Abstract
In the current multiplication technique first-generation seed tubers produced in the field by transplanting plants raised on peat in plastic rolls from plants cultured by repetitive multiplication using tip- and stem-cuttings and truncated plants are compared with in vitro micro-plants from the aspect of obtaining optimal-sized, disease-free seed tubers.
The objective of the study is to compare the dynamics of total plant dry mass and tuber dry mass of field-grown potato plants, and analyse the effect of the year and variety. Two local late-maturing potato varieties, Ants and Vigri, were used in the study. The field experiments were carried out in 2005–2007.
Significant impact of the multiplication method and experimental year on total plant dry mass and tuber dry mass was observed. The plants multiplied in vitro from micro-cuttings produced lower total dry mass and also lower tuber dry mass per m2. The plants multiplied by tip- and stem-cuttings as well as truncated plants proved to be more adaptable to unfavourable weather conditions than in vitro plants; in a favourable year, however, the differences were insignificant. In the early phase of growth the ratio of the tuber dry mass to total plant dry mass increased more rapidly in the case of in vitro plants, whereas by the end of the growing season the relevant ratio was similar for all multiplication methods and years.
All developed multiplication methods are suitable for practical seed potato production in the field and ensure a reasonable potato crop.
Introduction
Modern potato production involves cleaning infected potato plants of pathogens and micro-propagating planting material from the cleaned-up stock plants. The cuttings from in vitro plants are called micro-cuttings. In many countries multiplication with micro-cuttings in vitro in sterile conditions is used for multiplication of initial seed production material and new varieties. The method is safe but requires a hi-tech environment and well-trained staff, thus, it is expensive (Jeffries Citation1986, Mastenbroek and Eising Citation1987, Jones Citation1988, Ranalli et al. 1994, Sarkar et al. Citation1997, Haapala 2005). An advanced method of producing potato plantlets for further propagation based on a bioreactor (Paek et al. Citation2001) is used for reducing the cost of micro-propagation by means of the high efficiency of automated mass propagation (Paek et al. Citation2005). Also, propagation of different kind of cuttings, from sprouts or stems, is widely used (Goodwin et al. Citation1980, Bryan et al. Citation1981, Haapala 2005).
Several methods are available for growing seed tubers for multiplying potato plants, of which the following three methods are most common: micro tubers, mini tubers and tubers produced in vivo (Wattimena et al. 1983, Hussey and Stacey Citation1984, Estrada and Dodds Citation1986, Garner and Blake Citation1989, Struik and Lommen Citation1990, Citation1994, Nowak and Asiedu Citation1992, Ranalli et al. Citation1994, Donelly et al. Citation2003, Kawakami et al. 2003, Farran and Mingo-Castel Citation2006). However, it is necessary to introduce cheaper methods for propagating and growing of disease-free potatoes.
According to the technology developed at the Department of Biotechnological Research EVIKA of the Estonian Research Institute of Agriculture (ERIA) the first-generation seed tubers are grown in the field using plants produced from in vitro micro-plants pre-grown in plastic rolls on peat substrate, or increasing the number of plants by repetitive multiplication using truncated plants, tip- and stem-cuttings. Tubers of the first generation have to be harvested as early as possible to avoid re-infection. EVIKA technology has proved to be cheaper and simpler than multiplication of in vitro micro-plants and growing mini-tubers in the greenhouse (Rosenberg Citation1981, Rosenberg and Kotkas Citation1986, Kotkas 1987, Rosenberg et al. Citation1988, Kotkas and Särekanno 1996, Kotkas and Rosenberg 1999).
At EVIKA we have previously investigated the factors that influence the efficiency of the in vitro phase of multiplication (Rosenberg and Kotkas Citation1984, Rosenberg et al. Citation1997, Citation2005, Kotkas and Rosenberg Citation1999) and growth in plastic rolls in the greenhouse (Kotkas Citation1987, Särekanno Citation2000, Kotkas and Särekanno Citation2000, Citation2001), including the optimal composition of the multiplication medium in vitro, fertilizers in the peat substrate and the influence of growth regulators on the rooting of cuttings in plastic rolls (Kotkas and Särekanno Citation1996, 2001). However, so far less attention has been paid to the factors that influence the growth of meristem plants under field conditions.
Up to now the dynamics of the growth of potato vines and tuber yield has been investigated mostly in the cases of potatoes raised from tubers (micro-, mini- and conventional seed tubers) (Tooming et al. Citation1978, Wattimena et al. 1983, van Loon Citation1987, Naik et al. Citation1998, Kawakami et al. Citation2003, Citation2004, Haapala Citation2005, Eremeev Citation2007, Eremeev et al. Citation2008). Only a few reports of the studies on meristem culture of potato grown in the open field have been published (Wattimena et al. Citation1983, Tadesse et al. Citation2001).
From 2005 to 2007 a series of field trials was carried out with the objective of gathering knowledge of the growth of potato vines and tuber formation of potato plants multiplied by different methods and grown under field conditions. Some of the results of this study, namely how the leaf area index (LAI), the number of tubers per plant, mean fresh tuber mass, as well as the difference of fresh tuber mass and size distribution depend on the multiplication method, variety and experimental year have been published (Särekanno et al. 2010a, 2010b, 2010c). In this article we focus on the analysis of the dynamics of the total dry mass (DM) of the potato plant and tuber dry mass, their dependence on the multiplication method, experimental year and potato variety. The main objective of this paper is to analyse the difference between in vitro micro-plants and the plants raised by repetitive multiplication methods developed by EVIKA.
Materials and methods
Greenhouse and field trials were carried out during the growing seasons of 2005, 2006 and 2007 at EVIKA in Saku (59.3°N, 24.7°E). Four different multiplication methods (in vitro micro-plants, tip- and stem-cuttings and truncated plants grown in plastic rolls) were used.
Plant material
The local late-maturing potato varieties Ants and Vigri, both bred at the Jõgeva Plant Breeding Institute in Estonia, were used in the trials.
Potato multiplication methods and pre-growth under greenhouse conditions
| 1. | In vitro micro-plants. The potato plants were multiplied in vitro on artificial media. The 1–1.5 cm micro-cuttings (i.e stem-cut with 1 leaf) were cut from the preceding generation of in vitro micro-plants. These micro-cuttings were rooted and grown in vitro for 3 weeks after which, during the second half of May, the micro-plants were transplanted to plastic rolls filled with peat substrate and grown under greenhouse conditions for 2–3 weeks. | ||||
| 2. | Tip-cuttings in plastic rolls. Plants grown from in vitro micro-cuttings were transplanted to plastic rolls filled with peat substrate in the second half of April, followed by a growing period of 2–3 weeks under greenhouse conditions. In the second half of May, the shoot tips (1.5–2.0 cm, with leaves) were cut with scissors. The bottom end was treated with the rooting agent Juka-4 and the cuttings were planted in plastic rolls filled with peat substrate. The plants were grown in rolls for 2 weeks (including 5–6 days of rooting time). | ||||
| 3. | Stem-cuttings in plastic rolls. The stem-cuttings (1.5–2.0 cm, with 1 leaf) were cut from the same plants from which the tips had been used for treatment 2. The bottom end of the stem-cut was treated with the rooting agent Juka-4 and the cuttings were planted in plastic rolls filled with peat substrate. The plants were grown in the rolls for 2 weeks (including 5–6 days of rooting-time). | ||||
| 4. | Truncated plants grown in plastic rolls. The same plants that served as the source for both tip- and stem-cuttings were retained in the plastic rolls filled with peat for another 2 weeks. During that time, new shoots began to develop from axial buds of the source plants. | ||||
All the plants in plastic rolls were moved outdoors for acclimatization a week before transplanting them to the field.
Field trials
In the first half of June the plants were transferred to the field and planted individually by hand with a density of 7.1 plants m–2 and with a spacing of 70×20. A randomized block-design layout with 32 plots divided into four replications (2 varieties×4 multiplication methods×4 replications) with the total trial area of 336 m2 was used.
The soil type of the field trial area was a sandy loam soil, Sceletic Regosol by WRB classification (Citation2006). Routine agro-technological practices for seed potato production were followed. The field trial was discontinued in the first days of September even though the potato plants had not completed their vegetative growth by then. The intention was not to promote a further increase in tuber size, as the potatoes were to be used for seed production.
Measurements and computed parameters
The dynamics of potato shoot and tuber growth was measured 10–13 times during the vegetation period, at intervals of 5–7 days. Two weeks after transplanting, 10 control plants of each variety from each multiplication method were marked; those control plants were left to grow until the last harvest. The control plants were used for measuring some indicators of the plant growth before every sampling, and sample plants were chosen on the basis of these indicators. Plant height and the numbers of shoots per plant were observed as indicators before shoot lodging; later we used the distribution of shoots by their hardness (divided visually into four grades) and the sum of shoot lengths instead. Eight samples (two varieties, four multiplication methods) were examined at every observation, each sample consisting of four plants. The sampled plants were washed; their organs were separated and weighed. For the purpose of determining dry mass the transition coefficients from fresh to dry mass were established by drying out four random samples for each organ in a FD115 ‘‘Binder Gmbh’’ oven.
Since the samples were not large due to technical reasons, but the sampling was done frequently, a moving average of masses over three sample times (over two in the case of the first and the last dates) was used to decrease random fluctuations.
Meteorological background
The experimental years differed materially in terms of the amount and distribution of meteorological conditions (). Mean values of temperature, precipitation and solar radiation of the growing period of 2005 were quite similar to their long-term averages, but not very well balanced for different months. The year 2006 suffered from drought and was too hot during some periods. In 2007, despite dry conditions in June, the meteorological conditions onwards were well balanced; especially an equilibrium between solar radiation and precipitation was represented in the main tuber-growing period in August.
Table I. Mean temperature, total precipitation and total radiation from June to September in the experimental field at Saku.
Common methods involve expressing the progress of the season in terms of degree-days or cumulative incident solar radiation, in contrast to days after planting. The summation of daily effective temperature is defined as growing degree days, with units of degree-days (Yuan and Bland Citation2005) In the case of the potato crop; the best coincidence of experimental growth functions data for different years was achieved, using the cumulative daily mean temperatures above zero (Sepp et al. Citation1983, Kadaja and Tooming Citation2004). The sum of daily mean temperatures from planting to harvest was 1720 degree days in 2005, 1920 degree days in 2006 and 1650 degree days in 2007.
The analysis of experimental data
The dynamics of total plant dry mass (DM) production (including leaves, stems, roots and tubers) and tuber DM production (g m–2) was analysed for the entire growing period separately for each growing stage and multiplication method. The least significant difference (LSD05) was calculated to assess the differences between multiplication methods. In order to show that a factor (multiplication method, experimental year, variety) significantly influenced the total plant DM and tuber DM content the dispersion analysis was performed. The results of the dispersion analysis are presented as the calculation of F-statistic: F = MSmodel/MSerror (MS is mean square), the F sub-indexes representing the ‘model degrees of freedom’ and the ‘number of observed degrees of freedom’. The influence of a factor was taken as confirmed if the differences were significant at p<0.05 level.
As the total plant DM and tuber DM values are dependent on time, it was not possible to apply the same method for simultaneous analysis of the absolute values of the total DM and tuber DM production throughout the growing period. Since the main objective of this research was to compare different multiplication methods with the method of raising micro-plants in vitro, total plant DM and tuber DM index relative to in vitro micro-plants was calculated. The total plant DM and tuber DM values of every replication of every multiplication variant were divided by the corresponding mean values calculated for the in vitro micro-plants. The results show differences between the values of total plant DM and tuber DM of plants raised in vitro and the corresponding values of plants raised by other multiplication methods at the time of measurement. Such ratios make the values comparable and enable the analysis of the data to be made throughout the growing period.
Dispersion analysis was performed by Statistica 7.0 software (ANOVA, Fisher LSD test) (Statsoft Citation2005) with unsmoothed original data for dispersion analysis.
Results and discussion
The dynamics of total plant dry mass and tuber dry mass
The weather of the years 2005, 2006 and 2007 was considerably different in terms of plant growth and yield (Table I). June 2005 was cold and with sufficient rainfall, so the plants rooted normally. However, further plant growth became suppressed due to the deficit in moisture until the end of July. Precipitation in late July and August together with favourable temperature guaranteed the necessary growing conditions, which was evident in the efficient increment of the total plant DM and tubers DM that followed. The growing conditions in 2006 were unfavourable for the development of plants. After the plants had been transplanted to the open field, a long dry season with high temperatures followed.
Water deficit is a common stress factor in potato production, which leads to decrease in tuber quality and yield. In comparison with other species, the potato is very sensitive to water stress because of its shallow root system (Iwama Citation2008, Hassanpanah Citation2010). Water deficit strongly decreases plant water potential, growth rate, stem height, number of leaves, leaf area, ground coverage, canopy radiation interception, number of tubers and yield (Frensch Citation1997).
The plants suffered from post-rooting stress and remained depressed for most of the summer. At the end of August more significant increase in growth was observed. Stress that is likely to follow transplanting of potato plants to the open field has also been confirmed by trials carried out in other countries. According to Struik and Wiersma (1999) in the case of severe transplanting shock in vitro plantlets or cuttings will not root properly, development of the plants in the field will be slow and the yield low. Acclimation and transplanting of in vitro micro-plants to in vivo conditions has been and still is a serious problem in introducing meristem methods (Struik and Lommen 1999). The year 2007 was the most favourable of the three years observed for plant growth. The plants sprouted and forked abundantly, plant rows merged sooner than in previous years and the DM of the plants and tubers exceeded 1.9–2.5 and 1.6–2.5 times, respectively, that of the two previous years ().
Figure 1. The dynamics of total dry mass of potato plants (a–c) and dry mass of tubers (d–f) in 2005–2007 as the mean of two varieties. Plotted as moving average over three sample times (over two in the case of the first and the last dates). Multiplication methods: MP – in vitro micro-plants; TC – tip-cuttings; SC – stem-cuttings; TP – truncated plants.
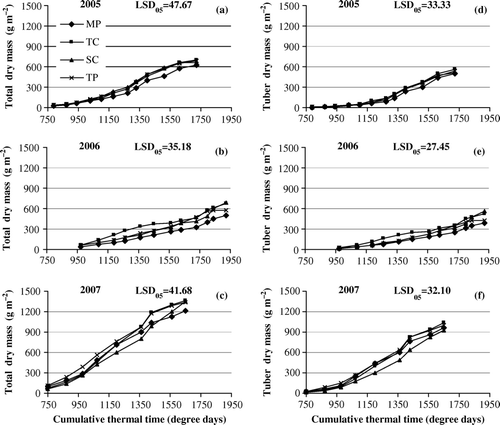
As a rule, plants raised in vitro achieved lower total plant DM as well as tuber dry mass than those raised by other tested multiplication methods ( and ). Since the size of potato plants varied considerably, no major statistical variability across different multiplication methods was observed at the time of several recordings. Significant statistical differences between multiplication methods occurred in complicated growing conditions in the year 2006.
Table II. The dynamics of total dry mass of potato plants multiplied as in vitro micro-plants (MP), and differences from it for other multiplication methods: tip-cuttings (TC), stem-cuttings (SC) and truncated plants (TP) in 2005–2007.
The year 2007 displayed slightly different dynamics of multiplication methods than the previous two years. The plants raised from tip- and stem- cuttings acquired lower total DM than the plants raised in vitro during most of the growing period. However, at the final harvest the total DM of in vitro plants was the lowest of the observed multiplication methods like in the previous two years. In 2007 the plants grown from stem-cuttings acquired lower tuber DM than the plants grown in vitro.
In 2007 development of the root system of plants raised from tip- and stem-cuttings was slower when compared with other multiplication methods. In the first two weeks of the pre-growing phase in the greenhouse the plants having been transplanted from test-tubes to plastic rolls and truncated plants develop more rapidly than the plants grown from stem- and tip-cuttings because the former already have an existing root system while plants grown from stem- and tip-cuttings only begin to form roots. As a rule, by the end of the pre-growing period (third week) before transplanting to the open field the differences in plant growth of different multiplication methods diminish. However, over the previous years of practice it has been observed that not all batches of plant cuttings develop equally in the same growing conditions. Particularly the development of plants grown from tip- and stem-cuttings is slowed down in the heat periods during a week after transplanting them to plastic rolls. When planted in the open field, differences between plants multiplied by different methods are smoothed within 10–14 days, when the growth in most cases is quite slow. In extremely favourable conditions, in 2007, intensive growth began sooner than usual (Table II), which is probably the reason why the plants lagging behind in development were not able to catch up throughout the growing period.
Table III. The dynamics of tuber dry mass of potato plants multiplied as in vitro micro-plants (MP), and differences from it for other multiplication methods: tip-cuttings (TC), stem-cuttings (SC) and truncated plants (TP) in 2005–2007.
On the basis of more significant differences between different multiplication methods in the unfavourable year of 2006, it can be concluded that in terms of productivity the plants multiplied by the methods developed at EVIKA tend to be more adaptable to unfavourable climate conditions when compared to in vitro plants, whereas the differences become insignificant in favourable years.
Ratio of tuber dry mass to total dry mass
The ratio of tuber DM to the total plant DM increased in the growing period and reached the final value 0.74–0.79, being similar both across different multiplication methods (a) and years (b). The differences occurred in the initial and middle phase of the growing period. By the end of the growing period, the ratio of tuber DM to the total plant DM was similar across all multiplication methods and years.
Figure 2. Ratio of tuber dry mass (DM) to the total dry mass of potato plant, averaged for multiplication methods over different years (a) and for different years over multiplication methods (b). Initial points in figure (b) reflect only the data of 2007. Multiplication methods: MP – in vitro micro-plants; TC – tip-cuttings; SC – stem-cuttings; TP – truncated plants.
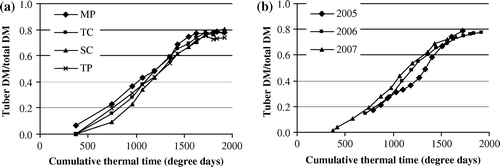
In the initial phase of the growing period the ratio of tuber DM to the total plant DM proved to be higher for the in vitro plants when compared with other multiplication methods. Earlier formation of tubers can be one of the reasons for lower biomass and lower yield of the in vitro plants when compared with other multiplication methods. Based on the published data (Struik and Lommen Citation1999) it is known that if tubers are formed at the time when LAI is still small, the formation of side-branches and leaves will be discontinued prematurely; also, the existing leaves are apt to age faster and, as a result, smaller yield will be achieved. In the case of stem-cuttings the ratio of tuber DM to the total plant DM proved to be to some extent lower than in the case of other multiplication methods.
In terms of years, the ratio of tuber DM to the total plant DM was different in relatively moderate 2005, whereas the droughty 2006 with its high temperatures and the favourable 2007 had similar and higher ratio of tuber DM to the total plant DM, respectively. The difference between 2005 and 2006 can be explained by the fact that in 2005 the plants were already well rooted when they had to suffer from drought, while in 2006 plant growth was suppressed after transplanting of plants to the field when the weather was already hot and dry. Therefore, it can be assumed that better-rooted plants reacted to drought by giving priority to foliage and root formation, whereas the plants transplanted to the field in hot and dry conditions reacted with faster formation of tubers.
Maximum daily increase of dry mass
In the favourable years (2005 and 2007) the greatest daily gain in the total plant DM and tuber DM as an average of different multiplication methods was recorded earlier than in the unfavourable year 2006 ().
Table IV. Mean maximum daily dry mass increase per year and per multiplication method of total potato plants and tubers.
At the same time, in 2006 maximum daily increase of total plant DM and tuber DM recorded as an average of different multiplication methods exceeded the daily maximum increase of total plant DM and tuber DM in the year 2005 (differences in the total plant DM 3.3 g m–2 and tuber DM 3.9 g m–2). This can be a result of the prolonged period of drought in 2006, when increase in dry mass was suppressed for a long time until precipitation in mid-August resulting in accelerated growth of plants. In more favourable conditions in the year 2005, accumulation of DM was distributed more evenly across the longer time period. Thus, it can be concluded that complicated weather conditions affect the rate of plant growth as well as accumulation of tuber DM making it significantly more uneven.
The highest maximum daily increase of DM as an average of the three years was achieved for plants raised from tip-cuttings and the lowest maximum daily increase of DM was achieved for plants raised in vitro. The difference in terms of total plant DM was 6.5 g m–2 and in terms of tuber DM 6.1 g m–2. Also, maximum increase of DM of truncated plants was lower than maximum increase of plants raised from tip- and stem-cuttings, therefore, it can be concluded that increase of plants having rooted earlier is distributed more evenly across the entire growing period, whereas maximum increase of DM of plants multiplied one cycle later is more fluctuating. Maximum increase of DM for plants raised from tip- and stem-cuttings having rooted one cycle later was achieved earlier than for in vitro and truncated plants, however, in the latter case increase of DM was more even.
The influence of multiplication method
To test whether the total DM and the tuber DM are influenced by the multiplication method, year and variety, we used the values relative to the values recorded in plants raised in vitro, a method that allows simultaneous analysis of the data for the entire experimental period. As the analysis indicated that the considered two late varieties had no significant influence either on total DM (F 1, 1229=0.07, p>0.05) or on tuber DM (F 1, 1116=2.41, p>0.05) the data of varieties were used together in the study of the influence of multiplication method and experimental year.
The results of the statistical analysis, using together the data of three years, revealed significant impact of the multiplication method both on the total DM (F 3, 1227=24.27, p<0.05) as well as tuber DM (F 3, 1114=14.01, p<0.05). In vitro plants had significantly smaller relative total plant DM and tuber DM than the plants grown from tip- and stem-cuttings and truncated plants. There were differences in the total plant DM and tuber DM also between EVIKA's own multiplication methods, stem-cuttings giving significantly lower results compared with tip-cuttings and truncated plants ().
Figure 3. Differences between multiplication methods in the total dry mass and tuber dry mass of potato plants determined relative to in vitro micro-plants multiplication method over three experimental years and two varieties. Multiplication methods: MP – in vitro micro-plants; TC – tip-cuttings; SC – stem-cuttings; TP – truncated plants. Different letters indicate significant differences (p < 0.05). Vertical bars denote 0.95 confidence intervals.
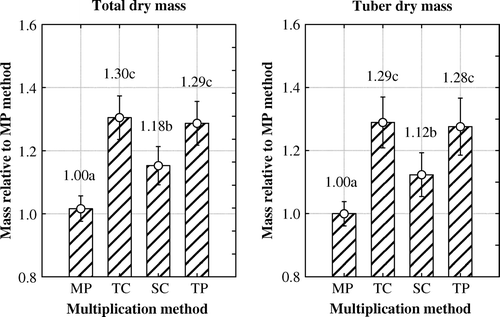
In previous articles the analysis of LAI, number of tubers per plant, tuber size, average tuber fresh mass and tubers per plant were presented using the data collected in the same trial (Särekanno et al. Citation2010a, Citation2010b, 2010c). It appeared that plants multiplied in vitro had smaller LAI, more tubers and smaller average tuber mass than plants multiplied by EVIKA multiplication methods. Increase in the number of tubers failed to compensate for the decrease in average tuber mass. In the case of in vitro multiplication method, tuber mass per plant remained significantly lower both in terms of fresh and dry mass, as well as the total plant DM, when compared with other multiplication methods.
The influence of year
In addition to the multiplication method, weather conditions had a notable impact on the accumulation of total plant DM and tuber DM.
The differences between years were determined by analysing together the relative DM values of four multiplication methods. Results of the dispersion analysis indicated significant influence of the observation year on relative total DM (F 2, 1228=87.03, p<0.05) and relative tuber DM (F 2, 1114=25.84, p<0.05) ().
Figure 4. Differences between years in the total dry mass and tuber dry mass of potato plants determined relative to in vitro micro-plants multiplication method over all multiplication methods and two varieties. Multiplication methods: MP – in vitro micro-plants; TC – tip-cuttings; SC – stem-cuttings; TP – truncated plants. Different letters indicate significant differences (p < 0.05). Vertical bars denote 0.95 confidence intervals.
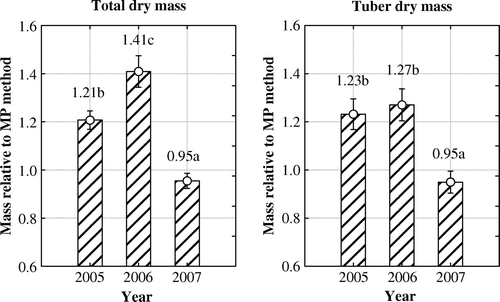
In the case of relative total plant DM, significant differences occurred across all trial years, whereas no significant difference was observed in terms of the relative value of tuber DM in the years 2005 and 2006. Both relative total plant DM and tuber DM proved to be the greatest in 2006 when the difference between EVIKA multiplication methods and in vitro methods in terms of total DM and tuber DM was most significant. In 2007, all observed multiplication methods in comparison with in vitro methods produced more or less similar results, and thanks to the variants of tip- and stem-cuttings, the sum of relative DM remained even below the value of 1.0.
The results of LAI and statistical analysis of relative tuber fresh mass per plant previously performed within the framework of the same trial, refer to tendencies similar to the results of the analysis of the total plant DM and tuber DM presented in this study (Särekanno et al. Citation2010c).
The advantages of EVIKA technology
The technology of multiplication of potato plants from tip- and stem-cuttings and truncated plants in plastic rolls is practical, easier, cheaper and more energy- and material-conserving compared with the common in vitro multiplication followed by growing of micro- or mini-tubers in vitro or in greenhouses.
Using EVIKA technology, the production of the first tuber generation in the fields allows one to receive seed tubers already in the first year, which can then be stored as regular seed potato, whereas the micro- and mini-tubers require specific storage conditions to preserve them as well as to suspend their resting period (Pruski et al. Citation2003, Suttle Citation2008). From the result of this study we conclude that plants multiplied by different multiplication methods by tip- and stem cuttings as well as by truncated plants achieve greater total plant DM and tuber DM compared with plants multiplied by in vitro micro-cuttings. All described multiplication methods were shown to be suitable for practical seed potato production.
Acknowledgements
The experiments on which the present study was based were made within the Target Research project no. 0442528s03, the national programme ‘Agricultural Applied Research and Development in 2004–2008’ and grants no. 6132 and 6092 of Estonian Science Foundation. The authors are grateful to Mrs Luule Kurimu and Mrs Erika Vesik for their generous technical assistance in sample processing.
References
- Bryan , J. E. , Jackson , M. T. and Melendez , N. G. 1981 . Rapid multiplication techniques for potatoes , Lima : International Potato Centre .
- Donelly , D. J. , Coleman , W. K, and Coleman , E. 2003 . Potato microtuber production and performance: A review . American Journal of Potato Research , 80 : 103 – 115 .
- Eremeev , V. 2007 . The influence of thermal shock and pre-sprouting of seed potatoes on formation of some yield structure elements . Ph.D. thesis. Tartu, Estonia .
- Eremeev , V. , Lõhmus , A. , Lääniste , P. , Jõudu , J. , Talgre , L. and Lauringson , E. 2008 . Influence of seed potato pre-planting treatments on leaf area and tuber yield formation . Acta Agriculturæ Scandinavica Section B – Soil & Plant Science , 48 : 35 – 42 .
- Estrada , R. P. and Dodds , J. H. 1986 . Induction of in vitro tubers in a range of potato genotypes . Plant Cell Tissue and Organ Culture , 7 : 3 – 10 .
- Farran , I. and Mingo-Castel , M. 2006 . Potato minituber production using aeroponics: effect of plant density and harvesting intervals . American Journal of Potato Research , 83 : 47 – 53 .
- Frensch , J. 1997 . Primary response of root and leaf elongation to water deficits in the atmosphere and soil solution . Experimental Botany , 48 : 985 – 999 .
- Garner , N. and Blake , J. 1989 . The induction and development of potato microtubers in vitro on media free of growth regulating substances . Annals of Botany , 63 : 663 – 674 .
- Goodwin , P. B. , Kim , Y. C. and Adisarwanto , T. 1980 . Propagation of potato by shoot-tip culture. 1. Shoot multiplication . Potato Research , 23 : 9 – 18 .
- Haapala , T. 2005 . Use of single-leaf cuttings of potato for efficient mass propagation . Potato Research , 48 : 201 – 214 .
- Hassanpanah , D. 2010 . Evaluation of potato cultivars for resistance against water deficit stress under in vivo conditions . Potato Research , 53 : 383 – 392 .
- Hussey , G. and Stacey , N. J. 1984 . Factors affecting the formation of in vitro tubers of potato (Solanum tuberosum L.) . Annals of Botany , 53 : 565 – 578 .
- Iwama , K. 2008 . Physiology of the potato: new insights into root system and repercussions for crop management . Potato Research , 51 : 333 – 353 .
- Jeffries , C. J. 1986 . “ The Scottish seed potato classification scheme and the production of nuclear stock using tissue culture ” . In Healthy planting material: strategies and technologies , Edited by: Rudd-Jones , D. and Langton , F. A. 14 Edinburgh : Scottish Agricultural Science Agency .
- Jones , E. D. 1988 . A current assessment of in vitro culture and other rapid multiplication methods in North America and Europe . American Journal of Potato Research , 65 : 209 – 220 .
- Kadaja , J. and Tooming , H. 2004 . Potato production model based on principle of maximum plant productivity . Agricultural and Forest Meteorology , 127 : 17 – 33 .
- Kawakami , J. , Iwama , K. , Hasegawa , T. and Jitsuyama , Y. 2003 . Growth and yield of potato plants grown from microtubers in field . American Journal of Potato Research , 80 : 371 – 378 .
- Kawakami , J. , Iwama , K. , Jitsuyama , Y. and Zheng , X. 2004 . Effect of cultivar maturity period on the growth and yield of potato plants grown from microtubers and conventional seed tubers . American Journal of Potato Research , 81 : 327 – 333 .
- Kotkas , K. 1987 . Kartuli meristeemtaimede paljundamine. (Propagation of potato meristem plants.) Teaduse soovitused tootmisele (Recommendations from Science to Practice) , pp. 12 – 15 . Infoleht, Eesti Maaviljeluse Institut (Newsletter, Estonian Research Institute of Agriculture), Tallinn, Estonia (in Estonian) .
- Kotkas , K. and Rosenberg , V. 1999 . Disease eradication and propagation of the initial seed potato material in Estonia . Potato Research , 42 : 577 – 583 .
- Kotkas , K. & Särekanno , M. 1996 . An effective and environmentally safe potato multiplication technology created in Research Centre EVIKA . Abstracts of 13th Triennial Conference of EAPR , pp. 198 – 199 . Veldhoven, the Netherlands .
- Kotkas , K. and Särekanno , M. 2000 . The influence of plant propagation method and planting density on the productivity of potato meristem plants . Transactions of the Estonian Academic Agricultural Society , 11 : 25 – 29 .
- Kotkas , K. and Särekanno , M. 2001 . The productivity of potato meristem plants depending on pre-planting treatment . Transactions of the Estonian Academic Agricultural Society , 14 : 105 – 109 .
- Mastenbroek , I. & Eising , J. 1987 . Optimum production of tubers from plantlets . Abstracts of the 10th Triennial Conference of EAPR , pp. 123 – 124 . Aalborg, Denmark .
- Nowak , J. and Asiedu , S. K. 1992 . Gelling agent and light effects on in vitro tuberization of potato cultivars . American Potato , 69 : 461 – 670 .
- Naik , P. S. , Sarkar , D. and Gaur , P. C. 1998 . Yield components of potato microtubers: in vitro production and field performance . Annals of Applied Biology , 133 : 91 – 99 .
- Paek , K. Y. , Hahn , E. J. and Son , S. H. 2001 . Application of bioreactors for large-scale micropropagation systems of plants . In vitro Cellular and Development Biology – Plant , 37 : 284 – 292 .
- Paek , K. Y. , Chakrabarty , D. and Hahn , E. J. 2005 . Application of bioreactor systems for large scale production of horticultural and medicinal plants . Plant Cell, Tissue and Organ Culture , 81 : 287 – 300 .
- Pruski , K. , Astatkie , T. , Duplessis , P. , Lewis , T. , Nowak , J. and Struik , P. C. 2003 . Use of jasmonate for conditioning of potato plantlets and microtubers in greenhouse production minitubers . American Journal of Potato Research , 80 : 183 – 189 .
- Ranalli , P. , Bassi , F. , Ruaro , G. , del Re , P. , Di Candilo , M. and Mandolino , G. 1994 . Microtuber and minituber production and field performance compared with normal tubers . Potato Research , 37 : 383 – 391 .
- Rosenberg , V. 1981 . Sposob polutsenie posadosnovo materjala kartofelja i sreda dlja razmnozenija regenerirovannōh rastenii . SU Autoritunnistus nr. 1025373 (in Russian) .
- Rosenberg , V. & Kotkas , K. 1984 . Sposoby uskorennovo razmnozenija rastenii kartofelja . Sposob nauchnyh trudov. LIII EMMTUI Zastsita rastenii , pp. 89 – 97 . Tallinn, Estonia (in Russian) .
- Rosenberg , V. & Kotkas , K. 1986 . Sposob razmnozenija posadosnovo materiala kartofelja v kulture tkani . SU Autoritunnistus nr. 15013118 (in Russian) .
- Rosenberg , V. , Kiisk , J. , Kotkas , K. , & Sarnet , V. 1988 . Sposob posadki rastenii-regenerantov kartofelja . SU Autoritunnistus nr. 1678255 (in Russian) .
- Rosenberg , V. , Kotkas , K. , & Särekanno , M. 1997 . Review about the research and production of seed potato in Estonia . Proceedings of the International Potato Symposium , pp. 10 – 13 . Brasov, Romania .
- Rosenberg , V. , Kotkas , K. and Särekanno , M. 2005 . The number of tubers per plant and tuber's weight formation dynamics of potato meristem plants . Transactions of the Estonian Agricultural University , 220 : 72 – 74 .
- Särekanno , M. 2000 . The productivity of disease-free potato plants . Transactions of the Jõgeva PBI III. Plant breeding and seed production , pp. 167 – 175 . Jõgeva, Estonia .
- Särekanno , M. , Kadaja , J. , Kotkas , K. , Rosenberg , V. , Vasar , V. , Ojarand , A. and Eremeev , V. 2010a . Dependence of leaf area index on different multiplication methods of potato meristem plants grown under field conditions. Acta Agriculturæ Scandinavica . Section B –Soil and Plant Science , 60 : 1 – 9 .
- Särekanno , M. , Kadaja , J. , Kotkas , K. , Rosenberg , V. , Vasar , V. , Saue , T. and Eremeev , V. 2010b . Potato seed from meristem plants using EVIKA multiplication methods. Acta Agriculturæ Scandinavica . Section B – Soil and Plant Science , 60 : 101 – 109 .
- Särekanno , M. , Kadaja , J. , Kotkas , K. , Rosenberg , V. , Vasar , V. , Saue , T. and Eremeev , V. 2010c . Yield potential and tuber-size distribution using EVIKA multiplication methods . Acta Agriculturæ Scandinavica Section B – Soil and Plant Science , 60 : 297 – 306 .
- Sarkar , D. , Chandra , R. and Naik , P. S. 1997 . Effect of inoculation density on potato micropropagation . Plant Cell Tissue and Organ Culture , 48 : 63 – 66 .
- Sepp , J. , Tooming , H. , & Karing , P. 1983 . Eksperimental'noe opredelenie funktsij rosta kartofelja (Experimentally determined growth functions for potato.) . Agroklimaticheskie Uslovija i Produktivnost' sel'skohozjaistvennyh kul'tur. Agroclimatic conditions and crops productivity. Trudy VNIISHM Proceedings of All-Union Research Institute of Agricultural Meteorology , 11 , 36 – 40 . Gidrometeoizdat, Leningrad (in Russian) .
- Statsoft 2005 . Statistica 7.0 , p. 716 . Tulsa, OK .
- Struik , P. C. & Lommen , W. J. M. 1990 . Production, storage and use of micro- and minitubers . Proceedings of the 11th Triennial Conference of EAPR, Edinburgh , pp. 122 – 133 .
- Struik , P. C. and Lommen , W. J. M. 1994 . Field performance of potato minitubers with different fresh weights and conventional seed tubers . Potato Research , 37 : 301 – 313 .
- Struik , P. C. and Lommen , W. J. M. 1999 . Improving the field performance of mini- and microtubers . Potato Research , 42 : 559 – 568 .
- Suttle , J. C. 2008 . Effects of synthetics Phenylurea and Nitroguanidine cytokinins on dormancy break and sprout growth in Russet Burbank minitubers . American Journal of Potato Research , 85 : 121 – 128 .
- Tadesse , M. , Lommen , W. J. M. and Struik , P. C. 2001 . Effect of temperature pre-treatment of transplants from in vitro produced potato plantlets on transplant growth and yield in the field . Potato Research , 44 : 173 – 185 .
- Tooming , H. G. , Mäetalu , H. I. , Kyjva , P. H. , Tammets , T. H. , & Rajg , H. G. 1978 . Programmirovanie maksimal'nyh urozhaev kartofelja (Programming of maximum potato yield) . Vestnik sel'skohozjajstvennyh nauk (Reports of Agricultural Sciences) 257 , 110 – 117 (in Russian) .
- van Loon , C. D. 1987 . Effect of physiological age on growth vigour of seed potatoes of two cultivars. 4. Influence of storage period and storage temperature on growth and yield in the field . Potato Research , 30 : 441 – 450 .
- Wattimena , G. , McCown , B. and Weis , G. 1983 . Comparative field performance of potatoes from microculture . American Journal of Potato Research , 60 : 27 – 33 .
- World Reference Base (WRB) 2006 . World reference base for soil resources. , 2nd edition . World Soil Resources. Report 103 , p. 128 . Rome : FAO .
- Yuan , F. M. and Bland , W. L. 2005 . Comparison of light- and temperature-based index models for potato (Solanum tuberosum L.) growth and development . American Journal of Potato Research , 82 : 345 – 352 .