Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or novel coronavirus disease 2019 (COVID-19) emerged from China in December 2019 and progressed to become a global pandemic. Our understanding of its pathophysiology and potential management was initially extrapolated from previous epidemics of coronaviruses like SARS and MERS. SARS-CoV-2 is asymptomatic or minimally symptomatic in more than 80% of patients and requires no additional management; however, the remaining patients progress to pneumonia and hypoxemia with ranging severity, including a smaller group that requires intensive care unit admission. To date, there are no approved treatments for SARS-CoV-2, and current management is focused on supplemental oxygen and supportive care. The antiviral medication remdesivir recently received emergency use authorization by the US Food and Drug Administration for patients with severe disease. Multiple clinical trials evaluating different treatment modalities such as antivirals, immunomodulators, convalescent plasma, and monoclonal antibodies, among others, are still ongoing. We believe that patients present with clinical phenotypes that correlate with the spectrum of disease. Each phenotype may benefit from one or multiple interventions. We discuss treatments under evaluation in clinical trials and their potential application based on clinical phenotype presentation.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or novel coronavirus disease 2019 (COVID-19) is caused by a betacoronavirus similar to SARS-CoV-1 and MERS-CoV. Since its origin in China at the end of 2019, it has spread throughout the world, resulting in a global pandemic. The understanding of the pathophysiology of the virus and its clinical implications is changing at a rapid pace as new data emerge almost daily.
There are multiple phases of the viral infection that correlate with clinical and laboratory manifestations of disease. SARS-CoV-2 uses the human angiotensin-converting enzyme 2 (ACE2) receptor (present in large quantities in the lung, heart, kidney, and bowel) to enter.Citation 1 This may explain the clinical manifestations of early stages (cough, diarrhea) and late stages of disease of SARS-CoV-2 infection. The initial viral phase is facilitated by escape mechanisms built into SARS-CoV-2 as well as many other coronaviruses, which blunt interferon-alpha and -beta production and evade immune recognition in initial phases. Ongoing viral replication is accompanied by cough, fever, malaise, diarrhea, and in some cases anosmia. Radiologic findings may include interstitial infiltrates in initial phases followed by alveolar infiltrates and sometimes consolidation, and laboratory findings may include elevated C-reactive protein (CRP) and variable elevation of lactate dehydrogenase or ferritin. It has been proposed that there may be an initial inflammatory response caused by down-regulation of ACE2 receptors and secretion of pro-inflammatory cytokines, followed by a secondary inflammatory response via activation of the complement cascade and antibody-mediated cellular cytotoxicity triggered by virus-antibody complex formation.Citation 2 The cytokine storm is caused by rapidly replicating virus, coupled with extensive cellular damage that leads to production of pro-inflammatory cytokines, down-regulation of the ACE2 receptor, and antibody-dependent enhancement of the immune system.Citation 2 , Citation 3 Thus, lung involvement reveals acute respiratory distress syndrome (ARDS) with variable levels of cytokine release. The radiological findings show infiltrates both in cases with elevated levels of CRP and ferritin and in cases with normal or mildly elevated cytokine levels. One mechanism of ARDS is related to increased vascular permeability, with either normal or mildly elevated inflammatory markers such as CRP and ferritin, with a second mechanism accompanied by high inflammatory markers including ferritin, CRP, D-dimer, and elevated interleukin (IL)-6.Citation 1 , Citation 3 This mechanism is believed to share pathophysiologic features with hemophagocytic lymphohistiocytosis.
A review of potential pharmacologic agents has recently been published.Citation 4 There have also been numerous open-label and single-arm trials, some unsuccessful and others with anecdotal success. However, to date, there is no proven effective treatment for SARS-CoV-2. As a result, the Infectious Diseases Society of America has outlined recommendations for treatment of symptomatic patients only in the context of clinical trials.Citation 5 A practical and systematic approach to manage patients with SARS-CoV-2 is necessary.
In this review, we summarize current therapies under evaluation in the setting of clinical trials that were recently completed or are still under way and propose treatment indications and combined management approaches, understanding that therapy is constantly changing as new evidence emerges.
BRIEF SUMMARY OF TREATMENTS UNDER EVALUATION
The use of supplemental oxygen is a key component in the treatment of SARS-CoV-2 infection. Patients may present with “silent hypoxemia,” without overt shortness of breath but with infiltrates on chest x-ray and oxygen saturation <95%. Severe disease with hypoxemia requiring oxygen supplementation may be present in 14% of patients, while 5% may require intensive care unit admission and mechanical ventilation.Citation 6 In this scenario, the most important intervention is the use of supplemental oxygen along with other supportive measures.Citation 7 , Citation 8
Antiviral medications
Ideally, antiviral medications should aim to reduce viral replication with a subsequent decrease in immune activation and progression to later stages of the disease. Several repurposed antivirals have been used either as salvage therapy or in open-label trials so far, with either modest success or no effectiveness at all.
Lopinavir-ritonavir, a potent protease inhibitor previously used for human immunodeficiency virus, has potent in vitro activity against the 3-chymotrypsin-like protease found in coronaviruses, although no data exist for SARS-CoV-2.Citation 4 , Citation 9 , Citation 10 A randomized open-label trial of 199 patients showed no difference in viral load reduction or mortality; however, there were increased rates of adverse effects (primarily gastrointestinal) when compared to standard of care.Citation 11
Antimalarial agents chloroquine and hydroxychloroquine have been proposed as potential therapeutic agents, as they block viral entry into cells and also have immunomodulatory effects by lowering cytokine levels.Citation 12 Hydroxychloroquine appears to be more potent than chloroquine, with lower effective concentrations required to inhibit viral replication.Citation 4 , Citation 13 Despite in vitro activity, neither has been shown to be effective in randomized controlled trials for SARS-CoV-2. One study in China failed to show clinical improvement or more rapid viral clearance when compared to standard of care.Citation 14 An open-label study in France seemed to offer better results with improved viral clearance in those who received hydroxychloroquine, although the lack of randomization and low number of patients, coupled with a lack of safety data and concerns of increased cardiotoxicity, preclude a recommendation to use this regimen.Citation 15 More recently, a retrospective observational report from the Veterans Affairs system where hydroxychloroquine was used with or without azithromycin showed no difference in cure rates or progression to severe disease and showed increased toxicity when compared to standard of care.Citation 16 In this report, the authors suggested that the lack of clinical benefit may arise from the inability to reach concentrations needed to realize antiviral activity with the current dosing regimens. Lastly, a retrospective review in New York on use of hydroxychloroquine with or without azithromycin, compared to no treatment, showed no difference in outcomes but did not show increased toxicity.Citation 17
Another potential antiviral agent is favipiravir, which is approved for the treatment of influenza in China and Japan. Favipiravir inhibits RNA-dependent RNA polymerase, has in vitro activity against many RNA viruses, and may have activity against SARS-CoV-2.Citation 18 , Citation 19 It was approved in China for treatment of SARS-Cov-2 at the end of March 2020, and several clinical trials are under way in China, with one at Stanford University in the United States.
Remdesivir seems to be the most promising agent of the antiviral class. It was initially discovered as an agent against flaviviruses and coronaviruses and was used during the Ebola outbreak in 2014.Citation 20 Remdesivir has been shown to be active in vitro against SARS-CoV and MERS-CoV. It works by inhibiting the RNA-dependent RNA polymerase and has been shown to have potent activity against SARS-CoV-2 in vitro and in a mouse model of lung infection.Citation 21 , Citation 22 Interestingly, there is a high barrier to resistance to the agent and, when present, it may be accompanied by a concomitant loss in viral fitness.Citation 23 Phase 1 clinical trials for Ebola have shown good tolerability with few side effects, good intracellular concentrations with an intracellular half-life of 40 h, and reversible liver function test elevations after multiple doses. Current clinical trials are evaluating a loading dose of 200 mg followed by 100 mg daily infusions. Its use is not recommended in those with a creatinine clearance <30 mL/min. Case reports of successful useCitation 24 as well as moderately encouraging results with compassionate use have been reported.Citation 25
Clinical trials have evaluated the safety and effectiveness of remdesivir both in moderate and severe disease. A randomized double-blind, placebo-controlled trial conducted in China on patients with severe disease showed a numerically faster time to clinical improvement in the remdesivir group, but the study was underpowered, as it did not attain the prespecified sample size.Citation 26 A recently published randomized double-blind clinical trial conducted by the National Institute of Allergy and Infectious Diseases that enrolled 1063 hospitalized patients with SARS-CoV-2 who had evidence of pneumonia showed that the use of remdesivir was associated with a faster time to recovery (11 vs 15 days; P < 0.05) and showed a trend toward improved mortality rates (8.0% vs 11.6%, P = 0.059) in those who received remdesivir when compared to placebo.Citation 27 In this trial, patients with severe disease who required supplemental oxygen via nasal cannula (ordinal scale 5) were more likely to benefit from remdesivir. The findings also suggest that once the disease advances to later stages, antiviral therapy may not be sufficient. Results of this trial led to emergency approval of the drug by the US Food and Drug Administration for hospitalized patients with oxygen saturation ≤94%.Citation 28 It seems that 5 days of therapy provides a benefit similar to that of 10 days of therapy, as recently highlighted in a randomized, open-label trial for patients with severe disease.Citation 29
Convalescent plasma
Emergent use of convalescent plasma has been shown to be effective against other lethal infectious diseases.Citation 30 It was used in patients with SARS and MERS with variable success and showed reduction in mortality, although data should be interpreted with caution as there were no control groups and the studies performed were deemed to have a high risk for bias.Citation 31 Initial reports from China have shown a potential benefit, with normalization of physiologic parameters including fever and oxygenation, ability to wean from the ventilator, seroconversion with increase in neutralizing antibody titers, and negative polymerase chain reaction test in nasopharyngeal swabs. Although encouraging, the data are anecdotal and have to be interpreted with caution.Citation 32–34
A clinical trial in China showed potential benefit in severe disease, although the difference was not statistically significant possibly due to the small sample size, the waning of the pandemic in China, and initiation of therapy in more advanced stages of the disease.Citation 35 On the other hand, a single center study that looked at 45 patients who received convalescent plasma, matched to a cohort of patients admitted during the same time period, showed improved oxygen requirements and survival in nonintubated patients who received convalescent plasma. A beneficial effect was not seen in those who required mechanical ventilation.Citation 36 The US Food and Drug Administration has approved the use of plasma from recovered patients to treat seriously ill COVID-19–infected patients.Citation 37
One potential challenge in using convalescent plasma is defining the titer of neutralizing antibodies necessary to have a significant effect in modifying or treating the disease. It was previously shown that some patients who recovered from viral disease may not have high enough titers of neutralizing antibody.Citation 38 Risks for plasma transfusion include viral infections, including transmission of SARS-CoV-2 as well as transfusion-related acute lung injury.Citation 30 Another possible side effect of convalescent plasma use relates to the phenomenon of antibody-dependent enhancement (ADE) of infection. For coronaviruses, several mechanisms for ADE have been described, and there is the concern that antibodies to one type of coronavirus could enhance infection to another viral strain.Citation 39 Overall, administration of convalescent plasma has been shown to be safe,Citation 40 and there were no reported adverse events in the case series from China.Citation 32–34 , Citation 40 There are areas of uncertainty such as efficacy and timing of administration that open the door for randomized clinical trials using this treatment modality against SARS-CoV-2.
IL-6 antagonists
Serum IL-6 concentrations are increased in SARS-CoV-2–mediated cytokine storm.Citation 41 , Citation 42 IL-6 blockers such as tocilizumab or sarilumab work by suppressing the inflammatory cascade mediated by IL-6 and are potential therapeutic options.Citation 43 It would seem that the use of IL-6 blockade would be more appropriate when increasing oxygen requirements and rising inflammatory markers are the predominant findings. Preliminary reports from a clinical trial suggest that tocilizumab improved clinical outcomes in patients with moderate or severe pneumonia,Citation 44 but interim data on sarilumab showed that only critically ill patients may benefit from its administration.Citation 45 Long-term adverse effects from these medications in this setting are unknown and should be an area for future research. While both agents show signs of improving outcomes in patients with COVID 19, both carry black box warnings concerning the risk of development of other infectious complications.
Corticosteroids
The routine use of corticosteroids is not recommended in patients with SARS-CoV-2. Its use may be associated with increased viral shedding and risk for other secondary infections.Citation 7 It was previously shown that patients with influenza who received steroids had increased mortality.Citation 46 There is also evidence from previous coronaviruses to suggest a detrimental effect of steroids in these patients,Citation 47 although a study on 201 patients in China showed that steroids improved mortality in those with ARDS secondary to SARS-CoV-2.Citation 48 Steroids are also recommended for certain indications, including concomitant septic shock, in ventilated patients with ARDS and those with evidence of cytokine storm resembling hemophagocytic lymphohistiocytosis.
Stem cell therapy
Mesenchymal stem cells have been used as salvage therapy in several patients with SARS-CoV-2 with variable success. These cells have potent anti-inflammatory and immunomodulating properties. They may block pro-inflammatory cytokine production (IL-1, IL-6, and IL-12, among others) and have the potential to inhibit aberrant activation of lymphocytes and macrophages, thus reducing the inflammatory cascade in the lung.Citation 49 When given intravenously, these cells get “trapped” in the lung, which may help achieve a local anti-inflammatory effect, promote lung healing, and prevent fibrosis.Citation 50 , Citation 51 A clinical trial to evaluate the use of stem cells in critically ill patients is under way (NCT04371393).
Monoclonal antibody therapy
Monoclonal antibodies may have therapeutic potential. SARS-CoV-2 has different viral epitopes that can be targeted; of those, the spike protein is the major inducer of neutralizing antibodies. The ACE2 receptor binding domain located in the spike protein is a potential therapeutic target for monoclonal antibody production.Citation 52 It should be mentioned that monoclonal antibody therapy has not been shown to be effective against all coronaviruses, as demonstrated in an animal model against MERS-CoV-2, where it failed to protect against severe disease or lung damage.Citation 53 More research is needed in this arena. It is possible that more than one monoclonal antibody against different targets would be necessary to have a neutralizing effect on SARS-CoV-2. Several clinical trials on the use of monoclonal antibodies for treatment of mild and severe disease have begun in the United States.
Anticoagulation
It is widely accepted that SARS-CoV-2 may induce a procoagulable state, affecting endothelial cells and leading to increased inflammatory markers and coagulation dysfunction, with increased levels of D-dimer and risk for thrombosis, including stroke and pulmonary microemboli as well as pulmonary macroemboli.Citation 54 , Citation 55 Development of disseminated intravascular coagulation, micropulmonary thrombosis, and cardiovascular complications, such as myocarditis and acute myocardial ischemia, have been reported as well.Citation 54 , Citation 56 A different approach may be necessary, including anticoagulation in those patients who are critically ill with high inflammatory states and on mechanical ventilation.Citation 57
CLINICAL PHENOTYPES OF SARS-COV-2
The clinical manifestations of SARS-CoV-2 range from symptomatic disease to hospitalization and the requirement for mechanical ventilation and even extracorporeal membrane oxygenation ( ). It is believed that comorbidities such as diabetes, heart disease, obesity, and possibly immunocompromise due to steroids or immunosuppressive agents can lead to more severe presentations.Citation 5 However, in one cohort of hospitalized patients in Georgia, it was found that >25% of patients did not meet predefined high-risk conditions.Citation 58
Table 1. Clinical phenotypes, laboratory markers, clinical characteristics, and interventions for SARS-CoV-2 disease
We believe that SARS-Cov-2 can manifest as distinct phenotypes, and each phenotype may respond to different therapies and have different clinical outcomes.Citation 59 Moreover, there is overlap between phenotypes, making treatment choices more complex. The mild disease phenotype usually presents with low-grade fever, cough, malaise, rhinorrhea, sore throat, nausea, vomiting, and diarrhea, but without radiological features of pneumonia and the absence of mental changes. The moderate disease phenotype adds respiratory symptoms such as cough and shortness of breath, along with x-ray features of early infiltrates. The severe phenotype includes dyspnea, tachypnea, oxygen saturation <94%, and lung infiltrates on >50% of lung fields.
The critically ill phenotype is usually a result of progression through the above mentioned stages of disease. We propose a subdivision of critical care SARS-CoV-2 into three subphenotypes that have distinct underlying pathophysiologic mechanisms: (1) features of ARDS requiring mechanical ventilation; (2) a high inflammatory state with fever, bilateral pulmonary infiltrates in about 50% of patients, immune activation with laboratory evidence of cytopenias, elevated ferritin, and elevated concentrations of cytokines such as IL-6 (cytokine storm), and features of hemophagocytic lymphohistiocytosis; and (3) microthrombosis, which is believed to be due to secondary compromise of endothelial cells by SARS-CoV-2, leading to an increase in inflammatory markers and coagulation diathesis.
Given these variable presentations, management of the disease should focus on reducing the rate of complications and avoiding progression to more severe stages ().Citation 60 The mild disease phenotype is best managed by clinical observation and rest. The moderate disease phenotype usually requires hospital admission, as these patients usually have radiographic findings of pneumonia and may develop hypoxemia without overt shortness of breath. This group should be observed for the need for oxygen supplementation and progression to more severe stages of the disease. It is not clear whether other therapeutic interventions would be beneficial or prevent deterioration or progression to severe stages, and thus participation in clinical trials to address this specific issue is key. Results of a clinical trial using remdesivir in moderate disease have shown more likelihood of clinical improvement for those who received 5 days of remdesivir and a trend for improvement in those receiving 10 days.Citation 61
Figure 1. Phenotypes and possible therapeutic approaches for SARS-CoV-2 disease. Adapted from Siddiqi (2020).Citation 60
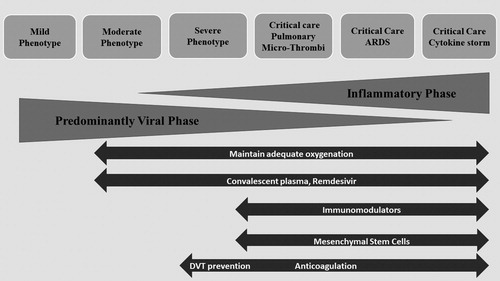
Patients with the severe phenotype may benefit from the use of remdesivir to decrease symptom duration and possibly mortality in those who require oxygen via nasal cannula. The best timing to start antiviral therapy is unclear, although it would seem best when the viral phase predominates. An overlapping severe phenotype could include both active viral replication and an inflammatory component, with progressive oxygen requirements including high-flow nasal cannula, in which case the combination of antiviral therapy with remdesivir or convalescent plasma and anti-inflammatory agents could be beneficial. A clinical trial with remdesivir with or without baricitinib (a Janus-Kinase inhibitor) is under way to test this hypothesis. Monoclonal antibodies will also be evaluated for management of patients with a moderate or severe phenotype.
All critical care phenotypes may benefit from antiviral agents. Although it may be important to reduce viral load in order to break the inflammatory cycle and direct lung damage, viral replication is not the main drive of these phenotypes. ARDS management should be used with low tidal volumes, prone positioning, and extracorporeal membrane oxygenation in selected cases. The use of convalescent plasma and mesenchymal cells may have value in this phase. Those with cytokine storm could benefit from strategies to reduce viral load as well as inflammation. It has been seen that the cytokine storm response is generally associated with very high viral loads, such that there may be a vicious cycle of viral replication leading to ongoing immune activation. It is possible that despite a need to reduce inflammation with different agents such as IL-6 blockers, adding antiviral therapy with either remdesivir or convalescent plasma may have a therapeutic role in decreasing viral load and breaking this cycle. One caveat is that because there may be multiple pathways leading to a common inflammatory activation cascade, one strategy alone may not be sufficient; data from IL-6 trials have not shown dramatic improvement in patients who received them, as was initially thought. Other therapies such as anti–granulocyte-macrophage colony-stimulating factor, mesenchymal cells, monoclonal antibodies against CCR5, and corticosteroids are being evaluated and may show value. One note should be made on the risk for immunosuppression with monoclonal antibodies, with risk for reactivation of hepatitis viruses and bacterial or fungal infections. Lastly, the phenotype manifesting with microthrombi and a procoagulable state may be further down the road, and anticoagulation and oxygenation support are the mainstays, while antivirals, convalescent plasma, or anti-inflammatory agents may not have a role at this stage.
DISCUSSION
SARS-CoV-2 has a wide spectrum of disease. Most patients are either asymptomatic or have a mild clinical course, and a fraction of those infected need hospitalization. For those who need hospitalization, not all disease behaves the same—with courses ranging in acuity from inpatient wards to progressive severe disease requiring mechanical ventilation and intensive care.
It is important to distinguish clinical phenotypes, since the approach to manage them is likely to vary. Although mild, moderate, and early severe phenotypes may have predominating viral effects, more advanced phenotypes are likely determined by an overlap in viral and inflammatory states. A less prominent viral phase, with a more predominant inflammatory state, may be correlated with progression of disease. It is also possible that down-regulation of IL-12 and interferon-gamma with a blunted CD4+ TH-1 cell response, increased CD4+ TH-2 cells, and IL-10 production may be responsible for impaired viral clearance. A similar mechanism has been proposed for MERS-CoV.Citation 62 With the exception of ARDS without marked elevation of ferritin and IL-6, other critical care phenotypes are determined by immune activation and hypercoagulable states. Risk factors for severe presentations and for progression of disease need to be determined. Ongoing clinical trials may shed some light on potentially effective therapies and are an important first step in the management of these patients.
There is a need to adapt multiple therapeutic approaches since phenotypic presentations are likely to overlap. A patient who presents with moderate disease may need antiviral therapy, while a patient with a mixed phenotype could benefit from both antiviral therapy and strategies to reduce immune activation. Adaptive clinical trials that use multiple modalities may show more success than using one strategy at a time. It is also unclear if antiviral agents have a role in severe disease once immune activation and severe inflammation are present. There may be a benefit in decreasing viral load and halting ongoing immune activation at any stage of the disease. Nevertheless, it must be stressed that our understanding of the pathophysiology of the disease is constantly changing. The precise utility of any of these modalities remains to be determined. It is clear, though, that both convalescent plasma and remdesivir are more likely to provide benefit in moderate or severe disease, before immune activation ensues.
SARS-CoV-2 has brought an enormous challenge. The lack of available therapies has placed us at a crossroad where we are faced with striving to provide the best patient care while at the same time helping to advance the science with the hope of finding effective treatment approaches.
DISCLOSURE STATEMET
Dr. Sandkovsky is an investigator for Gilead, Regeneron, Mesoblast, CytoDyn, and Karyopharm. Dr. Berhe is an investigator for Gilead, Regeneron, and Karyopharm. Drs. Modrykamien, Colley, and Sam have nothing to disclose. No compensation or grant was received for this work.
Acknowledgments
We thank our patients for placing their trust in our care, our frontline workers and critical care units for their commitment, and our pharmacy staff and North Texas Infectious Diseases for their invaluable support.
- Jin Y , Yang H , Ji W , et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses . 2020;12(4):372. doi:10.3390/v12040372.
- Fu Y , Cheng Y , Wu Y. Understanding SARS-CoV-2–mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin . 2020. doi:10.1007/s12250-020-00207-4.
- Ye Q , Wang B , Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect . 2020;80(6):607–613. doi:10.1016/j.jinf.2020.03.037.
- Sanders JM , Monogue ML , Jodlowski TZ , Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA . 2020; doi:10.1001/jama.2020.6019.
- Bhimraj A , Morgan RL , Shumaker AH , et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis . 2020;ciaa478. doi:10.1093/cid/ciaa478.
- Wu Z , McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA . 2020;323(13):1239. doi:10.1001/jama.2020.2648.
- Alhazzani W , Møller MH , Arabi YM , et al. Surviving Sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med . 2020;46(5):854–887. doi:10.1007/s00134-020-06022-5.
- Wang Y , Wang Y , Chen Y , Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol . 2020;92(6):568–576. doi:10.1002/jmv.25748.
- Chu CM , Cheng VC , Hung IF , et al ; HKU/UCH SARS Study Group . Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax . 2004;59(3):252–256. doi:10.1136/thorax.2003.012658.
- de Wilde AH , Jochmans D , Posthuma CC , et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother . 2014;58(8):4875–4884. doi:10.1128/AAC.03011-14.
- Cao B , Wang Y , Wen D , et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med . 2020;382(19):1787–1799. doi:10.1056/NEJMoa2001282.
- Devaux CA , Rolain JM , Colson P , Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents . 2020;55(5):105938. doi:10.1016/j.ijantimicag.2020.105938.
- Liu J , Cao R , Xu M , et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov . 2020;6(1):16. doi:10.1038/s41421-020-0156-0.
- Taccone FS , Gorham J , Vincent JL. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir Med . 2020;S2213-2600(20)30172-7. doi:10.1016/S2213-2600(20)30172-7.
- Gautret P , Lagier JC , Parola P , et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis . 2020;34:101663. doi:10.1016/j.tmaid.2020.101663.
- Magagnoli j , Narendran S , Pereira F , et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. Med . 2020. doi:10.1016/j.medj.2020.06.001.
- Rosenberg ES , Dufort EM , Udo T , et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA . 2020. doi:10.1001/jama.2020.8630.
- Delang L , Abdelnabi R , Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res . 2018;153:85–94. doi:10.1016/j.antiviral.2018.03.003.
- Furuta Y , Komeno T , Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad, Ser B, Phys Biol Sci . 2017;93(7):449–463. doi:10.2183/pjab.93.027.
- Siegel D , Hui HC , Doerffler E , et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem . 2017;60(5):1648–1661. doi:10.1021/acs.jmedchem.6b01594.
- Wang M , Cao R , Zhang L , et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res . 2020;30(3):269–271. doi:10.1038/s41422-020-0282-0.
- Zhai P , Ding Y , Wu X , Long J , Zhong Y , Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents . 2020;55(5):105955. doi:10.1016/j.ijantimicag.2020.105955.
- Agostini ML , Andres EL , Sims AC , et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio . 2018;9(2):e00221–18. doi:10.1128/mBio.00221-18.
- Holshue ML , DeBolt C , Lindquist S , et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med . 2020;382(10):929–936. doi:10.1056/NEJMoa2001191.
- Grein J , Ohmagari N , Shin D , et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med . 2020;NEJMoa2007016. doi:10.1056/NEJMoa2007016.
- Wang Y , Zhang D , Du G , et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet . 2020. doi:10.1016/S0140-6736(1020)31022-31029.
- Beigel JH , Tomashek KM , Dodd LE , et al ; ACTT-1 study group members . Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med . 2020;NEJMoa2007764. doi:10.1056/NEJMoa2007764.
- Hinton DM. Letter to A. Rhoades, Gilead sciences, granting emergency use authorization from the US Food and Drug Administration for remdesivir. https://www.fda.gov/media/137564/download. Published May 1, 2020.
- Goldman JD , Lye DCB , Hui DS , et al ; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med . 2020;NEJMoa2015301. doi:10.1056/NEJMoa2015301.
- Bloch EM , Shoham S , Casadevall A , et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest . 2020;130(6):2757–2765. doi:10.1172/JCI138745.
- Mair-Jenkins J , Saavedra-Campos M , Baillie JK , et al ; Convalescent Plasma Study Group . The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis . 2015;211(1):80–90. doi:10.1093/infdis/jiu396.
- Duan K , Liu B , Li C , et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA . 2020;117(17):9490–9496. doi:10.1073/pnas.2004168117.
- Shen C , Wang Z , Zhao F , et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA . 2020;323(16):1582. doi:10.1001/jama.2020.4783.
- Zhang B , Liu S , Tan T , et al. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest . 2020. doi:10.1016/j.chest.2020.03.039.
- Li L , Zhang W , Hu Y , et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA . 2020. doi:10.1001/jama.2020.10044.
- Liu STH , Lin HM , Baine I , et al. Convalescent plasma treatment of severe COVID-19: a matched control study. medRxiv . 2020. doi:10.1101/2020.05.20.20102236.
- Tanne JH. Covid-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ . 2020;368:m1256. doi:10.1136/bmj.m1256.
- Arabi YM , Hajeer AH , Luke T , et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerging Infect Dis . 2016;22(9):1554–1561. doi:10.3201/eid2209.151164.
- Wan Y , Shang J , Sun S , et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol . 2020;94(5):e02015–19. doi:10.1128/JVI.02015-19.
- Casadevall A , Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest . 2020;130(4):1545–1548. doi:10.1172/JCI138003.
- Conti P , Ronconi G , Caraffa A , et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents . 2020;34(2):1.
- Li X , Geng M , Peng Y , Meng L , Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal . 2020;10(2):102–108. doi:10.1016/j.jpha.2020.03.001.
- Tanaka T , Narazaki M , Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy . 2016;8(8):959–970. doi:10.2217/imt-2016-0020.
- Assistance Publique–Hôpitaux de Paris . Tocilizumab improves significantly clinical outcomes of patients with moderate or severe COVID-19 pneumonia. https://www.aphp.fr/contenu/tocilizumab-improves-significantly-clinical-outcomes-patients-moderate-or-severe-covid-19. Published April 27, 2020. Accessed June 5, 2020.
- BioSpace . Regeneron and Sanofi provide update on U.S. phase 2/3 adaptive-designed trial of Kevzara® (sarilumab) in hospitalized COVID-19 patients. https://www.biospace.com/article/releases/regeneron-and-sanofi-provide-update-on-u-s-phase-2-3-adaptive-designed-trial-of-kevzara-sarilumab-in-hospitalized-covid-19-patients. Published April 27, 2020. Accessed June 5, 2020.
- Ni YN , Chen G , Sun J , Liang BM , Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care . 2019;23(1):99. doi:10.1186/s13054-019-2395-8.
- Russell CD , Millar JE , Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet . 2020;395(10223):473–475. doi:10.1016/S0140-6736(20)30317-2.
- Wu C , Chen X , Cai Y , et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med . 2020; e200994. doi:10.1001/jamainternmed.2020.0994.
- Uccelli A , de Rosbo NK. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci . 2015;1351(1):114–126. doi:10.1111/nyas.12815.
- Golchin A , Seyedjafari E , Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep . 2020;16(3):427–433. doi:10.1007/s12015-020-09973-w.
- Lee JW , Fang X , Krasnodembskaya A , Howard JP , Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells . 2011;29(6):913–919. doi:10.1002/stem.643.
- Shanmugaraj B , Siriwattananon K , Wangkanont K , Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol . 2020;38(1):10–18. doi:10.12932/AP-200220-0773.
- Cockrell AS , Yount BL , Scobey T , et al. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat Microbiol . 2016;2(2):16226. doi:10.1038/nmicrobiol.2016.226.
- Han H , Yang L , Liu R , et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med . 2020. doi:10.1515/cclm-2020-0188.
- Tang N , Li D , Wang X , Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost . 2020;18(4):844–847. doi:10.1111/jth.14768.
- Guo T , Fan Y , Chen M , et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol . 2020. doi:10.1001/jamacardio.2020.1017.
- Bikdeli B , Madhavan MV , Jimenez D , et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, anti-thrombotic therapy, and follow-up. J Am Coll Cardiol . 2020;75(23):2950–2973.
- Gold JAW , Wong KK , Szablewski CM , et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19—Georgia, March 2020. MMWR Morb Mortal Wkly Rep . 2020;69(18):545–550. doi:10.15585/mmwr.mm6918e1.
- Rello J , Storti E , Belliato M , Serrano R. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J . 2020;55(5):2001028. doi:10.1183/13993003.01028-2020.
- Siddiqi HK , Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant . 2020;39(5):405–407. doi:10.1016/j.healun.2020.03.012.
- Gilead Sciences . Gilead announces results from Phase 3 trial of remdesivir in patients with moderate COVID-19. https://www.gilead.com/news-and-press/press-room/press-releases/2020/6/gilead-announces-results-from-phase-3-trial-of-remdesivir-in-patients-with-moderate-covid-19. Published June 1, 2020. Accessed June 5, 2020.
- Faure E , Poissy J , Goffard A , et al. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One . 2014;9(2):e88716. doi:10.1371/journal.pone.0088716.
