Abstract
Heavy metal pollution is a widespread global problem causing serious environmental concern. Cadmium, one of the heavy metals, is water soluble and can be transferred from soil to plants and enter into the food chain. It is detrimental to human health because it accumulates in the body and can cause renal tubular dysfunction, pulmonary emphysema and osteoporosis. This heavy metal needs to be cleaned up for a clean and safe environment. An experiment was conducted to evaluate the potential of Dyera costulata as a phytoremediator to absorb cadmium from contaminated soils. Dyera costulata seedlings were planted on six different growth media (soil + different levels of cadmium): Control, 25 ppm Cd, 50 ppm Cd, 75 ppm Cd, 100 ppm Cd and 150 ppm Cd. The highest growth performance mainly height, basal diameter and number of leaves were in the control, 50 ppm Cd and 25 ppm Cd treatments, respectively. The highest accumulation of cadmium (52.9 ppm) was in the 75 ppm Cd treatment. Among the plant parts, leaves showed the highest concentration of cadmium. Dyera costulata showed high translocation factor and low bioconcentration factor values in soil at high cadmium concentrations and was also able to tolerate and accumulate high concentrations of cadmium. The roots of Dyera costulata were found to be suitable for the absorption of cadmium in contaminated soils. This species can be an efficient phytoremediator for soils contaminated with cadmium.
Introduction
Sewage sludge, compost, mining waste, chemical fertilizers and other industrial wastes can be transported to natural vegetation and cultivated crops, causing widespread pollution in vast areas of land that eventually become non-arable and hazardous for both wildlife and human populations (Majid et al. Citation2011). The accumulation of heavy metals in agricultural lands can remain in the soil for many years (Alloway and Jackson Citation1991). Large areas of land contaminated with Cd were mainly caused by anthropogenic activities such as mining and mineral processing of metallic ores, waste disposal, phosphate fertilizer application and wastewater irrigation. Cadmium (Cd) is one of the most toxic heavy metals present in the environment (Wagner Citation1993). It is easily absorbed by roots and translocated to different plant parts and enters the human food chain (Baek et al. Citation2006, Xiao et al. Citation2008). High accumulation of Cd generally causes growth inhibition and even plant death (Khan and Khan Citation1983). Cadmium is of particular concern to human health, because it accumulates in the body with a half-life exceeding 10 years and has been linked with renal tubular dysfunction (Buchet et al. Citation1990), pulmonary emphysema (Ryan et al. Citation1982) and possibly osteoporosis (Bhattacharyya et al. Citation1988).
Cadmium is a non-essential element that possesses high toxicity and is easily accumulated from the environment by organisms (Rahimi and Nejatkhah Citation2010). Restoration of soils contaminated with potentially toxic metals and metalloids is of major concern (Shelmerdine et al. Citation2009).
Remediation of polluted soil could be carried out using physico-chemical processes such as ion-exchange, precipitation, reverse osmosis, evaporation and chemical reduction. However, these measures require external man-made resources and are costly (Mangkoedihardjo and Surahmaida Citation2008). Phytoremediation is a viable, relatively low-cost approach to remove heavy metals from soil and groundwater (Mojiri Citation2011). Plants have many properties that make them ideally suited to clean polluted soil, water and air, in a process called phytoremediation. Malaysia is considered one of the world's 12 leading mega-biodiversity countries with a high diversity of flora and fauna. There are several natural products such as medicinal plants that can be used as phytoremediators. D. costulata is easy to handle in the nursery, survives well when planted out, and has a good rate of growth. Some works of phytoremediation on polluted soils have been documented but phytoremediation with jelutong (Dyera costulata) has not been reported. The present study was therefore conducted to evaluate the heavy metal accumulation and translocation in jelutong (Dyera costulata) plant parts and to study the growth performance of D. costulata in contaminated soils.
Materials and methods
Site description and planting materials
The experiment was conducted at the glasshouse, Universiti Putra Malaysia, Serdang, Selangor, Malaysia for the period February to May 2010. The temperature in the morning was 26°C, 36 °C at noon and reduced to 30 °C in the evening. The growth media was a mixture of soil with different levels of cadmium, viz.: T0 (Control, soil), T1 (Soil + 25 ppm Cd), T2 (soil + 50 ppm Cd), T3 (soil + 75 ppm Cd), T4 (soil + 100 ppm Cd) and T5 (soil + 150 ppm Cd). A completely randomized design (CRD) was used with four replications. Dyera costulata was the test plant. Jelutong (Dyera costulata) is a large timber tree, preferring primary evergreen lowland or hill forest up to 300 m. It grows to approximately 60 m tall with diameters of 2 m and boles clear and straight for 30 m. It grows in Malaysia, Borneo, Sumatra and southern Thailand (Middleton Citation2004). It is considered low risk/least concern because this species regenerates readily in logged-over forest, it coppices well and it is extremely resistant to girdling (Anon. Citation2011).
Healthy seedlings of 3-month old and similar in form plants were selected for this study. After filling the pots (28.2 cm×34.2 cm size) with the growth media (10 kg pot-1) the seedlings of uniform age and size were transplanted into them (1 seedling pot−1). Weeding and watering were done when necessary to ensure normal growth of the seedlings. Twenty-four plants were used to measure growth parameters including basal diameter, number of leaves and height. Height was measured using a ruler and basal diameter by a caliper. The growth parameters were measured twice a month.
Plant and soil sampling and chemical analysis
Plant samples were collected at harvest and soil samples were collected from each pot before planting and at harvest and kept in standard plastic containers and air-dried before physico-chemical analysis. For analysis of heavy metals, 1.0 g dried plant sample and 20 ml aqua regia solution (mixture of concentrated HNO3 and HCl in a ratio of 3:1) was acid digested at 80 to 120 °C for 3 hours. After digestion the solution was transferred into a 100 ml beaker ready for analysis using an ICP-MS (Inductively Couple Plasma Mass Spectrometry) method (Sahoo et al. Citation2009). Particle size distribution was analysed by a pipette gravimetric method. Soil pH and total carbon were determined by using a glass electrode pH meter (Jackson Citation1973) and loss on ignition method, respectively.
Plant biomass measurement
Plant biomass was measured separately according to leaves, stems and roots and calculated. The loss in weight upon drying is the weight originally present. The moisture content of the sample was calculated using the following equation
Determination of bioconcentration factor (BCF) and translocation factor (TF)
The plant's ability to accumulate metals from soils and translocate metals from roots to shoots can be estimated using the bioconcentration factor (BCF) and translocation factor (TF), respectively. BCF and TF factors can be calculated as follows:
Statistical analysis
Analysis of variance for growth and heavy metals concentrations (in soil and plant parts) were done following the ANOVA test and the mean values were calculated by the DMRT (p=0.05) method (Steel et al. Citation1996). Comparison using t-test was also done to detect any significant differences between before planting and at harvest.
Results and discussion
Properties of the growth media
The growth media was a sandy clay texture which is suitable for seedling growth and development partly because of the high amount of nutrients and water-holding capacity is also high; however, drainage capacity is poor (Rice Citation2009). Moreover, clay soils also crack excessively while drying, and if they are very low in organic matter, clay soils may lose their structure and become cloddy and compacted (Aljibury Citation2011).
The soil pH ranged from 4.30 to 4.74 and 4.59 to 5.06 before planting and at harvest, respectively. pH of the growth media increased at harvest having the highest (5.06) in T2 followed by T1 (4.98). Before planting the highest pH was recorded in the control (4.87) followed by T1 (4.63) and the minimum was in T5 (4.05) (a). It was observed that pH was higher at harvest compared with before planting and this might be due to absorption of heavy metals (acidic elements) from the contaminated soil. Knight et al. (Citation1997) also found significant increment in soil pH grown with Thlaspi caerulescens on contaminated soils which corroborated the findings of our results. Soil pH affects all the chemical, physical and biological properties of soil (Brady and Weil Citation2002). Element accumulation in plant depends not only on their absolute content in a soil, but also on soil pH (Lorenz et al. Citation1994; Golovatyj Citation2002).
Figure 1. Change in pH (a), total carbon (b) and cadmium concentrations (c) in the growth media at harvest of Dyera costulata as influenced by different cadmium levels. Growth media indicates soil mixing with different levels of cadmium, i.e. T0=Control/soil, T1=Soil + 25 ppm Cd, T2=soil + 50 ppm Cd, T3=soil + 75 ppm Cd, T4 soil + 100 ppm Cd and T5=soil + 150 ppm Cd. Means±SE are shown in error bar (p = 0.05).
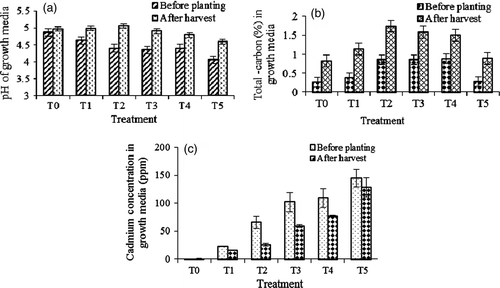
Total carbon was significantly different (p≤ 0.05) between before planting and at harvest (b). The total carbon content varied from 0.26 to 0.88% and 0.83 to 1.74% before planting and at harvest, respectively. Carbon content in the growth media was increased at harvest compared with before planting. The maximum increment (0.88%) was found in T2 followed by T1 (0.76%) and the minimum (0.57%) was in the control (b).
Growth performance
Growth parameters such as height, basal diameter and number of leaves were significantly influenced (p≤0.05) by the different treatments. The control showed the maximum height increment (4.13 cm) followed by T2 (3.75 cm) and T3 (3.16 cm). Minimum height increment (2.17 cm) was recorded in T5 (a). The highest height increment in the control might be due to lower concentration of cadmium and did not affect the absorption of other essential elements and physiological activities. The height decrease in T5 might be due to toxic effects of cadmium and shoot mortality. The highest basal diameter increment (3.12 cm) was observed in T2 followed by the control (2.44 cm). The lowest basal diameter increment (1.98 cm) was in T5 (b). The number of leaves ranged from 6 to 13. Treatment T1 produced the highest number of leaves followed by the control (9.75) and T2 (9.25). The minimum number of leaves (6.25) was recorded in T5. Plant growth decreased in T5 where Cd concentration was highest in the growth medium. Liu et al. (Citation2008) showed that height of ornamental plant decreased with increasing Cd concentrations, indicating that Cd restricted its growth.
Figure 2. Plant height (a), basal diameter (b) and number of leaves (c) of Dyera costulata at different months after planting as influenced by different treatments (increase per month). Growth media indicates soil mixing with different levels of cadmium, i.e. T0=Control/ soil, T1=Soil+25 ppm Cd, T2=soil+50 ppm Cd, T3=soil+75 ppm Cd, T4 soil+100 ppm Cd and T5 =soil+150 ppm Cd. Means±SE are shown in error bar (p=0.05).
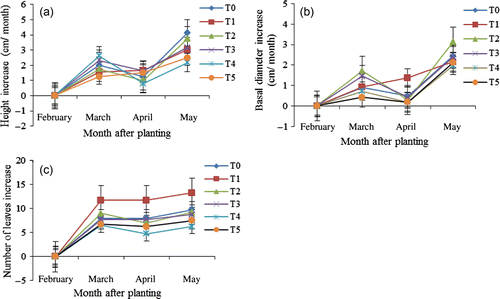
Seedlings were planted on February 2010. Height, basal diameter and number of leaves increment increased in March and decreased in April (). This decrease might be due to the toxic effects of cadmium 2 months after planting (in April). The maximum increments of height, diameter and leaves were in May (3 months after planting) for all treatments. T2 showed the highest increment in height (3.75 cm) and diameter (3.12 cm) while T1 produced the maximum leaves in May (). This is because the soil conditions became suitable for plant growth after 3 months.
Plant biomass
Treatment T1 produced the maximum root biomass (45.8 g) followed by the control (44.4 g) and the minimum was in T5 (28.3 g) (Table 1). There was no significant difference (p≤0.05) in stem biomass among all treatments. Stem biomass varied from 42.1 to 58.0 g having the highest (58.0 g) in T1 followed by T4 (51.4 g) and T2. The minimum (42.0 g) was observed in the control. Leaf biomass varied from 37.8 to 58.7 g with the highest in the control (58.75 g) followed by T1 (58.10 g), T2 (53.69 g) and T3 (51.34 g). The lowest leaf biomass (37.8 g) was in T4. The total biomass ranged from 111 g to 162 g, the highest in T1 followed by the control and the lowest was in T5 (Table 1). Mangkoedihardjo and Surahmaida (Citation2008) reported significant reduction in plant dry matter due to increased Cd concentration. Similar results were also reported by Arvind (Citation2004) and Tomar et al. (Citation2000) in Cd and Pb contaminated soil with Ceratophyllum demersum and Vigna radiata, respectively.
Table I. Dry biomass of leaves, stems and roots of Dyera costulata at harvest as influenced by different treatments (p = 0.05).
Heavy metal concentration in the growth media
Cadmium (Cd) concentration was significantly variable in the growth media (p ≤ 0.05). Before planting, T5 showed the highest concentration (144.8 ppm) followed by T4 (109.5 ppm). The lowest Cd concentration (0.30 ppm) was recorded in the control (c). After harvest Cd concentration decreased in all treatments (c). Treatment T3 showed the highest decrease (42.7 ppm) followed by T2 (40.2 ppm) and the minimum (0.09 ppm) was in the control. Plant growth was affected in high Cd concentration media and this is why the plants failed to absorb more Cd. On the other hand, Cd concentration was very low in the control hence absorption was also low. The concentration of Cd in normal soils ranged between 0.01 ppm to 2.0 ppm and the critical level ranged between 3.0 to 8.0 ppm (Alloway Citation1995). The concentrations of Cd in the growth media were above the critical level except the control.
Heavy metal concentration in plant parts
Cadmium concentration in plant parts was significantly different among the different treatments (p ≤ 0.05). In the leaves, the highest Cd concentration (52.9 ppm) was found in T3 followed by T2 (34.1 ppm). The lowest Cd concentration (0.07 ppm) was in the control. Cd concentrations in the stem ranged from 1.46 to 47.2 ppm having the highest in T5 (47.2 ppm) followed by T3 (40.0 ppm) and the lowest (1.46 ppm) was in the control (). Treatment T3 also showed the highest Cd concentration (44.1 ppm) in the roots which was significantly different (p≤0.05) from the other treatments. T2 gave the second highest concentration (31.2 ppm) in the roots followed by T1 (24.45 ppm) and the minimum (1.53 ppm) was also in the control. It was observed that Cd concentration in the leaves was higher than the roots (). However, Cd concentration in each plant part increased with increasing Cd in the growth medium up to a certain level after which increasing Cd in soil decreased its concentration in the plant parts. Liu et al. (Citation2008) also reported that Cd concentration in each plant part increased with increasing Cd in solution.
Table II. Cadmium accumulation in different parts and bioconcentration factor of Dyera costulata as influenced by different treatments (p=0.05).
Bioconcentration factor and translocation factor
Bioconcentration factor ranged 0.11 to 1.52. Treatment T1 showed significantly higher BCF (1.52) followed by T2 (1.21) and the minimum (0.11) was in T5 (). T1 and T2 treatments showed higher BCF values (1.52 and 1.21, respectively) but the other treatments showed very low values, having the lowest in T5 (), which may imply restriction in soil-root transfer at higher Cd concentrations in the soil. Similar results were reported by Yoon et al. (Citation2006). Ho et al. (Citation2008) reported 1.92–3.21 BCF in Pb treated kenaf (Hibiscus cannabinus L.).
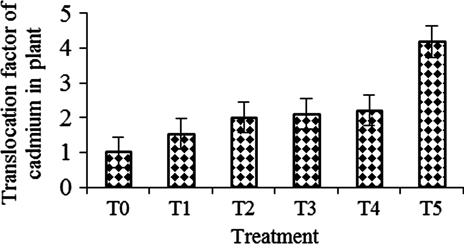
Translocation factor (TF) was also significantly influenced by the different levels of cadmium (p≤ 0.05). Translocation factor varied from 1.0 to 4.19 (). The highest TF (4.19) was in T5 which was significantly higher than the other treatments (p≤0.05). T4 showed the second highest TF which was similar to T2 and T3 but different from the remaining treatments. The lowest TF value (1.00) was in the control (). Translocation was more prominent in T5. TF of metal excluder species is < 1 whereas metal accumulator species have TF > 1 (Baker Citation1981). It was observed that all the treatments exhibited higher TF values (>1). Dyera cotulata has high TF and low BCF in soil at higher cadmium concentrations. Heavy metal tolerance with high TF and low BCF values was suggested for phytoaccumulators in contaminated soils (Yoon et al. Citation2006) and therefore, Dyera cotulata can be used as a potential phytoremediator for soils contaminated with cadmium.
Figure 3. Translocation factor of cadmium in Dyera costulata as influenced by different treatments. Growth media indicates soil mixing with different levels of cadmium, i.e. T0=Control/soil, T1=Soil + 25 ppm Cd, T2 =soil + 50 ppm Cd, T3=soil + 75 ppm Cd, T4 soil + 100 ppm Cd and T5=soil + 150 ppm Cd. Means±SE are shown in error bar (p = 0.05).
Acknowledgements
We are thankful to the Ministry of Higher Education Malaysia for the financial support through the Research University Grant Scheme (91784) to Universiti Putra Malaysia.
References
- Aljibury , F. K. 2011 . Managing clay soils in the home garden . Available at: http://vric.ucdavis.edu/pdf/soil_managingclay.pdf (Accessed 24 June 2011) .
- Alloway , B. J. 1995 . Heavy metals in soils , Blackie Academic & Professional .
- Alloway , B. J. and Jackson , A. P. 1991 . The behavior of heavy metals in sewage sludge amended soils . Journal of Science of the Total Environment , 100 : 151 – 176 .
- Anon . 2011 . Habitat and ecology . Available at: http://www.iucnredlist.org/apps/redlist/details/33212/0#top (Accessed 21 June 2011) .
- Arvind , P. 2004 . Zinc protects chloroplasts and associated photo-chemical functions in cadmium exposed Ceratophyllum demersum (L.) a fresh water macrophyte . Plant Science , 166 : 1321 – 1327 .
- Baek , K. H. , Chang , J. Y. , Chang , Y. Y. , Bae , B. H. , Kim , J. and Lee , I. S. 2006 . Phytoremediation of soil contaminated with cadmium and/or 2,4,6-Trinitrotoluene . Journal of Environmental Biology , 27 : 311 – 316 .
- Baker , A. J. M. 1981 . Accumulators and excluders – strategies in the response of plants to heavy metals . Journal of Plant Nutrition , 3 : 643 – 654 .
- Bhattacharyya , B. D. , Whelton , M. H. , Stern , P. H. and Peterson , D. P. 1988 . Cadmium accelerates bone loss in ovariectomized mice and fetal rat limb bones in culture . Proceedings of the National Academy of Sciences USA , 85 : 8761 – 8765 .
- Brady , N. C. and Weil , R. R. 2002 . The nature and properties of soils , New York : Macmillan Publishing Company .
- Buchet , J. P. , Lauwerys , R. , Roels , H. , Bernard , A. , Bruaux , P. , Claeys , F. , Ducoffre , G. , De Plaen , P. , Staessen , J. , Amery , A. , Lijnen , P. , Thijs , L. , Rondia , D. , Sater , F. , Saint , A. and Nick , L. 1990 . Renal effects of cadmium body burden of the general population . Lancet , 336 : 699 – 702 .
- Golovatyj , S. 2002 . Heavy metals in agroecosystems , Russia : Kojak Press .
- Ho , W. M. , Ang , L. H. and Lee , D. K. 2008 . Assessment of Pb uptake, translocation and immobilization in kenaf (Hibiscus cannabinus L.) for phytoremediation of sand tailings . Journal of Environmental Science , 20 : 1341 – 1347 .
- Jackson , M. L. 1973 . Soil chemical analysis , New Delhi : Prentice Hall of India Pvt. Ltd .
- Khan , S. and Khan , N. N. 1983 . Influence of lead and cadmium on the growth and nutrient concentration of tomato (Lycopersicon esculentum) and egg-plant (Solanum melongena) . Plant and Soil , 74 : 387 – 394 .
- Knight , B. , Zhao , F. J. and McGrath , S. P. 1997 . Zinc and cadmium uptake by the hyperaccumulator Thlaspi caerulescens in contaminated soils and its effects on the concentration and chemical speciation of metals in soil solution . Plant and Soil , 197 : 71 – 78 .
- Liu , J. , Zhou , Q. , Sun , T. , Lena , Q. M. and Wang , S. 2008 . Growth responses of three ornamental plants to Cd and Cd–Pb stress and their metal accumulation characteristics . Journal of Hazardous Materials , 151 : 261 – 267 .
- Lorenz , S. E. , Hamon , R. E. , McGrath , S. P. , Holm , P. E. and Christensen , T. H. 1994 . Applications of fertilizer cations affect cadmium, zinc concentrations in soil solutions and uptake by plants . Europe Journal Soil Science , 45 : 159 – 165 .
- Majid , N. M. , Islam , M. M. , Melina , E. N. , & Arifin , A. 2011 . Heavy metal uptake and translocation by Justicia gendarussa Burm F. from textile sludge contaminated soil . Acta Agriculturae Scandinavica, Section B – Soil & Plant Science . Available at: http://www.informaworld.com/smpp/title∼content=t713394126 (Accessed 15 June 2011) .
- Mangkoedihardjo , S. and Surahmaida , A. 2008 . Jatropha curcas L. for phytoremediation of lead and cadmium polluted soil . World Applied Sciences Journal , 4 : 519 – 522 .
- Middleton , D. J. 2004 . “ Apocynaceae ” . In Tree flora of Sabah and Sarawak , Edited by: Soepadmo , E. , Saw , L. G. and Chung , R. C. K. Kuala Lumpur : Government of Malaysia .
- Mojiri , A. 2011 . The potential of corn (Zea mays) for phytoremediation of soil contaminated with cadmium and lead . Journal of Biological and Environmental Sciences , 5 : 17 – 22 .
- Rahimi , B. and Nejatkhah , M.P. 2010 . Availability, accumulation and elimination of cadmium by Artemia urmiana in different salinities . Journal of Biological and Environmental Sciences , 4 : 149 – 157 .
- Rice , C. W. 2009 . Storing carbon in soil: Why and how? Available at: http://www.geotimes.org/jan02/feature_carbon.html#author (Accessed 25 November 2009) .
- Ryan , J. A. , Pahren , H. R. and Lucas , J. B. 1982 . Controlling cadmium in the human food chain: A review and rationale based on health effects . Environmental Research , 28 : 251 – 302 .
- Sahoo , S. K. , Muramatsu , Y. , Yoshida , S. , Matsuzaki , H. and Ruhm , W. 2009 . Determination of 129I and 127I concentration in soil samples from the Chernobyl 30-km zone by AMS and ICP-MS . Journal of Radiation Research , 50 : 325 – 332 .
- Shelmerdine , A. , Black , C. , McGrath , S. and Young , S. 2009 . Modelling phytoremediation by the hyperaccumulating fern, Pteris vittata, of soils historically contaminated with arsenic . Environmental Pollution , 157 : 1589 – 1596 .
- Steel , R. C. B. , Torrie , J. H. and Dickey , D. A. 1996 . Principles and procedures of statistics , New York : McGraw Hill .
- Tomar , M. , Kaur , I , Neelu and Bhatnagar , A. K. 2000 . Effect of enhanced lead in soil on growth and development of Vigna radiata (L.) Wilezek . Indian Journal of Plant Physiology , 5 : 13 – 18 .
- Wagner , G. J. 1993 . Accumulation of cadmium in crop plants and its consequences to human health . Advances in Agronomy , 51 : 173 – 212 .
- Xiao , X. , Tongbin , C. , Zhizhuang , A. and Mei , L. 2008 . Potential of Pteris vittata L. for phytoremediation of sites co-contaminated with cadmium and arsenic: The tolerance and accumulation . Journal of Environmental Sciences , 20 : 62 – 67 .
- Yoon , J. , Cao , X. D. , Zhou , Q. X. and Ma , L. Q. 2006 . Accumulation of Pb, Cu and Zn in native plants growing on a contaminated Florida site . Science of the Total Environment , 368 : 456 – 464 .