneration, as well as liposomes, which are mentioned in the literature as one of the most promising drug delivery systems. Due to the lack of data in the scientific literature on composites in which liposomes are dispersed in a calcium phosphate matrix, the authors sought to explain this by summarizing the literature that points to possible problems in obtaining such composites.
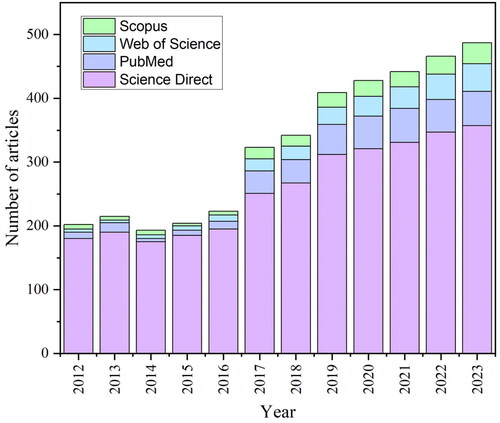
There has been a significant increase in the number of published articles about the calcium phosphates and liposomes (using key words: ‘Calcium Phosphates,’ ‘Hydroxyapatite,’ ‘Tricalcium Phosphates,’ ‘Amorphous Calcium Phosphate,’ ‘Drug Delivery,’ ‘Liposomes,’ ‘Calcium Ions,’ ‘Composites’), and the considerable diversity of results in various studies makes this topic attractive (). The process of article selection started with an initial screening based on the titles of relevant publications. Subsequent evaluation relies on the examination of abstracts, where the presence of essential keywords was assessed. The final inclusion of relevant articles relied on an initial assessment that clarified the content and extent of each publication. The comprehensive overview presented herein was developed through the incorporation of 125 distinct sources.
Figure 1. Calcium phosphates and liposomes related articles during the last decade in four databases: ScienceDirect, PubMed, Scopus, and Web of Science.
This review aims to provide an overview of currently obtained liposome and calcium phosphate composites as bioactive substance delivery systems, as well as to identify possible interactions between calcium ions and liposomes that could affect the possibilities of obtaining liposome-containing calcium phosphate composite materials. This review also summarizes the effects of liposomes and calcium phosphate material containing liposomes on drug delivery, bioavailability, and stability, while analyzing the benefits of such systems. The target audience for this paper is materials scientists, biomaterials researchers and doctors, who are engaged in the research and modification of calcium phosphates, as well as the study of liposomes and the development of liposomes and calcium phosphate composite materials. The analysis of the literature revealed a gap in knowledge opening the possibilities for future research.
2. Summary of calcium phosphates and comparison with natural bone
Different types of calcium phosphate biomaterials are used in bone regeneration, depending on the type, size, and shape of the bone defect, as well as the load-bearing capacity of the defected bone (cements, scaffolds, coatings, granules, and composites). These materials are made using different crystalline phases or amorphous phase of calcium phosphates, that have different properties depending on crystal structure, Ca/P ratio, solubility, and stability [Citation8,Citation30,Citation31]. All of the most popular calcium phosphates have been extensively reviewed elsewhere: hydroxyapatite (HAp) [Citation6,Citation30,Citation32,Citation33]; α-tricalcium phosphate (α-TCP) [Citation30,Citation32,Citation34–38]; β-tricalcium phosphate (β-TCP) [Citation30,Citation32,Citation38–41]; amorphous calcium phosphate (ACP) [Citation32,Citation34,Citation38,Citation42–46].
All the most popular types of calcium phosphates have different properties that also affect the application possibilities of these materials. Due to its brittleness and low mechanical properties, HAp is mainly used in coatings and composites. However, there are studies where HAp is used in non-load-bearing areas as a graft material – cement, granules, and pastes [Citation30]. α-TCP is used as injectable hydraulic bone cement, ceramics and composite for bone repair in non-load-bearing applications [Citation35]. β-TCP is widely used as a bone substitute material, in addition, its rate of resorption is comparable to that of natural bone. The application of β-TCP includes hydraulic bone cement, ceramics, granules, and scaffolds [Citation47]. ACP is most commonly used as a raw material for the synthesis of various other calcium phosphates, however, ACP is also used as coatings, self-setting injectable cements, ceramics, colloidal suspensions, and composites [Citation42].
The composition of the bone is directly related to the functions that the bone must provide. On the one hand, it must be durable, which is provided by the mineral part, but on the other hand, it must be flexible, which is provided by collagen fibrils. During human life, bones are subjected to two processes – modeling and remodeling. Modeling is the formation of new bone (during bone growth), but remodeling is the formation of bone after previous bone resorption (e.g. during the replacement of damaged bone with new bone). There are two types of bone structures with different characteristics: trabecular and cortical bone structure [Citation9,Citation48]. The trabecular structure is formed by a network of interconnected pores, but the cortical structure is relatively dense (it contains only ∼3–5% of free space). Due to these structural differences, the mechanical properties of these bones are not the same [Citation49].
3. Liposomes
3.1. Structure and properties of liposomes
Liposomes are closed spherical vesicles that form from phospholipids which are hydrated with an aqueous medium. Both natural (derived from natural sources such as egg yolk or soya beans) and synthetic phospholipids can be used to obtain liposomes.
In terms of bilayer structure, liposomes are classified into large or small unilamellar or multilamellar liposomes. Unilamellar liposomes are formed by a single spherical phospholipid bilayer, while multilamellar liposomes are formed by several concentrically arranged phospholipid bilayers. Liposome sizes usually range from 0.025 to 2.5 μm and the size directly affects the circulation time of liposomes in the body, as well as the amount of substance that can be encapsulated into liposomes [Citation50–52].
Phospholipids are classified according to their molecular structure: phosphatidylethanolamine (PE), phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidic acid (PA), and phosphatidylglycerol (PG). Liposomes are most commonly synthesized from PE and PC, as these lipids are represented in animals and plants. However, the synthetic form of lipids is mainly used because synthetic lipids have higher stability than natural lipids [Citation53,Citation54]. Natural phospholipids contain unsaturated fatty acids. Therefore, the gel phase transition temperature for these phospholipids is below 0 °C. This means that natural phospholipids in the body environment are in the liquid crystalline state resulting in flexible liposome structure and reduced physical stability [Citation55]. Examples of synthetic lipids are dimyristoyl phosphatidylcholine (DMPC), dipalmitoyl phosphatidylcholine (DPPC), distearoyl phosphatidylcholine (DSPC), 1-palmitoyl-2-oleoyl-snglycero-3-phosphocholine (POPC), 1,2-dioleoyl-snglycero-3-phosphocholine (DOPC), 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG), hydrogenated soy phosphatidylcholine (HSPC), 1,2-distearoyl-sn-glycero-3-phosphate (DSPA), and 1,2-distearoyl-sn-glycero-3-phospho-(10-rac-glycerol) (DSPG). During liposome formation, phospholipid molecules are arranged in two layers so that their hydrophilic heads are oriented toward the aqueous phase while the hydrophobic tails are oriented toward the inside of the bilayer. Cholesterol is also commonly introduced into the liposome structure to improve the stability of the phospholipid bilayer and reduce aqueous liposome content and surrounding body fluid permeability [Citation53,Citation54].
Liposome stability after intravenous injection is characterized by the circulation half-life of liposomes in the bloodstream. Since liposomes must be able to retain the encapsulated drug and reach the target cells after administration, it is important to study the leakage of the encapsulated drugs and the lifetime of the liposomes in the bloodstream to determine stability. Liposomes that are able to circulate in the bloodstream for longer time and retain the encapsulated content have higher stability and are more likely to reach the target cells. When liposomes are administered by other routes, for instance, orally, it is important to anticipate the stability of the liposomes in the gastrointestinal tract, thereby selecting an appropriate lipid composition capable of withstanding such an aggressive environments [Citation56].
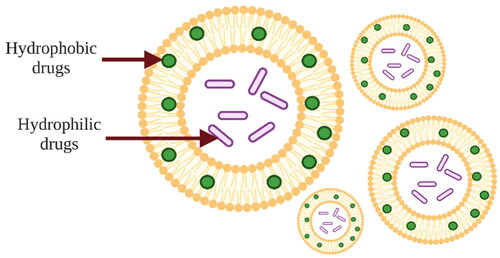
As liposomes are composed of a phospholipid bilayer, they have a unique property that distinguishes them from other drug delivery systems – liposomes can encapsulate both hydrophilic and hydrophobic substances. Water-soluble drugs are encapsulated in the aqueous phase of the liposome surrounded by a phospholipid bilayer, while fat-soluble substances are encapsulated inside the phospholipid bilayer (). Encapsulation of water-soluble drugs occurs during the hydration process, when spherical liposomes form from a thin film of lipids. In this process, the drug is dissolved in the aqueous medium and the liposomes encapsulate only as much drug as is entrapped into the aqueous core of the liposome. The phospholipid bilayer is permeable, so water-soluble drugs can diffuse out of the liposome, as a result high drug encapsulation efficiency cannot be achieved. Amphiphilic substances and substances containing ionizable groups can be encapsulated in liposomes with high efficiency. Fat-soluble drugs are dissolved in an organic solvent along with lipids and encapsulated in a phospholipid bilayer during the formulation of liposomes. As these drugs are poorly soluble in water, they remain entrapped in the liposome membrane during hydration but when they are in a suitable environment in vitro and in vivo, they are released in target tissues [Citation27,Citation50,Citation51,Citation57–60]. The release of hydrophilic drugs is facilitated by environmental conditions such as pH and the presence of plasma proteins [Citation61].
Figure 2. Schematic image of liposomes showing liposome structure and encapsulation of hydrophilic and hydrophobic active substances. The image is created with BioRender.com.
Liposome membranes have a selective permeability of substances, as a result the internal environment of the liposome may be different from the external environment [Citation27,Citation50,Citation51]. The rate of molecule passage through the liposome bilayer depends on the molecule’s type. For example, charged and large molecules pass through the membrane more slowly than small and non-polar molecules. Water and ions are also able to pass through the liposome membrane. Osmotic forces provide the movement of water molecules through the liposome membrane to a lower concentration region. In the case of solutions, the driving force of movement through the membrane is diffusion, which promotes the equalization of concentrations inside and outside the liposome [Citation50,Citation51,Citation58]. The degree of saturation and length of lipid chains also affect membrane permeability. Unsaturated fatty acids make the membrane more permeable because the double bond creates gaps between tightly packed phospholipid molecules. The longer the fatty acid chains, the lower the membrane permeability [Citation25,Citation27,Citation50,Citation51].
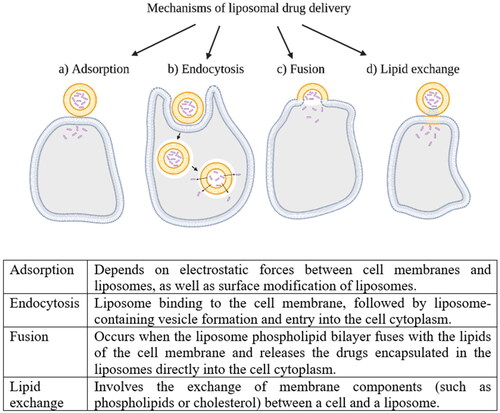
The liposomal drug delivery mechanism is possible through four major types of liposome interactions with body cells. These mechanisms are visualized in [Citation25,Citation27,Citation50,Citation60].
Liposome characteristics are highly tunable. Liposome properties such as flexibility, non-toxicity, non-immunogenicity, long circulation, and complete biodegradability can be obtained depending on the method of liposome preparation, the composition of the phospholipid bilayer, the size and the surface charge of the liposomes [Citation25,Citation27,Citation50,Citation51,Citation57].
There are several benefits to local liposome administration. Liposomes improve drug bioavailability and are able to provide the highest therapeutic effect with the lowest drug concentration. Liposomes ensure controlled drug delivery without damaging surrounding tissues because encapsulation in liposomes reduces the drug exposure to healthy tissues as well as reduces the toxicity of the drug and improves the stability of the pharmaceutical agent in the body environment [Citation50,Citation59,Citation62,Citation63]. As an example, can be mentioned a study where R. Krishna and colleagues compared the pharmacokinetics of liposome-encapsulated vincristine and conventional aqueous formulated vincristine in a mouse model of Lewis lung carcinoma. After injection of vincristine solution, they observed that the drug was distributed in a large volume of 145 mL, showing a low (0.59 μg × h/mL) plasma area under the plasma concentration-time curve (AUC) and a short half-life of 1.36 h. After encapsulation of vincristine in liposomes, the volume of distribution in the bloodstream decreased to 2 mL, the plasma AUC increased to 213 μg x h/mL, and the half-life reached 6.6 h. They observed that after liposome injection, the systemic exposure to free drug (plasma concentration of free drug) was 0.025–0.05 μg/mL over 4 h, but when vincristine solution was administered without the carrier, this concentration was higher – 0.1–0.35 μg/mL [Citation64].
3.2. Liposomal drug delivery – opportunities and challenges
In the human body, liposomes are administered by various routes: intravenously (the most common route), intramuscularly, intraperitoneally, and orally. Small liposomes (0.1–1.0 μm) are usually quickly perceived by the body as foreign cells. Then they are captured by the cells of the reticuloendothelial system and transported to the liver and spleen or other reticuloendothelial system organs, for example, kidney, bone marrow, lungs, and lymph nodes. This property can be used to treat various liver and spleen diseases, such as for the delivery of chemotherapeutic agents to treat a liver tumor. Larger liposomes (>3.0 μm) accumulate in the lungs [Citation65,Citation66].
Despite the positive properties of liposomes, they have limitations due to their low solubility, short circulation half-life, stability, susceptibility to lipid hydrolysis and oxidation, fusion and leakage, sterilization issues and high production costs [Citation27,Citation51]. The stability of liposomes during their production, administration and storage is related to the chemical and physical stability of the liposomes that includes the degradation of the phospholipids, leakage of the encapsulated substances and change in the liposome size distribution [Citation27,Citation51,Citation67]. Liposomes are also vulnerable to factors such as van der Walls forces [Citation27], hydrolysis of phospholipids [Citation68] and oxidative degradation [Citation27,Citation69].
To overcome limitations that the liposomes have while maintaining their stability, they can be stored in a lyophilized state using cryoprotectants such as trehalose, sucrose, or lactose aimed at preventing liposome fusion during dehydration, that may lead to changes in liposome size distribution after re-hydration of liposome powder in an aqueous medium [Citation27,Citation51,Citation67]. As the stability of liposomes is affected by several factors, their sterilization is challenging. The filtration is intended for sterilization of liposomes at ambient temperature, but the limitations of this method are related to the size of the liposomes (they should not exceed 200 nm) and selection of a suitable filter material (that does not interact with liposomes or liposome containing active agents). Other sterilization techniques can also be used for liposomes, such as UV-sterilization, γ-irradiation, dry heat, ethylene oxide and final steam sterilization, however, it should be taken into account that most of these methods are performed at elevated temperatures, which may result in phospholipid degradation, drug leakage or may promote the formation of undesirable residues in the liposome formulation [Citation27,Citation67].
Liposomes can be chemically modified, thus achieving their targeting to the specific site. The chemical modification also influences liposome stability and circulation time as well as changes the conditions of liposomal release. One of the liposome chemical modification methods is PEGylation. In this method, the surface of the liposome is coated with hydrophilic polyethylene glycol (PEG) coating, which provides steric stabilization of the liposomes and affects their drug delivery properties as well as increases the circulation time of liposomes in the blood. Alternatives to PEG polymer are polyvinylpyrrolidone (PVP) and polyacrylamide (PAA). Another option is the modification of the liposome surface with ligands (peptides, oligopeptides, antibodies, glycoproteins, polysaccharides, growth factors, folic acid, receptors, and carbohydrates) to ensure targeted delivery of liposomes. The main purpose of the modification is to provide targeting of liposomes to various tumor cells with the help of ligands. However, modification is also used to target liposomes to bone tissues as well as soft tissues such as the brain cells, liver cells, and lung cells. Types of liposome surface chemical modification that combine the two methods mentioned above are also being studied. However, surface modification of liposomes may affect liposome properties such as toxicity, biocompatibility and biodistribution, which needs to be studied and taken into account [Citation27,Citation66,Citation70–74].
The targeting of liposomes to specific cells or tissues is also achieved by physical methods. The distribution of liposomes in the body is affected by the liposome surface charge. If the surface charge of the liposome is negative, drugs are released into the cytoplasm of the cell as the liposome merges with the cell. If the charge on the liposome surface is positive or neutral, drugs are delivered to the cell by phagocytosis. Positively charged and neutral liposomes can stay in the body for a longer period of time, while negatively charged liposomes are degraded in a shorter timespan [Citation65]. Examples of the compositions of negatively and positively charged liposomes are PC and cholesterol liposomes in combination with dicetylphosphate and stearylamine respectively [Citation75], in contrast, neutral liposomes are obtained using, for example, DPPC phospholipid [Citation76].
3.3. Liposome-encapsulated biological agents and drugs for bone tissue regeneration
To ensure successful regeneration and healing of bone tissues, it is necessary to include four important elements in the therapy. The first element is the differentiation of stem cells into osteoblasts, that can be achieved by introducing osteoinductive growth factors into the bone defect [Citation77]. To achieve the previously mentioned cell differentiation, stem cells responsive to osteoinductive signals are required as the second element. Tissue vascularization is a very important element in bone regeneration, so an intact vascular supply must be ensured in the area of the bone defect. The fourth element is the osteoconductive matrix or scaffold, the main task of which is to perform the supporting function, as well as to ensure cell attachment, proliferation, and bone tissue ingrowth [Citation12].
The benefits of liposomes as a drug delivery system for bone tissues can be viewed from two sides. On the one hand, liposomes provide a local and controlled delivery of drugs and biologically active substances directly for the treatment of bone diseases and for more efficient bone regeneration [Citation78,Citation79]. On the other hand, liposomes can also indirectly improve the bone healing process by preventing the effect of drugs used for other diseases treatment on bone tissues. Several pharmacological agents administered intravenously in free form to treat various diseases, often have a negative effect on bone healing. For instance, chemotherapeutic agents, that inhibit bone formation, mineralization, and angiogenesis; corticosteroids that adversely affect osteoblast formation and cause osteocyte and osteoblast apoptosis; some antibiotics, for example, fluoroquinolones and ciprofloxacin, as well as anticoagulants [Citation11]. Therefore, encapsulation and targeted delivery of drugs only to the required tissues, using liposomes as drug carriers, reduces the adverse effects of these drugs on bone tissue regeneration because the drugs do not spread throughout the body thereby reducing their effects on other tissues [Citation78,Citation79].
Compared to other organ systems, bones are poorly vascularized because they receive only about 10% of the blood supplied by the heart, whereas cartilage contains no blood vessels at all and is not supplied with blood. In addition, as a person ages or suffers from pathologies such as cancer, diabetes, respiratory diseases, anemia and immobility, the bone vasculature tends to decline. In the case of bone damage, the bone vascular network is also lost, as a result the supply of nutrients, oxygen, drugs, or other biologically active substances to the bone tissues is interrupted. After systematic administration of therapeutic agents, they reach the bone tissue only through the circulatory system. If there is a bone defect, any medication therapy may be ineffective because the bone does not receive enough blood [Citation80]. This is a problem for which targeted or local drug delivery could be an alternative.
3.3.1. Treatment of bone fractures
In the case of bone fractures, biological adjuvants are used which include cell-based therapies, growth factor therapies, and anabolic therapies. Cell-based therapies are based on increasing the number of cells that promote bone regeneration in the defect area. In this treatment, autologous stem cells and/or progenitors are most often transplanted to the site of action, thus ensuring the physiologic remodeling of bone tissues, and promoting bone healing. At the same time adverse reactions in the body are avoided due to that stem cells are derived from the body’s tissues, such as the bone marrow, adipose tissue, muscle, dermis, periosteum, vascular pericytes, and peripheral blood. Growth factor therapies include the use of bone morphogenetic proteins, fibroblast growth factors, and platelet-derived growth factors. Anabolic therapy is associated with parathyroid hormone and recombinant parathyroid hormone delivery to bone defect [Citation12,Citation81]. The advantages of these growth factors and hormones are listed in .
Table 1. The advantages of growth factor and hormone therapies on bone fracture treatment.
In order to heal bone defects more effectively and increase the performance of biologically active substances, they can be encapsulated in liposomes and directly targeted to the site of action. The advantages of liposomes over other vectors used for gene delivery include a wide range of size variations that do not limit the size of biological adjuvants and simple preparation technology, as well as liposomes are more biocompatible and cause fewer immune responses [Citation12,Citation81].
3.3.2. Bone diseases
In the case of normal bone metabolism, two mutually balanced processes take place: osteoblast-regulated bone formation and osteoclast-regulated bone resorption. However, if abnormalities in bone metabolism occur, there is a risk of various bone diseases and skeletal disorders, such as osteoporosis, osteomyelitis, bone metastasis, osteosarcoma, Paget’s disease, rickets, and osteomalacia [Citation10].
3.3.2.1. Osteoporosis
One of the most common bone diseases that affects elderly people is osteoporosis. Osteoporosis results in a decrease in bone mass, as well as changes in bone structure (they become more porous), as a result, the bones become fragile and easy to break. Anabolic agents, estrogen or parathyroid hormone, and bisphosphonates are used as therapeutic agents for the treatment of osteoporosis. These pharmacological agents not only promote bone regeneration but also have a positive effect when bone implants are required, as they increase osseointegration and implant stability. Although these drugs are used in the treatment of osteoporosis, they also have significant drawbacks, such as renal toxicity as a result of intravenous bisphosphonate injection, and esophageal irritation as a result of oral bisphosphonate administration. Estrogen therapy can have a negative effect on blood vessels or even promote the development of breast carcinoma. For this reason, alternative medicines and drug delivery systems are explored for the treatment of osteoporosis [Citation10,Citation11,Citation88]. X. Sun and colleagues studied the effect of biomineral-binding liposome encapsulated drugs for the treatment of osteoporosis. The pharmacological agent icariin was used in the study. Icariin has potential use in the treatment of osteoporosis, but when administered in free form without a specific carrier, it has low bioavailability due to its poor solubility in water and its inability to accumulate in the affected bone tissue. In this research, the authors demonstrated that these limitations can be overcome by encapsulating icariin in bone-targeted liposomes thus providing not only treatment but also prophylaxis to prevent fractures and changes in bone structure in the future [Citation88].
3.3.2.2. Rickets and osteomalacia
Rickets and osteomalacia are bone diseases associated with delayed or lack of bone mineralization. These diseases are mainly caused by a lack of vitamin D, resulting in reduced absorption of calcium in the body. To treat these diseases vitamin D supplements are used [Citation10]. Encapsulation of vitamin D in liposomes, ensuring transdermal delivery of vitamin D, is not a new field of research. Due to the low solubility of vitamin D in water, liposomes provide improved vitamin D absorption in tissues. This vitamin can be encapsulated in DPPC and dilauroylphosphatidylcholine (DLPC) liposomes; DSPC poly(ethylene glycol)-distearoylphosphoethanolamine (PEG2000-DSPE) and sodium cholate liposomes; PS, PA and PE liposomes achieving up to ≥80% encapsulation efficiency [Citation89].
3.3.2.3. Bone tumors
Bone tumors are a serious condition that causes bone damage. Surgical resections are used to remove bone tumors, resulting in bone defects which are usually filled with implants. To promote the healing process, the administration of biological agents and drugs is required. After tumor removal the radiotherapy and chemotherapy are often needed. During chemotherapy, chemotherapeutic agents and other medical agents are administered to the affected area, for example, doxorubicin, methotrexate, cisplatin, etoposide, ifosfamide, cyclophosphamide, and vincristine [Citation90,Citation91]. Liposome formulations are available for all of these medical products [Citation62,Citation92]. Bone injuries, as well as bone surgical treatment, are associated with the risk of infections, so antibiotics are also delivered in addition to various drugs, including chemotherapeutic agents [Citation93,Citation94]. Research of liposomes for the treatment of bone tumors has been relevant for several decades, as liposomes can improve the delivery of necessary drugs to bone tissue. In recent years, research has been conducted on the modification of liposomes (conjugation with alendronate, low molecular weight heparin, or bisphosphonate), thus ensuring the targeted delivery of liposomes to bone tissue, as well as the co-encapsulation of drugs in liposomes, thus developing new treatment strategies and combining the simultaneous treatment of diseases [Citation90,Citation95–98]. An example of co-encapsulation can be mentioned in the study by M. Huang and colleagues investigating liposomes simultaneously containing curcumin and resveratrol. They showed that such liposomes can be obtained with high encapsulation efficiency as well as improved stability of the active substances [Citation99].
3.4. Liposome interaction with calcium ions
Understanding the impact of calcium ions on liposome phospholipid bilayers holds significant importance, as calcium is not only present in various types of synthetic biomaterials that can be combined with liposomes for drug delivery but is also naturally present within the body. Calcium ions exhibit strong binding properties due to their divalent nature. Their presence and variations in the calcium concentration play crutial roles in facilitating the transport of substances through cell membranes and regulating diverse cellular processes, including, proliferation, fertilization, differentiation, neuronal signaling, and muscle contraction. Calcium also maintains the fluid balance in the cells, the process of blood clotting, and the regulation of heartbeat, and is one of the main components of bone and teeth. Consequently, calcium ions are found throughout the body in both the intracellular environment and the extracellular space [Citation100–102] indicating that liposomes in the body environment will be exposed to calcium ions.
There is controversial information in the scientific literature regarding the interaction of liposomes and calcium ions. Each interaction alters the formulation stability as well as the biological stability of the liposomes. Direct interaction between the calcium ions and phospholipid bilayers has been reported indicating toward the biological stability of the liposomes after the administration. There are several possible mechanisms of interaction:
Szczes and a colleague studied the effect of DPPC liposomes on calcium carbonate precipitation and crystal growth, showing that calcium ions interact directly with the phospholipid bilayer. Calcium ions bind to the polar head groups of phospholipids, forming supersaturated areas with calcium ions on the phospholipid bilayer. In these supersaturated areas, calcium carbonate nucleation and crystal growth occur, leading to crystal aggregation [Citation103] ().
B. U.R. Pedersen and colleagues studied the properties of charged phospholipid bilayers under the influence of calcium ions. They observed that due to electrostatic forces, there is an interaction between negatively charged phospholipids and positively charged calcium ions, resulting in calcium ions being adsorbed on the surface of negatively charged membranes. This ion absorption significantly improves the stability of the phospholipid bilayer, as well as leads to lipid ordering and tighter packing of the phospholipid molecules [Citation104] ().
Figure 4. Interaction of calcium ions with polar phospholipid head groups leads to calcium carbonate precipitation and crystal growth. The image is created with BioRender.com.
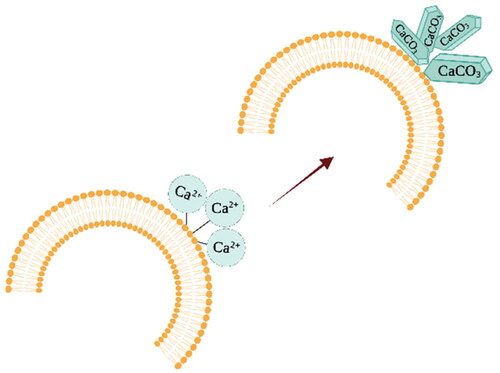
Figure 5. Electrostatic interactions between negatively charged phospholipid head groups and positively charged calcium ions result in improved liposome stability. The image is created with BioRender.com.
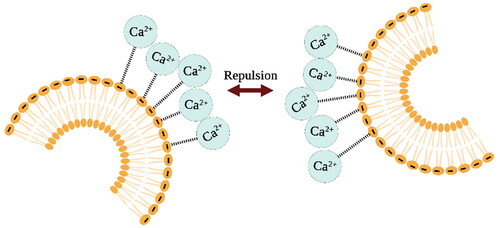
Similar results were obtained by A. Melcrova and colleagues who studied the binding of lipid membranes and calcium ions using a liposome phospholipid bilayer as a membrane model. They showed that calcium can cause negatively charged liposome aggregation. But when calcium ions were added to already aggregated liposomes, the opposite process was observed – the liposomes were deagglomerated. This suggests that calcium-induced liposome agglomeration is a reversible process and is not associated with liposome fusion.
C. Study by A. Melcrova et al also focused on the molecular level binding of the calcium ions to lipid bilayers, revealing adsorption of the calcium ions that restricts the mobility of both neutral and negatively charged lipid molecules through various binding sites and mechanisms. Calcium has the ability to bind to multiple lipid molecules concurrently and affects the mobility of lipid head groups and carbonyl groups. This binding leads to formation of calcium-lipid clusters, thus lipid molecules are rearranged resulting in membrane lateral compression and an increased bilayer thickness [Citation101]. O. Shih and colleagues also investigated the calcium binding to phospholipid membranes using phospholipid nanodiscs as a membrane model. They showed that calcium ions specifically bind to phospholipid head groups, leading to membrane charging and swelling. The lipid membrane swells, due to the adsorption of calcium ions on the lipid head groups, the phospholipid chains stretch, facing stronger van der Waals interactions [Citation105]. Similar processes of phospholipid bilayer and calcium ions interactions described above have also been documented by other researchers [Citation106–109].
The interaction of calcium ions and lipids depends on the type of lipids used for liposome preparation. PC phospholipids have a higher affinity to the calcium ions than PE phospholipids, so when PE lipids are used, the affinity of calcium to the membrane surface is lower. The adsorption of calcium ions on the phospholipid bilayer is reduced by the addition of cholesterol in the liposome composition. Cholesterol affects the orientation of phospholipid molecules, for example, DPPC phospholipid head groups are oriented inwards, thus reducing the interaction between liposomes and calcium ions. The addition of cholesterol also reduces the number of phospholipid head groups on the liposome surface, as some of these molecules are replaced by cholesterol molecules, thus reducing the surface area of contact between calcium ions and phosphate groups [Citation108,Citation110].
Although the available information does not provide a clear explanation for the adverse effects of calcium ions on the liposome bilayer, there is evidence of an interaction between them. By accurately selecting the composition of liposomes, as well as adjusting the parameters of liposome preparation, it is possible to predict the effect of calcium ions on the liposome formulation.
Furthermore, various composite materials have been obtained, containing both liposomes and calcium ions at the same time (described in more detail in Section 4. Liposome and calcium phosphate composites section). Calcium in these materials is used either in the process of manufacturing the scaffold, for example, production of calcium cross-linked alginate gels, where drug loaded liposomes are embedded [Citation72,Citation111], or calcium is present in a scaffold material itself such as hydroxyapatite or bioactive glass in which liposomes are then incorporated [Citation112]. These studies report successful production of liposome containing composites, which provide controlled and prolonged drug release. Although most studies show that the release of liposomal content occurs with burst release which could indicate the undesirable effect of calcium ions on scaffold-embedded liposomes [Citation72,Citation113].
4. Liposomes and calcium phosphate composites
Liposome-calcium systems refer to the complex interactions between liposomes and calcium ions in three categories, encapsulation of the calcium ion containing materials within the liposomes, calcium phosphate coatings on liposome bilayers and incorporation of the liposomes within the calcium ion containing scaffolds or matrices.
4.1. Encapsulation of calcium containing materials in liposomes
One type of composition described in the scientific literature where liposomes are exposed to calcium ions is the encapsulation of calcium or calcium-containing substances in liposomes. In this case, the liposomes are targeted to deliver calcium ions as crosslinking agents () in a gel system for the production of hydrogels or the liposomes are used as templates for the production of polymeric nanoparticles [Citation51].
Figure 6. Schematic image of liposome showing a calcium ions-containing liposome composition and the release of calcium ions through the phospholipid bilayer. The image is created with BioRender.com.
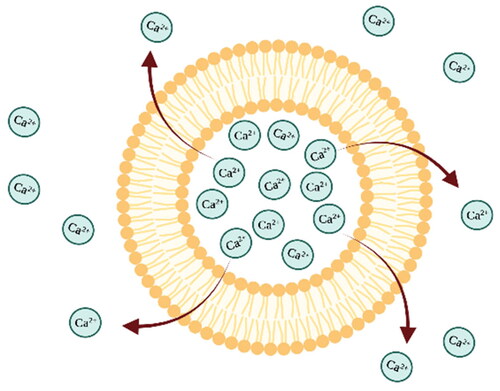
E. Westhaus and colleague studied the crosslinking of calcium alginate polysaccharide and protein-based hydrogels with thermally activated liposomes. They successfully formulated CaCl2-containing liposomes which when combined with and injectable gel precursor, remained fluid at room temperature for few days. However, upon heating to 37 °C system rapidly formed a gel due to the release of calcium ions from liposomes, initiating a crosslinking reaction. They also concluded that this injectable system could not be stored at room temperature for a extended periods of time because calcium ion leakage gradually increased viscosity over a few days [Citation114]. On the other hand J.S. Hong and colleagues developed a system involving liposometemplates to obtain calcium alginate nanogels. Initally, sodium alginate was encapsulated in the liposomes, which were then dispersed in a calcium chloride buffer. Theliposomes acted as a reaction vessels, permitting calcium ions to enter the liposomes through the lipid bilayer. By increasing the system’s temperature near the liposome bilayer’s melting point, the permeability to calcium ions was enhanced, leading to a crosslinking reaction inside the liposome. This process yielded nanogels at the same size as the liposome’s inner space [Citation111]. Although gel crosslinking using calcium ions was ensured in both studies, the use of liposomes was different in each research, resulting in the synthesis of hydrogels with completely different applications as nanogels or injectable hydrogels.
S. Yamasaki and colleagues used liposomes to synthesize calcium molybdate nanoparticles. In this study, 2 types of liposome suspensions were prepared: calcium ion-containing liposome suspension and molybdate ion-containing liposome suspension. To carry out the reaction where calcium molybdate nanoparticles precipitate inside the liposome membrane, the fusion of calcium ion-containing and molybdate ion-containing liposomes was achieved by mixing and heating both types of suspensions. In this study, liposomes played an important role not only in the delivery of the substances required for the reaction but also in the controlling size of the resulting nanoparticles, ensuring a homogeneous size distribution, which is considered to be a disadvantage of other calcium molybdate nanoparticles synthesis methods [Citation115].
J. Zhang et al. studied the encapsulation of small interfering ribonucleic acid (siRNA)-containing calcium phosphate nanoparticles in polycation liposomes. Calcium phosphate provides siRNA delivery to cells and stability against enzymatic degradation, but calcium phosphate nanoparticles tend to grow and aggregate after synthesis, reducing the potential for application of this drug delivery system. Liposomes were used in this study to limit the growth of calcium phosphate crystals [Citation116].
4.2. Incorporation of liposomes within calcium phosphate materials
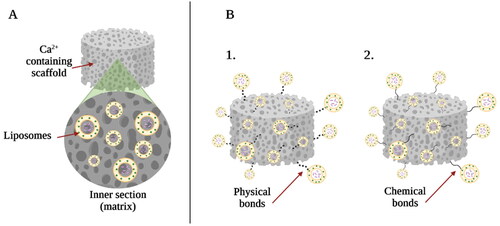
The aim of the incorporation of liposomes in the matrix of calcium-containing biomaterial scaffold is to modify the implant material by complementing their performance with local, controlled and sustained drug delivery [Citation51]. In most cases, the incorporation of liposomes in various polymer-based matrices, such as hydrogels which are used for tissue regeneration, is being studied [Citation117,Citation118]. The incorporation of liposomes into the matrix can vary: they can be dispersed in the volume of the matrix, as well as physically or chemically bonded to the surface of the implant material [Citation51] ().
Figure 7. Types of liposome and calcium phosphate scaffold composites: (A) liposomes embedded in the matrix of calcium phosphate scaffold; (B1) physically bounded liposomes to the surface of the calcium phosphate material and (B2) chemically bounded liposomes to the surface of the calcium phosphate material. The image is created with BioRender.com.
G. Wang and colleagues studied bisphosphonate decorated doxorubicin and model protein lysozyme-incorporated liposomes to create a bone mineral-binding delivery system of bone disease treatment agents. Two different liposome preparation methods were compared: lipid film hydration and reverse-phase evaporation method. The liposome preparation method affected the encapsulation efficiency of the active substances (doxorubicin and lysozyme). The liposome samples were tested in vitro on a collagen/HAp composite scaffold and the results showed that bisphosphonate decorated liposome affinity to HAp was much stronger than without the modification. Although the primary objective of this study was to develop a targeted drug delivery system that could be used for systemic administration and that would be capable of binding to bone tissues, the results proved that liposomes conjugated with bisphosphonates as bone-seeking ligands were able to bind to calcium phosphate-containing material surface. This leads to the conclusion that liposomes in combination with a bone substitute material can be used for local drug delivery [Citation119].
Composites of liposomes and calcium phosphates are mentioned in review articles and the benefits of such composites are defined, but they usually analyze liposomes coated with calcium phosphates rather than liposomes embedded in a calcium phosphate matrix [Citation120]. This means that either research in this direction is a relatively new field or it is difficult to obtain such hybrid composites. The lack of information on this topic could also be due to the effect of calcium ions on the phospholipid bilayer described above or due to poorly studied liposome incorporation technologies. However, more scientific research is needed in this direction to draw conclusions.
4.3. Calcium phosphate coatings on the surface of liposomes for efficient drug delivery
As mentioned in the previous section, the most commonly studied liposome-calcium phosphate composites are calcium phosphate coatings on the surface of the liposomes. To obtain these core-shell structure composites liposomes must be stable and negatively charged. During the process of coating liposomes are directly exposed to calcium ions because the calcium phosphate precipitation from supersaturated solution on the surface of liposomes is performed. The thickness of the precipitated layer depends on the reaction time (). HAp, partly crystalline HAp, and ACP are often used as the liposome surface coating material thus extending the use of such liposomes for the treatment and regeneration of bone tissue [Citation121–123].
Figure 8. Schematic representation of the formation of calcium phosphate coating on the surface of liposomes. During the reaction, liposomes are dispersed in a supersaturated calcium and phosphate ions containing solution. The thickness of the layer depends on the precipitation reaction time. The image is created with BioRender.com.
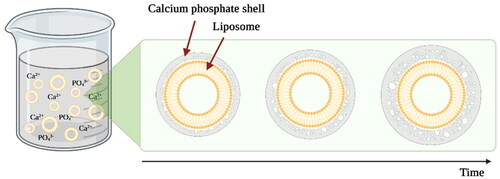
These calcium phosphate shells around liposomes not only specify the application possibilities but also affect the drug delivery system performance, for example, the drug release kinetics from the drug carriers. Q. Xu and colleagues in their study coated 1,2-dimyristoyl-sn-glycero-3-phosphate (DMPA) and DMPC liposomes with HAp coating. Indomethacin was encapsulated in liposomes as a model drug. They observed that the release of indomethacin from HAp-coated liposomes was 4 times slower compared to uncoated liposomes. 70% of the drug was released within 20 h. They also investigated that with HAp coating it is possible to change the release rate at different environmental pH values. As HAp has a higher solubility in acidic media, the drug release rate was faster at pH = 4 than at pH = 7.4. Such liposomes would be suitable for the local delivery of antibiotics and anti-inflammatory drugs for the treatment and prevention of bone tissue infections after various surgeries, usually associated with a high risk of postoperative infections. This is further emphasized by the fact that the pH of the inflammatory environment is acidic. Thus, in the case of inflammation, the release rate of antibiotics would be higher and more suitable for eradication of the infection [Citation122,Citation124].
But the main question that arises is why coat liposomes with HAp, if a simpler way would be to incorporate the drug into HAp implant materials itself? The main reason for this is that it is not possible to incorporate drugs into the HAp material with high efficiency. Also, drug release kinetics is not suitable for long-term drug delivery because the initial drug release mechanism is rapid. The burst release can be observed before a stable drug release profile is reached [Citation122,Citation124].
The use of calcium phosphate-coated liposomes is not limited to drug delivery. They are also used to deliver reporter molecules and imaging agents. In this case, the purpose of the calcium phosphate shell is to reduce the leakage of encapsulated substances. I. R. Berti and colleagues in their study encapsulated acridine orange (AO) and 5,10,15,20-Tetrakis(1-methyl-4-pyridinio)porphyrin (TMP) fluorophore dyes in 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA) liposomes to investigate the ability of this type of liposome composite to deliver active substances and to interact with bacterial biofilms. They observed that calcium phosphate-coated liposomes were able to adsorb and adhere on the surface of bacteria and concluded that the interaction between coating and bacteria is due to the calcium, which is also contained in Gram-positive bacterial cell walls. TMP release was observed only after the interaction of calcium phosphate coated liposomes and bacterial cells. Evidence of this interaction suggests that such liposome composites would be suitable for the delivery of active substances to bacterial cells as well as the calcium phosphate coating ensures the stability of the liposomes and the retention of the active substance in the liposomes until the target cells are reached [Citation125].
5. Benefits of liposome containing composite materials
Biomaterial scaffolds, which are used to fill tissue defects, provide various functions that improve and help with bone tissue regeneration. Scaffolds can provide mechanical properties as well as a suitable environment for cell proliferation in the body, but to improve the performance of biomaterial scaffolds, their modification can be accomplished [Citation112].
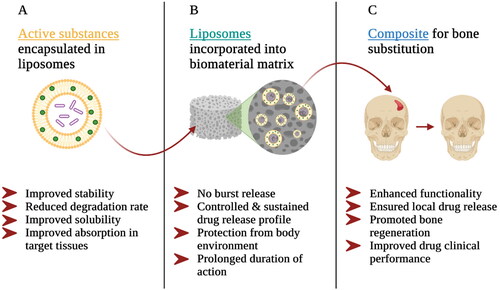
Calcium phosphate biomaterials as well as polymer scaffolds and gels are combined with liposomes to make local drug delivery more efficient. Firstly, the encapsulation of active substances in drug carriers offers several advantages to pharmaceutical agents (). It can improve the stability of drugs or biologically active substances and reduce the rate of degradation of the active substances, as well as improve the solubility and absorption of the drugs in target tissues [Citation72,Citation82,Citation92,Citation112].
Secondly, the incorporation of liposomes into the biomaterial matrix improves the performance of the drug delivery system (). Regarding the incorporation of liposomes as drug delivery systems into composites, the main goal is to prevent the burst release of liposome-encapsulated drugs. The initial rapid release of the drug observed after administration of the liposomes may lead to tissue toxicity or undesirable accumulation of the drug. The incorporation of liposomes into the matrix of another biomaterial delays the rapid release of the drug into tissues [Citation79]. Another aspect in favor of liposome incorporation in the matrix of another biomaterial is that in order to ensure sufficient accumulation of liposomes in the bone tissue where drugs need to be delivered, they must be able to circulate in the circulatory system for a reasonable time. When liposomes are incorporated into the matrix of bone substitute material, they are protected by the surrounding matrix. Liposomes do not need to circulate through the circulatory system to the bone defect zone, but they are already in the pathological site of disease where the drugs can be released in a controlled manner at the desired therapeutic concentration level. As a result, the duration of action of liposomes may be prolonged leading to prolonged drug release [Citation29,Citation82,Citation112].
Thirdly, the incorporation of drug-containing liposomes into the biomaterial matrix improves the functionality of the scaffold itself (). Such a composite material ensures the local delivery of drugs and biologically active substances in the required concentration for the treatment of various diseases and the promotion of tissue regeneration. It is possible to encapsulate several drugs simultaneously in drug carriers, as well as to achieve uniform distribution of drug carriers in the biomaterial matrix, thus improving clinical performance. As an example, may be mentioned composite materials that combine calcium phosphate implant materials and drug-containing liposomes. These composites can promote more efficient and faster bone regeneration, especially in cases of large and complex bone defects where it is essential, as well as to locally supply prophylactic agents for prolonged periods of time, such as antibiotics, to prevent the risk of infections in the operation site [Citation72,Citation82,Citation112].
6. Conclusions
Although various calcium phosphate bone substitute materials have proven their potential applications in filling bone defects, research aimed at developing and improving the properties and application possibilities of these materials continues. To promote bone tissue regeneration more efficiently and faster, as well as to treat and prevent various bone diseases locally, the combination of biologically active substances and drugs with bone substitutes is being studied. The main reason why composites of calcium phosphate materials and drug carriers should be obtained instead of incorporating free drugs into the material matrix is the efficiency of drug entrapment and release kinetics of the active substance, which, in the case of composites, can be adjusted and controlled more extensively. Liposomes, due to their similar chemical and structural properties to the body, as well as their wide range of modification possibilities, are potentially suitable candidates for this type of application. In addition, liposomes improve the efficacy of pharmacological agents in terms of both stability and absorption capacity. With liposomes, it is possible to achieve local, controlled, and sustained drug release directly into the desired bone site.
Regarding the interaction of liposomes and calcium ions, there is a controversial information in the scientific literature. The development of various composite materials where liposomes are directly exposed to calcium ions has been reported. However, rapid initial drug release from this type of composites has also been reported, which could indicate an undesirable interaction between calcium ions and liposomes. Several other studies have found that calcium ions tend to form bonds with the polar head groups of phospholipids. Adsorption of calcium ions to a negatively charged phospholipid bilayer is also reported, contributing to improved liposome stability, and is considered a positive aspect. Although calcium ions initiate structural transformations of phospholipid molecules resulting in tighter packing and decreased mobility of these phospholipids, no study emphasizes the destructive effects of calcium ions on liposomes. It should also be emphasized that the interaction of calcium ions on the phospholipid bilayer depends on the composition of the liposomes, especially the presence of cholesterol. There are various compositions of liposomes and calcium phosphates in which liposomes are directly exposed to calcium ions. In these composites, liposomes serve various purposes, such as: delivering ions to complete chemical reactions, as templates for nanoparticle production, modifying calcium phosphate materials, and providing modified drug release kinetics.
In summary, liposome-calcium phosphate composites have potential for the treatment and prevention of various bone diseases, but more research is needed particularly on liposomes incorporated in the matrix of calcium phosphate scaffolds to evaluate the efficacy of such composites.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dimitriou R, Jones E, McGonagle D, et al. Bone regeneration: current concepts and future directions. BMC Med. 2011;9(1):66. doi: 10.1186/1741-7015-9-66.
- Collon K, Gallo MC, Lieberman JR. Musculoskeletal tissue engineering: regional gene therapy for bone repair. Biomaterials. 2021;275:120901. doi: 10.1016/j.biomaterials.2021.120901.
- Miramond T, Corre P, Borget P, et al. Osteoinduction of biphasic calcium phosphate scaffolds in a nude mouse model. J Biomater Appl. 2014;29(4):595–604. doi: 10.1177/0885328214537859.
- Parent M, Baradari H, Champion E, et al. Design of calcium phosphate ceramics for drug delivery applications in bone diseases: a review of the parameters affecting the loading and release of the therapeutic substance. J Control Release. 2017;252:1–17. doi: 10.1016/j.jconrel.2017.02.012.
- Su Y, Cockerill I, Zheng Y, et al. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact Mater. 2019;4:196–206. doi: 10.1016/j.bioactmat.2019.05.001.
- Lu J, Yu H, Chen C. Biological properties of calcium phosphate biomaterials for bone repair: a review. RSC Adv. 2018;8(4):2015–2033. doi: 10.1039/c7ra11278e.
- Zhang D, Wu X, Chen J, et al. The development of collagen based composite scaffolds for bone regeneration. Bioact Mater. 2018;3(1):129–138. doi: 10.1016/j.bioactmat.2017.08.004.
- Bose S, Tarafder S, Edgington J, et al. Calcium phosphate ceramics in drug delivery. JOM. 2011;63(4):93–98. doi: 10.1007/s11837-011-0065-7.
- Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair : a review. Bioact Mater. 2017;2(4):224–247. doi: 10.1016/j.bioactmat.2017.05.007.
- Sharma G, Alle M, Chakraborty C, et al. Strategies for transdermal drug delivery against bone disorders: a preclinical and clinical update. J Control Release. 2021;336:375–395. doi: 10.1016/j.jconrel.2021.06.035.
- Tarantino U, et al. Pharmacological agents and bone healing. Clin Cases Min Bone Metab. 2009;6(2):144–148.
- Virk MS, Lieberman JR. Biologic adjuvants for fracture healing. Arthritis Res Ther. 2012;14(6):225. doi: 10.1186/ar4053.
- Spitzmüller R, Gümbel D, Güthoff C, et al. Duration of antibiotic treatment and risk of recurrence after surgical management of orthopaedic device infections: a multicenter case-control study. BMC Musculoskelet Disord. 2019;20(1):184. doi: 10.1186/s12891-019-2574-4.
- Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386–1397. doi: 10.2106/JBJS.N.01141.
- Singh JA, et al. Rates of total joint replacement in the United States: future projections to 2020-2040 using the national inpatient sample. J Rheumatol. 2019;46:1–7. doi: 10.3899/jrheum.170990.
- Heitzmann LG, et al. Postoperative chronic osteomyelitis in the long bones – current knowledge and management of the problem. Revista Brasileira De Ortopedia. 2019;54:627–635.
- Mifsud M, McNally M. Local delivery of antimicrobials in the treatment of bone infections. Orthop Trauma. 2019;33(3):160–165. doi: 10.1016/j.mporth.2019.03.007.
- Ginebra M-P, Canal C, Espanol M, et al. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev. 2012;64(12):1090–1110. doi: 10.1016/j.addr.2012.01.008.
- Huang D, He B, Mi P. Calcium phosphate nanocarriers for drug delivery to tumors: Imaging, therapy and theranostics. Biomater Sci. 2019;7(10):3942–3960. doi: 10.1039/c9bm00831d.
- Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021;5(9):951–967. doi: 10.1038/s41551-021-00698-w.
- Serwer L, Hashizume R, Ozawa T, et al. Systemic and local drug delivery for treating diseases of the Central nervous system in rodent models. JoVE. 2010;42(42):1–6. doi: 10.3791/1992.
- Verron E, Khairoun I, Guicheux J, et al. Calcium phosphate biomaterials as bone drug delivery systems: a review. Drug Discov Today. 2010;15(13–14):547–552. doi: 10.1016/j.drudis.2010.05.003.
- Ng CY, Chen C-B, Wu M-Y, et al. Anticancer drugs induced severe adverse cutaneous drug reactions: an updated review on the risks associated with anticancer targeted therapy or immunotherapies. J Immunol Res. 2018;2018:5376476–5376479. doi: 10.1155/2018/5376476.
- Vostinaru O. Adverse effects and drug interactions of the non‐steroidal anti‐inflammatory drugs. In: Gamal AA, editor. Nonsteroidal anti-inflammatory drugs. Rijeka: InTech Open, 2017. p. 17–31. doi: 10.1007/978-1-349-05952-2_31.
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8.
- Tiwari G, Tiwari R, Sriwastawa B, et al. Drug delivery systems: an updated review. Int J Pharm Investig. 2012;2(1):2–11. doi: 10.4103/2230-973x.96920.
- Cagdas M, Sezer AD, Bucak S. Liposomes as potential drug carrier systems for drug delivery. In: Sezer AD, editor. Application of nanotechnology in drug delivery. Rijeka: InTech Open, 2014.
- Gadekar V, Borade Y, Kannaujia S, et al. Nanomedicines accessible in the market for clinical interventions. J Control Release. 2021;330:372–397. doi: 10.1016/j.jconrel.2020.12.034.
- Ordikhani F, Zandi N, Mazaheri M, et al. Targeted nanomedicines for the treatment of bone disease and regeneration. Med Res Rev. 2021;41(3):1221–1254. doi: 10.1002/med.21759.
- Jeong J, Kim JH, Shim JH, et al. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23(1):4. doi: 10.1186/s40824-018-0149-3.
- Canillas M, Pena P, De Aza AH, et al. Calcium phosphates for biomedical applications. Boletín De La Sociedad Española De Cerámica y Vidrio. 2017;56(3):91–112. 10.1016/j.bsecv.2017.05.001
- Dorozhkin SV. A detailed history of calcium orthophosphates from 1770s till 1950. Mater Sci Eng C Mater Biol Appl. 2013;33(6):3085–3110. doi: 10.1016/j.msec.2013.04.002.
- Kattimani VS, Kondaka S, Lingamaneni KP. Hydroxyapatite–past, present, and future in bone regeneration. Bone Tissue Regen Insights. 2016;7:BTRI.S36138. doi: 10.4137/BTRI.S36138.
- Antoniac IV. 2016) Handbook of bioceramics and biocomposites. Cham: Springer International Publishing Switzerland.
- Carrodeguas RG, De Aza S. α-tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater. 2011;7(10):3536–3546. doi: 10.1016/j.actbio.2011.06.019.
- Elliot JC. 1994) Structure and chemistry of the apatites and other calcium orthophosphates. Amsterdam: Elsevier Science B.V. doi: 10.1016/B978-0-444-88534-0.50001-1.
- Kokubo T. 2008) Bioceramics and their clinical applications. Cambridge: Woodhead Publishing and Maney Publishing on behalf of The Institute of Materials, Minerals & Mining.
- Laskus A, Kolmas J. Ionic substitutions in non-apatitic calcium phosphates. Int J Mol Sci. 2017;18(12):2542–2564. doi: 10.3390/ijms18122542.
- Liu B, Lun D. Current application of β-tricalcium phosphate composites in orthopaedics. Orthop Surg. 2012;4(3):139–144. doi: 10.1111/j.1757-7861.2012.00189.x.
- Nahar U K, B S, Chandra D R, et al. Characterization of beta-tricalcium phosphate (β- TCP) produced at different process conditions. J Bioeng Biomed Sci. 2017;07(02):1000221–1000224. doi: 10.4172/2155-9538.1000221.
- Tanaka T, Komaki H, Chazono M, et al. Basic research and clinical application of beta-tricalcium phosphate. Morphologie. 2017;101(334):164–172. doi: 10.1016/j.morpho.2017.03.002.
- Combes C, Rey C. Amorphous calcium phosphates: synthesis, properties and uses in biomaterials. Acta Biomater. 2010;6(9):3362–3378. doi: 10.1016/j.actbio.2010.02.017.
- Du L-W, Bian S, Gou B-D, et al. Structure of clusters and formation of amorphous calcium phosphate and hydroxyapatite: from the perspective of coordination chemistry. Cryst Growth Des. 2013;13(7):3103–3109. doi: 10.1021/cg400498j.
- Valian A, Moezzyzadeh M, Moravejsalehi E. Role of amorphous calcium phosphate in dentistry. Beheshti Univ Dent J. 2014;31(4):243–253.
- Zhao J, Liu Y, Sun W-B, et al. First detection, characterization, and application of amorphous calcium phosphate in dentistry. J Dent Sci. 2012;7(4):316–323. doi: 10.1016/j.jds.2012.09.001.
- Wu M, V, Uskoković V. Is there a relationship between solubility and resorbability of different calcium phosphate phases in vitro? Biochim Biophys Acta. 2016;1860(10):2157–2168. doi: 10.1016/j.bbagen.2016.05.022.
- Bohner M, Santoni BLG, Döbelin N. β-Tricalcium phosphate for bone substitution: synthesis and properties. Acta Biomater. 2020;113:23–41. doi: 10.1016/j.actbio.2020.06.022.
- Salmasi S, Nayyer L, Seifalian AM, et al. Nanohydroxyapatite effect on the degradation, osteoconduction and mechanical properties of polymeric bone tissue engineered scaffolds. Open Orthop J. 2016;10(1):900–919. doi: 10.2174/1874325001610010900.
- Qu H, Fu H, Han Z, et al. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019;9(45):26252–26262. 10.1039/c9ra05214c35531040
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102–110. doi: 10.1186/1556-276X-8-102.
- Monteiro N, Martins A, Reis RL, et al. Liposomes in tissue engineering and regenerative medicine. J R Soc Interface. 2014;11(101):20140459. doi: 10.1098/rsif.2014.0459.
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632.
- Bulbake U, Doppalapudi S, Kommineni N, et al. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2):12. doi: 10.3390/pharmaceutics9020012.
- Popovska O, et al. An overview: methods for preparation and characterization of liposomes as drug delivery systems. Int J Pharm Phytopharm Res. 2013;3(3):182–189.
- van Hoogevest P, Wendel A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur J Lipid Sci Technol. 2014;116(9):1088–1107. doi: 10.1002/ejlt.201400219.
- Taira MC, Chiaramoni NS, Pecuch KM, et al. Stability of liposomal formulations in physiological conditions for oral drug delivery. Drug Deliv. 2004;11(2):123–128. doi: 10.1080/10717540490280769.
- Deodhar S, Dash AK. Long circulating liposomes: challenges and opportunities. Ther Deliv. 2018;9(12):857–872. doi: 10.4155/tde-2018-0035.
- Disalvo A, de los Angeles Frias M. The role of water in the responsive properties in lipid interphase of biomimetic systems. In: Catala A, editor. Liposomes - advances and perspectives. Rijeka: InTech Open, 2019. p. 1–21. doi: 10.5772/intechopen.85811.
- Li J, Wang X, Zhang T, et al. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10(2):81–98. doi: 10.1016/j.ajps.2014.09.004.
- Mishra H, Chauhan V, Kumar K, et al. A comprehensive review on liposomes: a novel drug delivery system. J Drug Delivery Ther. 2018;8(6):400–404. doi: 10.22270/jddt.v8i6.2071.
- Joguparthi V, Xiang T-X, and D, Anderson B. Liposome transport of hydrophobic drugs: gel phase lipid bilayer permeability and partitioning of the lactone form of a hydrophobic camptothecin, DB-67. J Pharm Sci. 2008;97(1):400–420. doi: 10.1002/jps.
- Beltrán-Gracia E, et al. 2019) Nanomedicine review: clinical developments in liposomal applications, cancer nanotechnology. Vienna: Springer. doi: 10.1186/s12645-019-0055-y.
- Shepherd SJ, Issadore D, Mitchell MJ. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials. 2021;274:120826. doi: 10.1016/j.biomaterials.2021.120826.
- Krishna R, Webb MS, St Onge G, et al. Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J Pharmacol Exp Ther. 2001;298(3):1206–1212.
- Ranade VV, Hollinger AM. 2004) Drug delivery systems. 2nd ed. London, New York and Washington: CRS Press: Boca Raton. doi: 10.1007/978-1-4939-1998-7.
- Sercombe L, Veerati T, Moheimani F, et al. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286.
- Vemuri S, Rhodes CT. Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharm Acta Helv. 1995;70(2):95–111. doi: 10.1016/0031-6865(95)00010-7.
- Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27(4):1–23. doi: 10.3390/molecules27041372.
- Nakhaei P, Margiana R, Bokov DO, et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol. 2021;9:705886. doi: 10.3389/fbioe.2021.705886.
- Gao X, Li L, Cai X, et al. Targeting nanoparticles for diagnosis and therapy of bone tumors: opportunities and challenges. Biomaterials. 2021;265:120404. doi: 10.1016/j.biomaterials.2020.120404.
- Khan AA, Allemailem KS, Almatroodi SA, et al. Recent strategies towards the surface modification of liposomes: an innovative approach for different clinical applications. 3 Biotech. 2020;10(4):163. doi: 10.1007/s13205-020-2144-3.
- Mufamadi MS, Pillay V, Choonara YE, et al. A review on composite liposomal technologies for specialized drug delivery. J Drug Deliv. 2011; 2011:939851–19. doi: 10.1155/2011/939851.
- Samad A, Sultana Y, Aqil M. Liposomal drug delivery systems: an update review. Curr Drug Deliv. 2007;4(4):297–305. doi: 10.2174/156720107782151269.
- Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23(9):3319–3329. doi: 10.1080/10717544.2016.1177136.
- Villasmil-Sánchez S, Drhimeur W, Ospino SCS, et al. Positively and negatively charged liposomes as carriers for transdermal delivery of sumatriptan: in vitro characterization. Drug Dev Ind Pharm. 2010;36(6):666–675. doi: 10.3109/03639040903419640.
- Zhao W, Zhuang S, Qi XR. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int J Nanomedicine. 2011;6:3087–3098. doi: 10.2147/IJN.S25399.
- Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10(0):S96–S101. doi: 10.1007/s005860100282.
- Large DE, Abdelmessih RG, Fink EA, et al. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851. doi: 10.1016/j.addr.2021.113851.
- Leggio L, Arrabito G, Ferrara V, et al. Mastering the tools: natural versus artificial vesicles in nanomedicine. Adv Healthc Mater. 2020;9(18):e2000731. doi: 10.1002/adhm.202000731.
- Marenzana M, Arnett TR. The key role of the blood supply to bone. Bone Res. 2013;1(3):203–215. doi: 10.4248/BR201303001.
- Park J, Ries J, Gelse K, et al. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10(13):1089–1098. doi: 10.1038/sj.gt.3301960.
- Chen J, Ashames A, Buabeid MA, et al. Nanocomposites drug delivery systems for the healing of bone fractures. Int J Pharm. 2020;585:119477–119488. doi: 10.1016/j.ijpharm.2020.119477.
- Marongiu G, Dolci A, Verona M, et al. The biology and treatment of acute long-bones diaphyseal fractures: overview of the current options for bone healing enhancement. Bone Rep. 2020;12:100249. doi: 10.1016/j.bonr.2020.100249.
- Xiang Q, Xiao J, Zhang H, et al. Preparation and characterisation of bFGF-encapsulated liposomes and evaluation of wound-healing activities in the rat. Burns. 2011;37(5):886–895. doi: 10.1016/j.burns.2011.01.018.
- Cheng X, Tsao C, M. Saul J, et al. Comparison of two nanoparticle formulations for localized delivery of platelet-derived growth factor (PDGF) from aligned collagen fibers. PNT. 2013;1(2):105–114. doi: 10.2174/2211738511301020006.
- Mallorie A, Shine B. Normal bone physiology, remodelling and its hormonal regulation. Surgery. 2022;40(3):163–168. doi: 10.1016/j.mpsur.2021.12.001.
- Fukunaga M, Miller MM, Deftos LJ. Liposome-entrapped calcitonin and parathyroid hormone are orally effective in rats. Horm Metab Res. 1991;23(4):166–167. doi: 10.1055/s-2007-1003642.
- Sun X, Wei J, Lyu J, et al. Bone-targeting drug delivery system of biomineral-binding liposomes loaded with icariin enhances the treatment for osteoporosis. J Nanobiotechnology. 2019;17(1):10. BioMed Central, doi: 10.1186/s12951-019-0447-5.
- Glowka E, Stasiak J, Lulek J. Drug delivery systems for vitamin D supplementation and therapy. Pharmaceutics. 2019;11(7):347. doi: 10.3390/pharmaceutics11070347.
- Quadros M, Momin M, Verma G. Design strategies and evolving role of biomaterial assisted treatment of osteosarcoma. Mater Sci Eng, C. 2021;121:111875. doi: 10.1016/j.msec.2021.111875.
- Rajani R, Gibbs CP. Treatment of bone tumors. Surg Pathol Clin. 2012;5(1):301–318. doi: 10.1016/j.path.2011.07.015.
- Antimisiaris SG, Marazioti A, Kannavou M, et al. Overcoming barriers by local drug delivery with liposomes. Adv Drug Deliv Rev. 2021;174:53–86. doi: 10.1016/j.addr.2021.01.019.
- Darley ESR, MacGowan AP. Antibiotic treatment of gram-positive bone and joint infections. J Antimicrob Chemother. 2004;53(6):928–935. doi: 10.1093/jac/dkh191.
- Lomas J. Antimicrobial treatment in bone and joint infections. Orthop Trauma. 2019;33(3):153–159. doi: 10.1016/j.mporth.2019.03.002.
- Anada T, Takeda Y, Honda Y, et al. Synthesis of calcium phosphate-binding liposome for drug delivery. Bioorg Med Chem Lett. 2009;19(15):4148–4150. doi: 10.1016/j.bmcl.2009.05.117.
- Caliskan Y, Dalgic AD, Gerekci S, et al. A new therapeutic combination for osteosarcoma: gemcitabine and clofazimine co-loaded liposomal formulation. Int J Pharm. 2019;557:97–104. doi: 10.1016/j.ijpharm.2018.12.041.
- Skubitz KM. Phase II trial of pegylated-liposomal doxorubicin (DoxilTM) in sarcoma. Cancer Invest. 2003;21(2):167–176. doi: 10.1081/CNV-120016412.
- Wu H, Luo Y, Xu D, et al. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int J Biol Macromol. 2020;164:2583–2597. doi: 10.1016/j.ijbiomac.2020.08.068.
- Huang M, Liang C, Tan C, et al. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019;10(10):6447–6458. doi: 10.1039/c9fo01338e.
- Lytton J. 2013. Membrane transporters: Na+/Ca2+ exchangers. In: Encyclopedia of biological chemistry. 2nd ed. Amsterdam: Elsevier Inc. doi: 10.1016/B978-0-12-378630-2.00132-8.
- Melcrová A, Pokorna S, Pullanchery S, et al. The complex nature of calcium cation interactions with phospholipid bilayers. Sci Rep. 2016;6(1):38035. doi: 10.1038/srep38035.
- Pravina P, Sayaji D, Avinash M. Calcium and its role in human body. Int J Res Pharm Biomed Sci. 2013;4(2):659–668.
- Szcześ A, Sternik D. Properties of calcium carbonate precipitated in the presence of DPPC liposomes modified with the phospholipase A2. J Therm Anal Calorim. 2016;123(3):2357–2365. doi: 10.1007/s10973-015-4958-5.
- Pedersen UR, Leidy C, Westh P, et al. The effect of calcium on the properties of charged phospholipid bilayers. Biochim Biophys Acta. 2006;1758(5):573–582. doi: 10.1016/j.bbamem.2006.03.035.
- Shih O, Yeh Y-Q, Liao K-F, et al. Membrane charging and swelling upon calcium adsorption as revealed by phospholipid nanodiscs. J Phys Chem Lett. 2018;9(15):4287–4293. doi: 10.1021/acs.jpclett.8b01651.
- Bo T, Pawliszyn J. Role of calcium binding in protein structural changes and phospholipid-protein interactions studied by capillary isoelectric focusing with whole column imaging detection. Anal Chim Acta. 2006;559(1):1–8. doi: 10.1016/j.aca.2005.11.047.
- Hagge SO, Hammer MU, Wiese A, et al. Calcium adsorption and displacement: characterization of lipid monolayers and their interaction with membrane-active peptides/proteins. BMC Biochem. 2006;7(1):15. doi: 10.1186/1471-2091-7-15.
- Iraolagoitia XLR, Martini MF. Ca2+ adsorption to lipid membranes and the effect of cholesterol in their composition. Colloids Surf B Biointerfaces. 2010;76(1):215–220. doi: 10.1016/j.colsurfb.2009.10.037.
- Martín-Molina A, Rodríguez-Beas C, Faraudo J. Effect of calcium and magnesium on phosphatidylserine membranes: experiments and all-atomic simulations. Biophys J. 2012;102(9):2095–2103. doi: 10.1016/j.bpj.2012.03.009.
- Szcześ A. Effects of DPPC/cholesterol liposomes on the properties of freshly precipitated calcium carbonate. Colloids Surf B Biointerfaces. 2013;101:44–48. doi: 10.1016/j.colsurfb.2012.06.013.
- Hong JS, Vreeland WN, Lacerda SHD, et al. Liposome-templated supramolecular assembly of responsive alginate nanogels. Langmuir. 2008;24(8):4092–4096. doi: 10.1021/la7031219.
- Cheng R, Liu L, Xiang Y, et al. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials. 2020;232:119706. doi: 10.1016/j.biomaterials.2019.119706.
- Dhoot NO, Wheatley MA. Microencapsulated liposomes in controlled drug delivery: strategies to modulate drug release and eliminate the burst effect. J Pharm Sci. 2003;92(3):679–689. doi: 10.1002/jps.19104.
- Westhaus E, Messersmith PB. Triggered release of calcium from lipid vesicles: a bioinspired strategy for rapid gelation of polysaccharide and protein hydrogels. Biomaterials. 2001;22(5):453–462. doi: 10.1016/S0142-9612(00)00200-3.
- Yamasaki S, Kurita S, Ochiai A, et al. Calcium molybdate nanoparticles formation in egg phosphatidyl choline based liposome caused by liposome fusion. J Colloid Interface Sci. 2018;530:473–480. doi: 10.1016/j.jcis.2018.07.007.
- Zhang J, Sun X, Shao R, et al. Polycation liposomes combined with calcium phosphate nanoparticles as a non-viral carrier for siRNA delivery. J Drug Delivery Sci Technol. 2015;30:1–6. doi: 10.1016/j.jddst.2015.09.005.
- Cheng R, Yan Y, Liu H, et al. Mechanically enhanced lipo-hydrogel with controlled release of multi-type drugs for bone regeneration. Appl Mater Today. 2018;12:294–308. doi: 10.1016/j.apmt.2018.06.008.
- Liu L, Xiang Y, Wang Z, et al. Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia Mater. 2019;11(1):1–18. Springer US, doi: 10.1038/s41427-019-0185-z.
- Wang G, Mostafa NZ, Incani V, et al. Bisphosphonate-decorated lipid nanoparticles designed as drug carriers for bone diseases. J Biomed Mater Res A. 2012;100(3):684–693. doi: 10.1002/jbm.a.34002.
- Zhang M, Kataoka K. Nano-structured composites based on calcium phosphate for cellular delivery of therapeutic and diagnostic agents. Nano Today. 2009;4(6):508–517. doi: 10.1016/j.nantod.2009.10.009.
- Patel H, et al. Preparation and characterization of calcium phosphate coated vinblastine sulfate liposomes. Int J Pharm Res Biosci. 2013;2(2):263–280.
- Xu Q, Tanaka Y, Czernuszka JT. Encapsulation and release of a hydrophobic drug from hydroxyapatite coated liposomes. Biomaterials. 2007;28(16):2687–2694. doi: 10.1016/j.biomaterials.2007.02.007.
- Yeo C-H, Zein SHS, Ahmad AL, et al. Comparison of DOPA and DPPA liposome templates for the synthesis of calcium phosphate nanoshells. Ceram Int. 2012;38(1):561–570. doi: 10.1016/j.ceramint.2011.07.044.
- Erra Díaz F, Dantas E, Geffner J. Unravelling the interplay between extracellular acidosis and immune cells. Mediators Inflamm. 2018;2018:1218297–11. doi: 10.1155/2018/1218297.
- Rivero Berti I, Dell’ Arciprete ML, Dittler ML, et al. Delivery of fluorophores by calcium phosphate-coated nanoliposomes and interaction with Staphylococcus aureus biofilms. Colloids Surf B Biointerfaces. 2016;142:214–222. doi: 10.1016/j.colsurfb.2016.03.003.