Abstract
Limited data exist on asthma and chronic obstructive pulmonary disease (COPD) management—major drivers of healthcare resource utilization (HCRU) in the USA. We describe prevalence and exacerbation rates, therapeutic interventions, and HCRU for asthma and/or COPD within a large, integrated healthcare system. Patients with asthma, COPD, and asthma + COPD were identified from retrospective electronic health record data (2016–2018) of >1.7 million patients. Descriptive analysis of disease prevalence and exacerbation frequencies, pharmacotherapies, and HCRU was performed. Time-to-event analysis of time to first exacerbation was performed in patients with asthma and/or COPD. Exacerbation rates, pharmacotherapies, and HCRU were examined by exploratory analysis in an outpatient subset. Overall, 149,086 unique patients (8.6%) had encounters for asthma, COPD, or asthma + COPD. Acute care utilization was high, including emergency department visits (asthma, 52.9%; COPD, 35.1%) and hospitalizations (asthma, 26.7%; COPD, 65.7%). Many patients were prescribed short-acting therapies (asthma, 45.3%; COPD, 40.0%; asthma + COPD, 54.7%). Prescription rates for maintenance therapies were low (17.1%, 20.8%, 31.7%) and annual exacerbation rates were 0.65, 0.80, and 1.33. This analysis showed a substantive prevalence of pulmonary disease, variability between documented prescriptions and pharmacotherapy guidelines, and high HCRU. Appropriate tailoring of pharmacotherapies and management of asthma and COPD over a continuum are opportunities to improve patient care.
The prevalence of asthma and chronic obstructive pulmonary disease (COPD) is increasing in the US and is an important source of healthcare resource utilization (HCRU) and costs.Citation1–6 Currently, in the US, 19.2 million adults have been diagnosed with asthma and 12.8 million adults have been diagnosed with COPD.Citation7,Citation8 These chronic lower respiratory diseases were the sixth leading cause of death in the US in 2020Citation9 and result in over 18 million emergency department (ED) and physician visits each year.Citation7,Citation8 The direct discounted medical cost from 2019 to 2038 attributable to COPD in the US is estimated to be $800.9 billion (95% credible interval: $565.29–$1081.29 billion).Citation10 New population-level approaches are needed to help patients better manage these chronic conditions and avoid unnecessary HCRU. However, there is limited availability of pharmacoepidemiological data related to the incidence, prevalence, and current treatment of asthma and COPD within large populations that can be used to develop strategies to improve care and health outcomes for patients with these diseases.Citation11–13 The objectives of this study were to delineate the real-world period prevalence of asthma and COPD and describe exacerbations, pharmacoepidemiology, and associated HCRU for these conditions within Baylor Scott & White Health (BSWH), a large, integrated healthcare delivery system in North/Central Texas.
METHODS
This retrospective observational cohort study examined the prevalence of asthma and COPD or a concomitant diagnosis of asthma and COPD as well as outcomes and trends in disease management and HCRU in patients receiving care within BSWH. BSWH currently includes 52 owned, operated, joint-ventured, and affiliated hospitals with >5000 licensed beds, >800 patient care sites with >7 million patient encounters annually, and >7000 affiliated physicians. This study was approved by the BSWH Research Institute Institutional Review Board (#019-132) under a waiver of informed consent.
The overall study population included patients with ≥1 BSWH facility encounter (office visit to primary care/pulmonologist/allergist, ED visit, or hospital stay) between January 1, 2016, and December 31, 2018, who were ≥6 years of age with a diagnosis of asthma (asthma cohort) or ≥40 years of age with a diagnosis of COPD. Patients were subdivided into cohorts based on diagnosis (asthma, COPD, or asthma + COPD for patients with a concomitant diagnosis of asthma and COPD). Diagnoses were determined based on the presence of the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) codes for asthma (J45.X) and COPD (J41.X, J42.X, and J44.X).
Patients were considered to have asthma or COPD if they had ≥1 inpatient/ED admission with a primary diagnosis of asthma or COPD and/or ≥1 outpatient service date with a diagnosis of asthma or COPD at any point. Patients with diagnoses of both asthma and COPD at any point during the study period were classified as having a concomitant diagnosis of asthma and COPD; the individual diagnosis could occur during the same or separate BSWH visits.
Demographic data included age, sex, race, ethnicity, Elixhauser Comorbidity Index score, insurance provider, smoking status, and number of BSWH encounters. Medication classes included inhaled corticosteroids (ICS), long-acting muscarinic antagonists (LAMAs), long-acting beta2-agonists (LABAs), short-acting muscarinic antagonists, short-acting beta2-agonists (SABAs), methylxanthines, oral/intravenous corticosteroids, phosphodiesterase type 4 inhibitors, biologics, leukotrienes, and antibiotics. The index date for an asthma and/or COPD diagnosis was the earliest inpatient admission date, ED date, or outpatient service date with a corresponding diagnosis code during the study period.
Data were extracted from the electronic health records (EHRs) in place during the study period at BSWH wholly owned hospitals (ED visits and inpatient stays) and BSWH outpatient practices (employed or affiliated physicians using the system’s EHR) as well as administrative databases. Data elements included patient demographics; pulmonary pharmacotherapy (by medication class); patient outcomes, including rates of exacerbation, pneumonia diagnosis, and inpatient mortality; and HCRU.
Since asthma and COPD exacerbations are commonly defined as a worsening of respiratory symptoms that leads to a change in medications, exacerbations in this study were defined as an order entry within the BSWH EHR of oral or intravenous corticosteroids and/or antibiotic therapy administered in any BSWH setting, followed by an outpatient visit and/or related ED visits or inpatient admissions.
HCRU included primary care provider (PCP), pulmonologist, or allergist visits and ED visits; hospitalizations; intensive care unit (ICU) utilization; nebulizer/inhaler or ventilator use; and 30- and 90-day all-cause readmissions and only diagnosis-related readmissions. Episodes were measured from the diagnosis index date until a patient was censored following the last documented BSWH visit during the study timeframe. Episodes encompassed all exacerbation events until a 14-day clean period occurred (i.e., if two or more events occurred <14 days apart, they were considered part of the same exacerbation episode). The end of the episode was determined by the service date of an office/ED visit or discharge date of an inpatient stay, whichever was latest. Exacerbation episodes were classified according to the most severe event during the episode. A severe exacerbation was defined as a hospital inpatient admission with an asthma or COPD diagnosis in the primary position. A moderate exacerbation was defined as an ED visit with a primary diagnosis of asthma or COPD and an entry of an oral corticosteroid or antibiotic within 7 days of the date of an office visit with a diagnosis of asthma or COPD in any position.
Readmissions were classified as “diagnosis related” if an ICD-10 code for asthma or COPD was listed as the primary or secondary diagnosis code for patients with a hospital readmission within 30 or 90 days having an existing diagnosis of asthma or COPD, respectively.
Descriptive statistics were used to characterize the patient population with asthma, COPD, and asthma + COPD; quantify disease prevalence and exacerbations; assess pharmacotherapies; and examine HCRU. Categorical data were analyzed as frequencies and percentages, and continuous data were analyzed as means with standard deviations. Annual exacerbation rates were calculated by dividing the total number of exacerbations by the total observation time (days)/365. Survival analysis was used to assess time to first exacerbation only in patients with either asthma or COPD. Time to first exacerbation from the index date was reported in days and plotted as a Kaplan-Meier curve for each cohort. Patients were considered censored as of the last visit within BSWH before January 1, 2019, if they did not experience an exacerbation during the study period. Patients with only one visit within BSWH (on the index date) were excluded from the exacerbation analysis, as it was more likely that the lack of observed exacerbations for these patients may have been due to a change in provider. An exploratory analysis was conducted to examine exacerbation rates, pharmacotherapies, and HCRU in patients who visited BSWH providers at an outpatient clinic, and findings for patients with ≥1 outpatient visit are reported. Data were analyzed using SAS statistical software (SAS Institute Inc.), and survival estimates were calculated using PROC LIFETEST.
RESULTS
Approximately 1.7 million unique patients had a visit (for any reason) captured in the EHR at the BSWH hospital and outpatient practice sites used in the study during 2016 to 2018. Approximately 9% of this BSWH patient population had ≥1 inpatient, ED, or outpatient visit to a BSWH facility with a primary diagnosis of asthma (n = 90,569), COPD (n = 48,302), or asthma + COPD (n = 10,215) during the study (). The period prevalence of these diseases among the ∼1.7 million unique BSWH patients during the study was 5.2% for asthma, 2.8% for COPD, and 0.6% for asthma + COPD. Patients with asthma were younger, more frequently of a nonwhite race, and more likely to be of Hispanic ethnicity than patients with COPD and asthma + COPD. The mean number of comorbidities (Elixhauser Comorbidity Index score) in hospitalized study patients was 3.3 for patients with asthma and 4.3 each for patients with COPD and asthma + COPD. Most patients with asthma had commercial insurance. Most patients with COPD and asthma + COPD were covered by Medicare (61.8% and 58.5%, respectively) or had commercial insurance (22.6% and 24.9%, respectively). Smoking status was not recorded in a structured EHR data field (and was thus unknown) for almost half of the study population. Most patients with asthma (58.2%) or COPD (51.7%) had only one encounter at a BSWH facility during the 3-year study period, while the majority of patients with asthma + COPD (67.5%) had ≥3 encounters.
Table 1. Patient demographics and baseline characteristics
In the exploratory outpatient subanalysis, 47,805 patients had ≥1 visit to a PCP or pulmonologist/allergist captured within the BSWH EHR with a primary diagnosis of asthma (n = 31,494), COPD (n = 12,301), or asthma + COPD (n = 4010; ). Within this subanalysis group, the number of patients whose documented contact with an outpatient PCP or pulmonologist/allergist comprised only 1 visit over the 3-year study period was 15,288 (48.5%), 3727 (30.3%), and 496 (12.4%) for the asthma, COPD, and asthma + COPD cohorts, respectively. The demographics of the outpatient population were similar to those of the overall study population. A higher percentage of patients (∼12%–15%) with COPD or asthma + COPD were covered by Medicare in the outpatient population vs the overall population. Most patients with asthma were never smokers, while the majority of patients with COPD and asthma + COPD in the outpatient population were former smokers.
Differences in documented pharmacotherapies were observed in the outpatient population. The percentage of patients with documented prescriptions of SABAs and/or short-acting muscarinic antagonists within 7 days of their index encounter was higher for patients with asthma, COPD, and asthma + COPD who had ≥1 outpatient visit vs the overall study population (). Prescription rates for maintenance therapies were also higher in the outpatient subset. Likewise, a larger percentage of the outpatient population had documented prescriptions for systemic corticosteroids vs the overall study population.
Table 2. Pharmacotherapy at baseline
Approximately 33% each of patients with asthma and COPD and 49% of patients with asthma + COPD who had ≥2 encounters during the study period had a documented exacerbation (). Exacerbations were more likely to be severe for patients with COPD and asthma + COPD than for patients with asthma. The annual rate of exacerbations was similar for patients with asthma and COPD (0.65 and 0.80 episodes/year, respectively) and higher for patients with asthma + COPD (1.33 episodes/year). The estimated median time to first exacerbation from an index encounter was 690 days (95% confidence interval [CI], 679–700 days) and 654 days (95% CI, 640–670 days) for patients with asthma and COPD, respectively. The estimated probability of patients not having an exacerbation after 900 days was <35% in both cohorts (). Patients with ≥1 outpatient visit (and ≥2 BSWH encounters) were more likely to have a documented exacerbation vs the overall population; however, exacerbations were less likely to be severe.
Figure 1. Kaplan-Meier estimates of the probability of no exacerbation in patients with asthma or chronic obstructive pulmonary disease (COPD) only and ≥2 visits. The number of patients at risk is displayed for each time point. Patients with asthma and COPD were not included in the analysis.
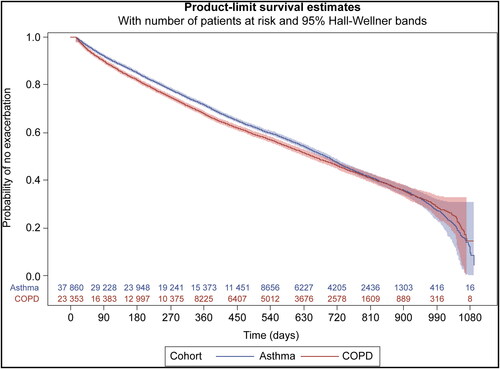
Table 3. Summary of exacerbations
HCRU varied between patients with asthma and COPD (). A higher percentage of patients with asthma had at least one primary care visit in the overall population, but patients with COPD were more likely to have a visit to a pulmonary specialist. Patients with asthma were more likely to have an ED visit than patients with COPD (52.9% vs 35.1%) but less likely to be hospitalized (26.7% vs 65.7%). ICU utilization was much higher for patients with COPD vs patients with asthma. Ventilator, inhaler, or nebulizer use was lower for patients with asthma vs patients with COPD. A higher percentage of patients with COPD had pneumonia and inpatient mortality compared with those with asthma or asthma + COPD. All-cause readmission rates were higher for patients with COPD at 30 and 90 days than for patients with asthma or asthma + COPD, as were diagnosis-related readmissions.
Table 4. Healthcare resource utilization
Total captured HCRU was higher in the outpatient subset population vs the overall population. However, as indicated in , compared with the overall study population, use of higher-acuity care (ED utilization, hospitalizations, and ICU utilization) and inpatient mortality were lower for patients with asthma and COPD followed up in BSWH outpatient clinics. All-cause readmission rates to BSWH hospitals at 30 and 90 days were higher for patients with COPD in the outpatient population vs the overall study population.
DISCUSSION
This analysis of an EHR-derived dataset from a large, integrated healthcare system indicates a high prevalence of pulmonary disease (∼9%) among patients and provides insights into potential care improvement opportunities.
Less than one-third of the patients had documented outpatient encounters for their pulmonary condition. Although total visit frequency (all inpatient + outpatient visits) was higher in this outpatient subset, utilization of acute care (ED visits + inpatient stays) was almost twofold lower. Furthermore, recorded use of maintenance pharmacotherapies was more frequent in this population. While some patients may have received outpatient pulmonary care from providers not using the BSWH EHR, our study data suggest the importance and benefit of connecting individuals with acute pulmonary disease visits to PCPs and pulmonary specialists for management across a continuum of care. Early engagement of patients and healthcare providers and clear communication to target barriers may facilitate care transitions between the ED and the primary care setting for acute asthmaCitation13 and COPD.Citation14
Approximately half of the overall study population did not have a documented smoking status, compared with <2% of patients with a documented BSWH outpatient visit. Prompts within the BSWH outpatient EHR may be responsible for this higher documentation rate.Citation15 Awareness of smoking status and facilitation of smoking cessation are essential for the management of asthma and COPD,Citation16,Citation17 as smoking is associated with a higher prevalence of pulmonary symptoms and greater mortality in patients with COPD.Citation18,Citation19 The Global Initiative for Asthma (GINA) report indicates that smoking cessation advice should be given to the patient at every visit.Citation17 Studies have shown that counseling delivered by clinicians significantly increases quit rates.Citation20
Among patients with documented outpatient clinic encounters, 21.5% of the individuals with asthma had a captured encounter with an allergist or pulmonologist, whereas 48.7% of those with COPD had a captured subspecialist encounter; these findings are in accord with those of two other recent studies.Citation21,Citation22
Although we did not have the ability to determine GOLD scores and assess medication appropriateness for individual patients, we believe that opportunities exist to improve pharmacotherapy as part of a disease management program. The 2017 GOLD recommendations suggest that almost all patients with COPD experiencing more than occasional dyspnea should be prescribed long-acting bronchodilator therapy with a LABA, LAMA, or both.Citation23 Studies have reported population-level variance between pharmacotherapies and clinical guidelines.Citation22,Citation24–28
For patients with asthma, observed pharmacotherapy prescribing patterns showed significant rates of short-acting therapies being used alone without ICS. The 2017 GINA reportCitation17 (the version in effect during the study) recommended SABAs alone or with an ICS for patients with mild asthma, and ICS use rates observed in our dataset are within the range of the rates reported in other studies (10.9%Citation24 to 37%).Citation29 Of note, several recent large clinical studies found no increase in serious asthma-related side effects in patients using ICS/LABA compared with those using ICS monotherapy,Citation30–32 prompting a recommendation in the 2019 GINA update to use low-dose ICS/LABA combination therapy as needed for all therapeutic steps for breakthrough asthma symptoms in lieu of a SABA.Citation17 The evolution of management guidelines for pulmonary disease highlights the need to regularly revisit provider and patient knowledge surrounding therapeutic options and to pair education with other strategies that facilitate the adoption of evidence-based practices.
This retrospective study has several limitations, as data were entered during the provision of routine care within the BSWH EHR, and given the population size, we could only retrieve information from structured data fields. Data may be missing, particularly information contained in narrative, unstructured fields, and follow-up may be incomplete for some patients; for example, discernment of smoking status was not possible for 50% of patients. We were unable to capture HCRU occurring outside of the BSWH EHR; thus, utilization rates for both inpatient and outpatient care are likely underestimated. Included hospital and ED HCRU following the qualifying index visit were not limited to COPD and asthma as primary admitting diagnoses and may have been for nonpulmonary issues. The only captured care encounter for many patients was in a BSWH ED, and documentation regarding medications and HCRU for these patients may have been limited. We were unable to determine disease onset for all patients, as COPD or asthma may have been present before a visit to a BSWH facility with a relevant diagnosis code. Additionally, the results reflect structured data capturable in an electronic format from one healthcare system and may not be generalizable to other settings. Inferential statistics were not performed owing to the descriptive and observational nature of this study, limiting our ability to draw conclusions from comparisons between the general and outpatient populations.
We view our findings as a basis for prioritizing specific care redesign initiatives for both patients and providers, including wider education about asthma and COPD and the development of approaches for better management of these conditions over a continuum. While this was an observational study with many limitations, it provides an overview of pulmonary care within a healthcare system that can be used to formulate quality improvement initiatives in both the inpatient and outpatient settings.
Acknowledgments
The authors thank Jami Weiner, MSc, CCRP, for providing project management support. Editorial support and formatting assistance were provided by Suchita Nath-Sain, PhD, of Cactus Life Sciences (part of Cactus Communications), which was contracted and compensated by Boehringer Ingelheim Pharmaceuticals, Inc. for these services.
Additional information
Funding
- Centers for Disease Control and Prevention. COPD death rates in the United States. https://www.cdc.gov/copd/data.html. Accessed April 16, 2021.
- Centers for Disease Control and Prevention. Asthma surveillance data. https://www.cdc.gov/asthma/asthmadata.htm. Accessed April 16, 2021.
- Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi:10.1378/chest.14-0972.
- Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348–356. doi:10.1513/AnnalsATS.201703-259OC.
- Wallace AE, Kaila S, Bayer V, et al. Health care resource utilization and exacerbation rates in patients with COPD stratified by disease severity in a commercially insured population. J Manag Care Spec Pharm. 2019;25(2):205–217. doi:10.18553/jmcp.2019.25.2.205.
- Yaghoubi M, Adibi A, Safari A, FitzGerald JM, Sadatsafavi M. The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med. 2019;200(9):1102–1112. doi:10.1164/rccm.201901-0016OC.
- Centers for Disease Control and Prevention. Faststats asthma. https://www.cdc.gov/nchs/fastats/asthma.htm. Accessed April 16, 2020.
- Centers for Disease Control and Prevention. Faststats COPD. https://www.cdc.gov/nchs/fastats/copd.htm. Accessed April 16, 2020.
- Murphy SL, Kochanek KD, Xu J, Arias E. Mortality in the United States, 2020. NCHS Data Brief, no 427. Hyattsville, MD: National Center for Health Statistics; 2021. https://www.cdc.gov/nchs/products/databriefs/db427.htm.
- Zafari Z, Li S, Eakin MN, Bellanger M, Reed RM. Projecting long-term health and economic burden of COPD in the United States. Chest. 2021;159(4):1400–1410. doi:10.1016/j.chest.2020.09.255.
- Centers for Disease Control and Prevention. COPD costs. https://www.cdc.gov/copd/infographics/copd-costs.html. Accessed April 16, 2020.
- Lee TM, Tu K, Wing LL, Gershon AS. Identifying individuals with physician-diagnosed chronic obstructive pulmonary disease in primary care electronic medical records: a retrospective chart abstraction study. NPJ Prim Care Respir Med. 2017;27(1):34. doi:10.1038/s41533-017-0035-9.
- Villa-Roel C, Ospina M, Majumdar SR, et al. Engaging patients and primary care providers in the design of novel opinion leader based interventions for acute asthma in the emergency department: a mixed methods study. BMC Health Serv Res. 2018;18(1):789. doi:10.1186/s12913-018-3587-7.
- Michas M, Deuchar L, Leigh R, et al; COPD PRIHS-2 Group. Factors influencing the implementation and uptake of a discharge care bundle for patients with acute exacerbation of chronic obstructive pulmonary disease: a qualitative focus group study. Implement Sci Commun. 2020;1:3. doi:10.1186/s43058-020-00017-5.
- Millard M, Priest E, Cole L, Mularski R, Hazlehurst B, Masica A. Impact of an EMR documentation template as clinical decision support for outpatient asthma management. Chest. 2013;144(4):68A. doi:10.1378/chest.1703596.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020. https://goldcopd.org/. Accessed April 18, 2020.
- Global Initiative for Asthma. Global strategy for asthma management and prevention, 2020. https://ginasthma.org/. Accessed April 17, 2020.
- Liu Y, Pleasants RA, Croft JB, et al. Smoking duration, respiratory symptoms, and COPD in adults aged ≥45 years with a smoking history. COPD. 2015;10:1409–1416. doi:10.2147/COPD.S82259.
- Lou P, Chen P, Zhang P, et al. Effects of smoking, depression, and anxiety on mortality in COPD patients: a prospective study. Respir Care. 2014;59(1):54–61. doi: 10.4187/respcare.0248.
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;2013(5):CD000165. doi:10.1002/14651858.CD000165.pub4.
- Price C, Agarwal G, Chan D, et al. Large care gaps in primary care management of asthma: a longitudinal practice audit. BMJ Open. 2019;9(1):e022506. doi:10.1136/bmjopen-2018-022506.
- Ragaišienė G, Kibarskytė R, Gauronskaitė R, et al. Diagnosing COPD in primary care: what has real life practice got to do with guidelines? Multidiscip Respir Med. 2019;14:28. doi:10.1186/s40248-019-0191-6.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP.
- Baldacci S, Simoni M, Maio S, et al; ARGA Collaborative Group. Prescriptive adherence to GINA guidelines and asthma control: an Italian cross sectional study in general practice. Respir Med. 2019;146:10–17. doi:10.1016/j.rmed.2018.11.001.
- Chinai B, Hunter K, Roy S. Outpatient management of chronic obstructive pulmonary disease: physician adherence to the 2017 Global Initiative for Chronic Obstructive Lung Disease guidelines and its effect on patient outcomes. J Clin Med Res. 2019;11(8):556–562. doi:10.14740/jocmr3888.
- Ding B, Small M, Holmgren U. A cross-sectional survey of current treatment and symptom burden of patients with COPD consulting for routine care according to GOLD 2014 classifications. Int J Chron Obstruct Pulmon Dis. 2017;12:1527–1537. doi:10.2147/COPD.S133793.
- Surani S, Aiyer A, Eikermann S, et al. Adoption and adherence to chronic obstructive pulmonary disease GOLD guidelines in a primary care setting. SAGE Open Med. 2019;7:2050312119842221. doi:10.1177/2050312119842221.
- Weber H, Bassett G, Bartl D, et al. Successful implementation of evidence-based guidelines in a regional emergency department for children presenting with acute asthma. Aust J Rural Health. 2019;27(6):557–562. doi:10.1111/ajr.12544.
- Watson L, Kerstjens HA, Rabe KF, Kiri V, Visick GT, Postma DS. Obtaining optimal control in mild asthma: theory and practice. Fam Pract. 2005;22(3):305–310. doi:10.1093/fampra/cmi013.
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev. 2010;5:CD005535. doi:10.1002/14651858.CD005535.pub2.
- Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respir Med. 2013;1(1):23–31. doi:10.1016/S2213-2600(13)70012-2.
- Patel M, Pilcher J, Pritchard A, et al; SMART Study Group. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med. 2013;1(1):32–42. doi:10.1016/S2213-2600(13)70007-9.
