Abstract
We tested the hypothesis that the fungivorous nematodes Aphelenchoides spp. and Aphelenchus avenae can suppress damping-off caused by Rhizoctonia solani in cauliflower seedlings, and enhance the disease-suppressive effect of compost. In greenhouse experiments, we used two different composts mixed with peat (20% + 80%) and pure peat as growth substrates in growing pots. In each substrate, treatments were: (A) with R. solani and nematodes, (B) with R. solani, (C) with nematodes, (D) control without R. solani or nematodes. Treatment effects were measured as percentage of healthy seedlings 7, 10 and 14 days after start of the experiment. We conducted two different experiments with the treatments A–D; one with Aphelenchoides spp. and one with Aphelenchus avenae. Aphelenchoides spp.+R. solani (treatment A) had 85% healthy plants (= control without addition of fungi (D)) compared with 45% in R. solani without nematodes (B). Aphelenchus avenae suppressed damping-off significantly in all substrates, from almost 100% dead plants in peat with R. solani to 65% healthy plants in R. solani+A. avenae. One compost mixture had an intrinsic suppressive effect on damping-off, while plant health in the other compost mixture was not better than in 100% peat as growing substrate. There were no additive suppressive effects (enhancement) between nematode effects and the suppressive compost. The results demonstrate the ability of fungivorous nematodes to suppress plant diseases. The effects of fungivorous nematodes in combination with compost and other control measures on disease suppression need further attention. The usefulness of fungivorous nematodes in agriculture and horticulture is discussed.
Introduction
The potential of fungivorous nematodes to reduce plant disease and thereby improve plant health has been demonstrated in several studies. The first systematic study was performed by Rhoades & Linford (Citation1959), who found that Aphelenchus avenae Bastian was efficient in controlling Pythium root rot in maize (Zea mays L.) under greenhouse conditions. Since then A. avenae and to some extent also the fungivorous species in the genera Aphelenchoides and Ditylenchus have been tested in lab, greenhouse and field experiment to control a range of plant pathogenic fungi in crops (Friberg et al., Citation2005). Fungi used in these experiments include Rhizoctonia solani and Fusarium spp. Fungivorous nematodes have a mouth stylet by which they penetrate fungal cells and ingest the cell contents, thereby damaging the mycelium. Population densities of fungivorous nematodes in soil are usually lower than those of bacterivorous or phytoparasitic nematodes (Freckman & Caswell, Citation1985) but in the presence of good fungal hosts, their populations may increase several-fold within a short time period (Hoffman & S’-Jacob, Citation1989; Arancon et al., Citation2003).
The disease-suppressive effect of compost added to growth substrates has gained much interest in recent years (Hoitink & Boehm, Citation1999; Noble & Coventry, Citation2005). The underlying mechanisms seem to involve both chemical and biological processes. In a broad overview of disease suppression involving 18 composts and 7 pathosystems, disease suppression was found in 54% of the cases and some of the disease-suppressive composts contained high densities of fungivorous nematodes. When disease suppressiveness was tested using redundancy analysis (RDA) with the compost/peat mixture, 31% of the variation was explained by the density of nematodes (Termorshuizen et al., Citation2006). If the disease suppression by compost is caused by antagonistic fungi, such as Trichoderma spp., the presence of fungivorous nematodes in the compost could counteract the suppression effect, if the nematodes feed on these fungi. If the antagonists are non-hosts the addition of fungivorous nematodes could add to the disease suppression effect of the compost. The combined effect of disease suppressive compost and fungivorous nematodes have been studied earlier only in a few cases (e.g. Bae & Knudsenm 2001; Hasna et al., Citation2008).
In the present paper, we report the results of greenhouse experiments, in which we investigated the effects of fungivorous nematode species on damping-off caused by Rhizoctonia solani in cauliflower. The growth substrate consisted of pure peat or two different disease-suppressive composts made from garden waste and/or wood mixed with peat. Our hypothesis was that the fungivorous nematodes would have a disease-suppressive effect and enhance the disease-suppressive effect of the composts.
Materials and methods
The effects of fungivorous nematodes on damping-off of cauliflower (Brassica oleracea L. var botrytis cv. Menovi) in soil infested with Rhizoctonia solani were tested in peat as growing substrate and in mixtures of compost and peat. The composts used (referred to here as m-compost and q-compost) were obtained from the Netherlands. By volume, the m-compost consisted of wood chips (88%), manure (2.5%) and clay (10%), while the q-compost consisted of garden waste. Other characteristics of the composts are described in Termorshuizen et al. (Citation2006). Both composts had high abundances of the fungivorous nematodes Ditylenchus sp. and Aphelenchoides spp. In the experiments we also used Aphelenchus avenae, which was isolated from a Swedish potato field.
Aphelenchoides spp. and A. avenae were propagated on the saprophytic fungus Pochonia bulbillosa (W. Gams & Malla) Zare & W. Gams on Malt Extract Agar (Difco Lab., USA) amended by streptomycin and ampicillin, 50 ppm L−1. Nematode extractions were made by Baermann funnel for 24 h (Sohlenius, Citation1979).
The composts and peat were mixed with fertilizer and lime to reach a similar nutritional level for all mixtures. The mixtures were incubated under 70% RH for one week at 20 °C before the bioassays were performed. For each treatment, mixtures of 20% compost with 80% peat (volume basis) were prepared. The two composts contained fungivorous nematodes (58 specimens g−1 d.w. in m-compost and 38 specimens g−1 d.w. in q-compost). Around 10% of these were Aphelenchoides spp. and the remainder belonged to the genus Ditylenchus. Bacterivorous nematodes, predominantly Rhabditida were present with populations of 45 individuals g−1 d.w. in the m-compost mixture, and 10 g−1 d.w. in the q-compost mixture. Composts were kept at 4 °C and periodically analysed for nematodes. No significant variation in the number of fungivorous or bacterivorous nematodes was found during the storage period (data not shown).
The experiment combined three substrates (100% peat and the mixtures of 80% peat and 20% m-compost or q-compost) and four treatments (A–D), with eight replicates of each. The following treatments were tested in each substrate:
| A. | With R. solani and nematodes (Aphelenchoides spp. or Aphelenchus avenae). | ||||
| B. | With R. solani. | ||||
| C. | With nematodes (Aphelenchoides spp. or A. avenae). | ||||
| D. | Without R. solani or nematodes (control). | ||||
Two hundred mL of substrate were mixed with 50 mL of water and potted in 200 mL pots. In the pots treated with R. solani, 0.2 g of the fungal inoculum was mixed through the upper half of the potting mix. The pots treated with the nematodes were inoculated with 20000 nematodes per pot suspended in tap water and pipetted into five holes of 3 cm depth distributed over the surface (175 nematodes g−1 f.w. substrate). Sowing and addition of nematodes was done at day one of the experiment.
All treatments were sown with nine seeds of cauliflower in each pot and placed in random order in the greenhouse at 20 °C and 70% air humidity and watered daily. The experiment 1 with Aphelenchoidesspp. was performed nine weeks before the experiment 2 with A. avenae.
Cauliflower seedlings were counted after 7, 10 and 14 days, both as the total number of emerged seedlings and number of damped-off seedlings. For statistical analysis, only the number of non-damped-off (healthy) seedlings was considered.
Statistical analysis
A repeated measure of analysis of variance was performed using the PROC mixed-model in SAS. The model used arcsin-transformed data obtained from three different times to determine whether the various substrates and treatments could suppress fungal plant diseases and whether the suppression was time-dependent (d.f.=287). Fisher's protected least significant difference (LSD) was used for comparing treatment means of healthy seedlings over time (p < 0.05).
Results
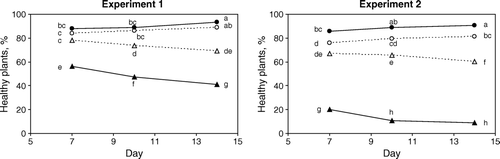
There was a strong treatment effect in both experiments 1 (with addition of Aphelenchoides spp.) and 2 (with addition of Aphelenchus avenae) (: p < 0.001). In the overall treatment effect, inoculation with R. solani decreased the number of healthy plants, and in experiment 2, the disease incidence was higher than in experiment 1. The damping-off effect increased over time in both experiments, in treatments with and without nematodes (; ). After 14 days, in experiment 2, treatment with R. solani but without nematode addition, 97% of seedlings were dead in the q-compost mixture and 99% in peat alone. In experiment 1, the fungus was less virulent with c. 40% live plants after 14 days on average in the R. solani only treatment.
Table I. Analysis of variance of a three-factorial pot experiment with cauliflower (Brassica oleracea) seedlings infected with Rhizoctonia solani in three substrates and inoculated with two fungivorous nematodes: 1 = Aphelenchoides spp.; 2 = Aphelenchus avenae. Effect on proportion of non-wilted plant, n = 8.
Addition of nematodes suppressed the R. solani damping-off effect in both experiments, i.e. the plants stayed healthy to a greater extent when nematodes were added in addition to R. solani as compared with R. solani addition alone. Aphelenchus avenae was a more effective suppressor of the damping-off than Aphelenchoides spp. on average and over the experimental time ( and ). Inoculation with nematodes alone had no effect on the number of healthy plants compared with the control in the Aphelenchoides spp. experiment (experiment 1) but had a weak negative effect in the A. avenae experiment (experiment 2; and : p < 0.05). In all substrates, A. avenae were able to suppress damping off (experiment 2; ). In the Aphelenchoides spp. experiment, the nematodes were able to suppress damping-off significantly only in the 100% peat substrate, while in the q- and m-compost substrates, the nematodes did not improve plant health significantly further (experiment 1; ).
Figure 1. Effect of substrate×treatment on the proportion of healthy cauliflower (Brassica oleracea) seedlings in pot experiments with three substrates inoculated with Rhizoctonia solani and nematodes Aphelenchoides spp. (Experiment 1) or Aphelenchus avenae (Experiment 2), n = 8. Substrates: black bars = q-compost 20% + peat, grey bars = m-compost 20% + peat, white bars = peat 100%. Bars marked with different letters are significantly different (p<0.05).
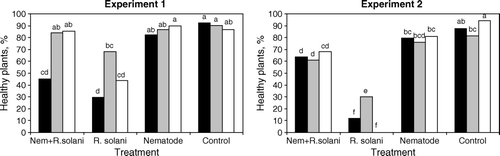
Figure 2. Effect of day×treatment on proportion of healthy cauliflower seedlings after 7, 10 and 14 days, n = 8. Treatments: Δ = substrate inoculated with Rhizoctonia solani and nematodes, ▴ = substrate inoculated with Rhizoctonia solani, ○ = substrate inoculated with nematodes, • = only substrate (Control). Points marked with different letters (both vertical and horizontal comparisons) are significantly different (p<0.05). Nematodes inoculated: Aphelenchoides spp. (Experiment 1) or Aphelenchus avenae (Experiment 2).
The m-compost mixture had an intrinsic suppressive effect on damping-off as compared to 100% peat substrate, which is evident from the result of the R. solani treatment in both experiments. This suppressive effect was significantly different from that of the q-compost mixture in both experiments (), which had no suppressive effect as compared with the pure peat. In experiment 1, mixing peat with q-compost even decreased the number of healthy plants compared with 100% peat. This effect was significant only in the treatment with Aphelenchoides spp. and R. solani ().
Aphelenchus avenae was able to suppress damping-off significantly in all substrates but there was no additive effect (enhancement) to the suppressive effect of m-compost. Aphelenchoides spp. was able to suppress damping-off significantly only in the pure compost substrate () and was not able to enhance the suppressive effect of the m-compost.
Discussion
In agreement with our hypothesis, the fungivorous nematodes were able to improve plant health by suppressing disease in all three tested growing substrates, but the addition of nematodes could not enhance the disease-suppressive effect of the m-compost substrate.
Inoculating once with a high number of nematodes in the seedbed was a good biological control strategy – inundative biological control, i.e. the control is achieved exclusively by the released organisms themselves (Eilenberg et al., Citation2001) – as R. solani was in a stage with actively growing hyphae that could rapidly infect the emerging cauliflower seedlings. The nematodes inoculated with a high density were probably able to consume the mycelium at once, before the hyphae infected the seedlings.
Experiments 1 and 2, with Aphelenchoides spp. and A. avenae respectively, were conducted at different times and this could explain why the virulence of R. solani differed. In the Aphelenchoides experiment, the virulence was lower and in this experiment the m-compost was so disease-suppressive that the plant health in the treatment with R. solani alone was only slightly worse that in the non-inoculated. In experiment 2, the m-compost was suppressive and the 100% peat substrate was highly conducive to the disease. Irrespective of substrate, A. avenae was able to suppress the disease and maintained the number of healthy plants at around 60% compared with a very low level of healthy plants in the fungus-only treatment. That A. avenae was more efficient in controlling damping-off than Aphelenchoides spp. was shown clearly in the q-compost mixture, in which the disease developed fast and the Aphelenchoides spp. could never keep pace.
The difference in nematode efficiency between the species in our experiments may have been due to their different feeding behaviour or differing preference for fungi (Nickle & Mc Intosh, Citation1968). These results are both in agreement with, and contradictory to, the results of in vitro preference and population growth tests undertaken by Hasna et al. (Citation2007), where A. avenae was more attracted to R. solani than was Aphelenchoides spp., although both developed very well on the pathogen. In the prevailing temperature ranges of the experiment (20–26 °C) both nematode species reproduce very well (Hunt, Citation1993; Okada, Citation2006).
The lack of a disease suppressive effect of q-compost is not in agreement with the results of previous experiments, where q-compost suppressed this disease (Termorshuizen et al., Citation2006). The suppressive effect of the composts could have decreased during the storage period. Such a change in the suppressiveness of composts over time was shown by Termorshuizen et al. (Citation2006). Differences in the inoculation methods used in our experiments and others’ may also have been responsible for differences in the results obtained. Rhizoctonia solani was inoculated into substrate that was immediately sown with cauliflower in the present experiment, while Termorshuizen et al. (Citation2006) inoculated one-week old seedlings individually with discs of PDA with actively growing mycelium of R. solani just below the soil surface.
In contradiction to our hypothesis, we did not find that any of the nematodes reinforced the suppressive effect of the compost substrate. Such an effect could be expected if the suppressive effect of the compost was due to fungi antagonistic to the pathogen, such as Trichoderma and if the fungivorous nematodes would prefer the pathogenic fungus before the antagonistic one. However, Trichoderma spp. were present in both composts used in the experiments and in attraction tests on agar plates with T. harzianum (isolated from m-compost) versus R. solani, A. avenae was significantly more attracted to the antagonistic fungus than to the plant pathogenic one, while Aphelenchoides spp. did not show any difference in preference (Hasna et al., Citation2007).
Jun and Kim (Citation2004) tested the control effect of A. avenae and different Trichoderma species, in combination and alone, on damping off of radish caused by Pythium spp. One combination (nematodes plus T. harzianum) showed an enhanced control effect while five other Trichoderma-species in combination with the nematodes gave no additional effect or reduced effect as compared to that of the nematodes or antagonistic fungus being used alone. In contradiction to their hypothesis the T. harzianum was the most preferred among the tested Trichoderma-species as a food source for the nematodes and supported the highest nematode population growth. Their explanation to this unexpected result is that the activity of the nematodes was enhanced by T. harzianum and that other Trichoderma species (on which the nematodes grow less well) had some toxic effects on the nematodes. Hasna et al. (Citation2007) found that T. harzianum had a certain inhibitory effect compared to other fungi (saprophytic or plant pathogenic) used as food on population growth of Aphelenchoides spp. in a growth experiment on agar plates. Bae and Knudsen (2001) found that Aphelenchoides spp. suppressed T. harzianum in soil cultures in the laboratory and suggest that fungivorous nematodes may be a significant biotic constraint on activity of biocontrol fungi in the field.
Compost amended soils have been shown to contain smaller populations of plant parasitic nematodes than inorganic-fertilizer treated soils and to hold higher populations of fungivorous and bacterivorous nematodes (Arancon et al., Citation2003). Probably this can contribute to improved plant health without actively adding fungivorous nematodes as biocontrol agents of fungal diseases.
Although the biocontrol capacity of fungivorous nematodes, and especially of A. avenae, is well-documented, they have so far not been extensively used in practical agriculture or horticulture in the form of nematode applications or of systematic creation of favourable conditions for beneficial nematodes. This is somewhat surprising, since the nematodes we used are easy to propagate in large numbers and can be stored in a dormant stage (anhydrobiosis). The nematodes could be distributed to growers in this form and applied in the field, especially in the establishment phase of high-value crops (Ishibashi et al., Citation2000; Ishibashi, Citation2005). The explanation for this reluctance to commercialize fungivorous nematodes is probably the current high costs associated with reproduction, storage and distribution. If the beneficial effects of nematodes could be combined in growing systems with other beneficial practices or other biocontrol agents, their use could be more interesting. For instance, the nematodes could be used in combination with disease-suppressive compost amendments and certain antagonistic fungi (Jun & Kim, Citation2004) or with other fungivorous soil animals (Lootsma & Scholte, Citation1997a, Citation1997b). In this perspective their use would probably be economically viable and environmentally friendly, particularly in horticultural production systems.
Acknowledgements
We thank Lena Färeby for excellent help with rearing of the nematodes and fungi cultured in the laboratory and with the greenhouse work, Prof. B. Eriksson (SLU) for providing us with Aphelenchus avenae and Dr Björn Sohlenius (Swedish National Museum of Natural History) for providing us with the fungus Pochonia bulbillosa that was used for propagation of nematodes in cultures. We are grateful to Bengt Eriksson, Hanna Friberg and Aad Termorshuizen for helpful discussions and valuable comments on the manuscript. This research was funded by the European Commission (Management of Soil Health in Horticulture Using Compost, Project QLK5-CT-2001-01442) and by SLU (Swedish University of Agricultural Sciences).
References
- Arancon , N. Q. , Galvis , P. , Edwards , C. and Yardim , E. 2003 . The trophic diversity of nematode communities in soils treated with vermicompost . Pedobiologia , 47 : 736 – 740 .
- Bae , Y-S. and Knudsen , G. R. 2001 . Influence of a fungus-feeding nematode on growth and biocontrol efficacy of Trichoderma harzianum . Phytopathology , 91 : 301 – 306 .
- Eilenberg , J. , Hajek , A. and Lomer , C. 2001 . Suggestions for unifying the terminology in biological control . BioControl , 46 : 387 – 400 .
- Friberg , H. , Lagerlöf , J. and Rämert , B. 2005 . Influence of soil fauna and fungal plant pathogens in agricultural and horticultural systems . Biocontrol Science and Technology , 15 : 641 – 658 .
- Freckman , D. W. and Caswell , E. P. 1985 . The ecology of nematodes in agroecosytems . Annual Review of Phytopathology , 23 : 275 – 296 .
- Hasna , M. K. , Insunza , V. , Lagerlöf , J. and Rämert , B. 2007 . Food attraction and population growth of fungivorous nematodes with different fungi . Annals of Applied Biology , 151 : 175 – 182 .
- Hasna , M. K. , Lagerlöf , J. and Rämert , B. 2008 . Effects of fungivorous nematodes on corky-root disease of tomato grown in compost amended soil . Acta Agriculture Scandinavica, Section B., Plant and Soil Science , 58 : 145 – 153 .
- Hoffman , T. W. and S’-Jacob , J. J. 1989 . Distribution and dynamics of mycophagous and microvorous nematodes in potato fields and their relationship to some food sources . Annals of Applied Biology , 115 : 291 – 298 .
- Hoitink , H. A. J. and Boehm , M. J. 1999 . Biocontrol within the context of soil microbial communities: A soil-dependent phenomenon . Annual Review of Phytopathology , 37 : 427 – 446 .
- Hunt , D. J. 1993 . Aphelenchida, Longidoridae and Trichodoridae. Their Systematics and Bionomics , Cambridge : Cambridge University Press .
- Ishibashi , N. 2005 . “ Potential of fungal-feeding nematodes for the control of soil-borne pathogens ” . In Nematodes as biocontrol agents , Edited by: Grewal , P. S. , Ehlers , R. U. and Shapiro , D. I. 467 – 476 . Georgia : USDA ARS .
- Ishibashi , N. , Rustom , A. and Saramoto , M. 2000 . Mass-production of fungivorous nematode Aphelenchus avenae Bastian 1865, on industrial vegetable/animal wastes . Japanese Journal of Nematology , 30 : 8 – 17 .
- Jun , O. K. and Kim , Y. H. 2004 . Aphelenchus avenae and antagonistic fungi as biological control agents of Pythium spp . Plant Pathology Journal , 20 : 271 – 276 .
- Lootsma , M. and Scholte , K. 1997a . Effects of the springtail Folsomia fimetaria and the nematode Aphelenchus avenae on Rhizoctonia solani stem infection of potato at temperatures of 10 and 15°C . Plant Pathology , 46 : 203 – 208 .
- Lootsma , M. and Scholte , K. 1997b . Effect of soil moisture content on the suppression of Rhizoctonia stem canker on potato by the nematode Aphelenchus avenae and the springtail Folsonia fimetaria . Plant Pathology , 46 : 209 – 215 .
- Nickle , W. R. and Mc Intosh , P. 1968 . Studies on the feeding and reproduction of seven mycophagus nematodes on Rhizoctonia, Fusarium and Verticillium . Nematologica , 14 : 11 – 12 .
- Noble , R. and Coventry , E. 2005 . Suppression of soil-borne plant diseases with composts: A review . Biocontrol Science and Technology , 15 : 3 – 20 .
- Okada , H. H. 2006 . Ecology of fungivorous nematodes and their use for suppression of plant diseases . Bulletin of the National Agricultural Research Centre for Tohoku Region , 105 , 155 197 ( In Japanese with summary in English ).
- Rhoades , H. L. and Linford , M. B. 1959 . Control of Pythium root rot by the nematode Aphelenchus avenae . Plant Disease Reporter , 43 : 323 – 328 .
- Sohlenius , B. 1979 . A carbon budget for nematodes, rotifers and tardigrades in a Swedish coniferous forest soil . Holartic Ecology , 2 : 30 – 40 .
- Termorshuizen , A. J. , van rijn , E. , van der Gaag , D. J. , alabouvette , C. , Chen , Y. , Lagerlöf , J. , Malandrakis , A. A. , Paplomatas , E. J. , Rämert , B. , Ryckeboer , J. , Steinberg , C. and Zmora-Nahum , S. 2006 . Suppressiveness of 18 composts against 7 pathosystems: Variability in pathogen response . Soil Biology and Biochemistry , 38 : 2461 – 2477 .