Abstract
Thrombotic complications such as venous thromboembolism, ischemic stroke, and myocardial infarction have emerged as causes of significant morbidity and mortality in patients infected with COVID-19. We present a 32-year-old man who developed a large saddle pulmonary embolus secondary to COVID-19 infection and underwent successful bilateral percutaneous pulmonary artery mechanical thrombectomy.
Fever, cough, dyspnea, and bilateral infiltrates on chest imaging are the most common findings of COVID-19. Serious complications include acute respiratory distress syndrome, acute cardiac injury, shock, inflammatory response, and thromboembolic events. The incidence of pulmonary embolus (PE) with COVID-19 is estimated at around 22%.Citation 1–3 Studies previously indicated that PE was associated only with severe COVID-19 and intensive care unit admissions, but newer studies are suggesting the development of PE even in mild infections.Citation 1 , Citation 2 With this case report, we aim to sensitize physicians to the risk of deep vein thrombosis and PE in COVID-19 patients.
CASE PRESENTATION
A 32-year-old man presented with chest pain and hemoptysis for 2 days. He had been diagnosed with COVID-19 3 days earlier when he visited his primary care doctor with symptoms of malaise, fatigue, and dyspnea of 10 days’ duration. On arrival, his heart rate was 130 beats/minute; respiratory rate, 28 breaths/minute; and oxygen saturation, 92% on room air. He appeared to be in respiratory distress. His D-dimer was >20 µg/mL. On the electrocardiogram, sinus tachycardia was noted (), and a chest radiograph demonstrated a left lower lobe infiltrate. Computed tomography pulmonary angiography revealed a large saddle PE with extension into segmental branches of the lower lobes bilaterally and the right middle lobe with infarct of the left lung base posteriorly; the right-to-left ventricular size ratio was 1.2, suggestive of right heart strain (). Echocardiography showed right ventricular dilation and dysfunction, although troponin and brain natriuretic peptide levels were normal. Doppler ultrasound of the bilateral lower extremities was negative for acute lower-extremity deep venous thrombosis.
Figure 1. The patient’s electrocardiogram on presentation, showing normal sinus rhythm with sinus tachycardia.
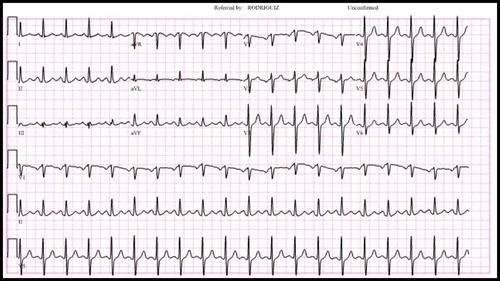
Figure 2. CT pulmonary angiography showing (a) a large saddle embolus and (b) extension of the saddle pulmonary embolus. (c) Pulmonary angiogram revealing lack of blood flow. (d) Restoration of blood flow in the pulmonary circuit after mechanical thrombectomy.
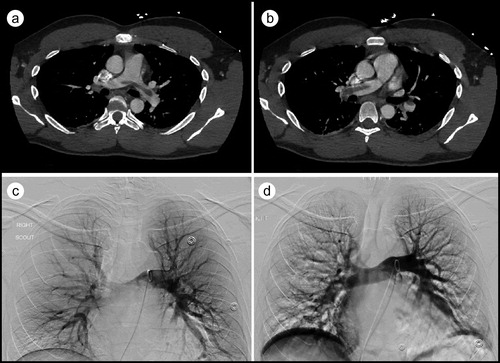
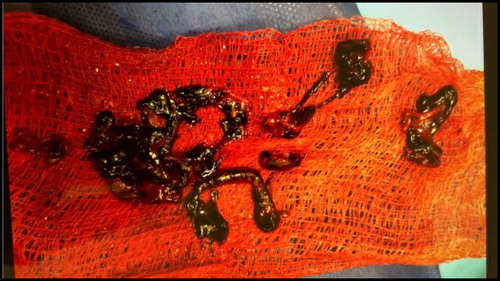
The patient was started on an intravenous infusion of unfractionated heparin and underwent percutaneous mechanical thrombectomy of the right interlobar, truncus anterior, and left interlobar pulmonary arteries using a 20 French Flowtriever mechanical thrombectomy catheter (Inari Medical, Irvine, CA). A large clot burden was removed (), and subsequent pulmonary arteriogram demonstrated resolution of the filling defects (). Pulmonary artery systolic pressures were noted to be elevated to 32 mm Hg before the procedure and dropped to 22 mm Hg afterward. The patient’s hemoglobin was 14.6 g/dL and 11.6 g/dL before and after the procedure, respectively. Following the thrombectomy, the patient remained stable and was subsequently transitioned to rivaroxaban. He was discharged 3 days later, with instructions to take rivaroxaban for at least 6 months.
DISCUSSION
A significant proportion of COVID-19–infected individuals develop a hypercoagulable state. The postulated mechanisms include the direct effects of the virus on endothelial cells, prolonged hypoxia, severe inflammation, cytokine release, critical illness, and immobilization, leading to venous stasis and endothelial injury.Citation 4–8 Elevated fibrinogen levels, elevated D-dimer, prolonged prothrombin time, and the international normalized ratio in COVID-19 disease suggest a coagulopathic process, but the exact mechanism remains uncertain.Citation 6 , Citation 7 Our patient did not have any of the classic risk factors for an acute PE except for obesity, with a body mass index of 29 kg/m2; thus, his PE was attributed to the systemic inflammation caused by COVID-19 infection.
The diagnosis of PE can be challenging in COVID-19 patients because of overlapping clinical signs and symptoms and the inability to obtain timely imaging studies due to the risk of infection transmission. Also, an elevated D-dimer level is common in COVID-19 patients and does not necessarily warrant investigation for PE in the absence of clinical suspicion.
The treatment of PE with concomitant COVID-19 is essentially the same as that in patients without COVID-19. Thrombolysis is usually the treatment of choice for hemodynamically unstable acute PE. Catheter-directed therapy and surgical embolectomy can be used in submassive and massive PE in selected patients who are not candidates for systemic thrombolysis. Even though our patient’s PE severity index score was low, we performed mechanical thrombectomy because it can reliably remove the thrombi in one session without requiring interruption of systemic anticoagulation therapy, thereby minimizing transport and recurrent exposures of health care professionals to an infected patient.
- Poyiadji N , Cormier P , Patel PY , et al. Acute pulmonary embolism and COVID-19. Radiology . 2020;201955. doi:10.1148/radiol.2020201955.
- Grillet F , Behr J , Calame P , Aubry S , Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology . 2020;201544. doi:10.1148/radiol.2020201544.
- Leonard-Lorant I , Delabranche X , Severac F , et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology . 2020;201561. doi:10.1148/radiol.2020201561.
- Klok FA , Kruip M , van der Meer NJM , et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res . 2020;191:148–150. doi:10.1016/j.thromres.2020.04.041.
- Bikdeli B , Madhavan MV , Jimenez D , et al ; Global COVID-19 Thrombosis Collaborative Group . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol . 2020;75(23):2950–2973. doi:10.1016/j.jacc.2020.04.031.
- Panigada M , Bottino N , Tagliabue P , et al. Hypercoagulability of COVID-19 patients in intensive care unit: areport of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost . 2020;18(7):1738–1111. doi:10.1111/jth.14850.
- Ranucci M , Ballotta A , Di Dedda U , et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost . 2020;18(7):1747–1751. 10.1111/jth.14854. doi:10.1111/jth.14854.
- Tang N , Li D , Wang X , Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost . 2020;18(4):844–847. doi:10.1111/jth.14768.