Abstract
Microbial communities are integral parts of the soil and their activity is very important to the functioning of the soil but the impact of different factors on soil microbial community composition is not well researched. Many studies have focused only on a few species. The aim of this study was to investigate the impact of treatment and sampling date on soil pH, abundance of total number of bacteria, molds, yeasts, mesophilic spore-forming bacteria, Fusarium spp., actinomycetes, azotobacteria, cellulose decomposers, denitrifying and nitrifying bacteria in organically (ORGGRM – green manure, and ORGFYM – green manure, solid cattle manure) and conventionally (CONFYM – solid cattle manure + mineral fertilizer and pesticides) cultivated soil.
Fertilization with manure had positive direct- and after-effect (p<0.05) on the total number of bacteria, mesophilic spore-forming bacteria, nitrifying bacteria and cellulose decomposers, even in the CONFYM treatment. The abundance of yeasts was higher (p<0.05) in ORGFYM treatment (3.35×103) and 34–48% lower in CONFYM and ORGGRM treatments.
The abundance of molds, Fusarium spp., and actinomycetes during the study in different treatments was similar but their abundance was significantly higher (p<0.05) when the weather in the sampling time was warm and humid.
The negative impact of pesticides in CONFYM treatment occurred on the total number of bacteria, yeasts, molds, and denitrifying bacteria. The yeasts were most sensitive to pesticides; in study years, when the use of pesticides was very intensive, their abundance in CONFYM treatment decreased 72% compared with the ORGFYM treatment.
The 4-year test results showed that although green manuring is considered an important management practice in organic cultivation, to maintain and increase the abundance of microbes in different microbial communities it is important to use other organic fertilizers such as animal manure in addition to green manure.
Introduction
The diversity and abundance of life in the soil is richer than in any other ecosystem. Microorganisms play a critical role in soil quality in supporting plant growth. They stimulate plant growth by facilitating the assimilation of phosphorus and iron, nitrogen fixation, releasing phytohormones, inhibiting root pathogens, and synthesizing antibiotics (Glick Citation1995).
Microbial communities adapt sensitively to changing environmental conditions by varying individual activity (Novak et al. Citation1993). Microorganisms respond sensitively to changes and environmental stress because they have intimate relations with their surroundings due to their high surface-to-volume ratio. The season, soil humidity, pH, fertilization, and other factors determine the number and species composition of microorganisms in soil. For example, the supplement of organic fertilizers particularly stimulates bacteria and actinomycetes, reducing the fungal population (Novak et al. Citation1993). In some instances, changes in microbial communities can precede detectable changes in soil properties or in plant and animal communities, thereby providing an early sign of soil improvement or an early warning of soil deterioration (Pankhurst et al. Citation1995).
Within the last 50 years, farmers in some parts of the world have been able to markedly increase total crop yields. Farmers in these areas have managed to intensify farming systems using technologies that rely on agricultural chemicals, mechanization, and plant breeding. Unfortunately, intensification in many cases has come with an environmental price, caused by the overuse of agricultural inputs, the application of practices which lead to the deterioration of soils and the mismanagement of natural resources. Synthetic pesticides are intentionally introduced into agricultural systems to protect crops against weeds, insects, fungi, and other pests. However, the majority of the applied pesticides, even if sprayed on foliage of crop plants and weeds, will eventually reach the soil, which may affect the growth and activity of soil microbial communities (Omar and Abdel-Sater Citation2001, Singh and Singh Citation2005).
Numerous studies have indicated that organic farming has higher potential to accommodate biological concerns than conventional farming (Stolze et al. Citation2000). Plant production in organic farming mainly depends on nutrient release as a function of mineralization processes in soils. Therefore, active soil microflora and a considerable pool of accessible nutrients is an important priority in organic farming. Fertilizing the soil rather than the plant is an organic farmer's goal to assure sufficient nutrient mineralization to meet his economic needs (Fliessbach and Mäder Citation2000). The most important driving factors for these services are the amount and quality of organic manure and mulch, soil tillage, crop rotation, and crop diversity. Legumes in rotation supply symbiotically fixed nitrogen to the system, aid in maintaining proper water status and reduce pathogen load. Studies have shown the positive effects of crop rotation on crop growth, attributing this to changes in the bacterial community composition (Shipton Citation1977). Plant residues supplement the soil with organic matter and improve its microbiological and biochemical characteristics (Perucci et al. Citation1997).
The development of Estonian organic farming began over 20 years ago in 1989. Since 2000, the organically farmed land has expanded more than ten times. In 2010, organic land (121 817 ha) was about 13% of all agricultural land in use, but only nearly two-thirds of organic farmers in Estonia keep animals (Vetemaa and Mikk Citation2011). The greatest challenge for stockless organic farming is management of the nutrient supply. There is greater emphasis on alternative fertility-building strategies, such as the use of green manure, and the import of manure, compost, and other acceptable fertilizers. Unfortunately in Estonia manures are most often not sufficiently available in organic arable farming. Green manure commonly replaces farmyard manure amendments on stockless farms and a question of great concern is whether green manure can be considered to be equal to farmyard manure as a carbon source for improving and sustaining soil biological properties and fertility.
Inputs of green manure or crop residues can increase the size and activity of soil microbial communities (Bolton et al. Citation1985, Martens et al. Citation1992, Kirchner et al. Citation1993, Fauci and Dick Citation1994, Kautz et al. Citation2004, Manici et al. Citation2004), but the impact on soil microbial community composition and function is not well understood.
The aim of this study was to investigate the impact of the cultivation methods on the abundance of total number of bacteria, molds, yeasts, mesophilic spore-forming bacteria, Fusarium spp., actinomytcetes, azotobacteria, cellulose decomposers, denitrifying and nitrifying bacteria in organically (with and without farmyard manure) and conventionally (manure, mineral fertilizer, and pesticides were used) cultivated soil.
Materials and methods
Experimental site
The field trial was performed in Central Estonia at Olustvere (58° 33′ N, 25° 34′ E) during 2007–2010. The soil type of the Olustvere field was loamy sod-podzolic soil according to the WRB 1998 classification (FAO, ISSS, ISRIC. Citation1998). In the trial area the field crops have been cultivated according to the principles of organic farming since 2002. Conventional tillage was used in all treatment variants.
Experimental set up
Since 2007 there was a five-field crop rotation; potato (Solanum tuberosum L., 2007), oats (Avena sativa L., 2008), barley (Hordeum vulgare L.) with undersown red clover (Trifolium pratense L., 2009), red clover (2010) and winter rye (Secale cereale L., 2011, data not included in present paper). The size of each field in the crop rotation was 1.2 ha, which was divided into three equal parts (4000 m2) between the cultivation methods. Since 2007 the following cultivation methods were carried out: organic (ORGGRM) with green manure; organic (ORGFYM) with solid cattle manure and green manure; and conventional (CONFYM) – green manure, cattle manure, mineral fertilizers, and pesticides were used. Solid cattle manure at the rate of 60 t ha−1 was applied in spring 2007. The clover was cut and ploughed under the soil in the beginning of July 2010. The total input of plant nutrients and pesticides is shown in .
Table I. Input of plant nutrients (with solid cattle manure and mineral fertilizers) and pesticides. The input of plant nutrients and active ingredients is the average of 2007–2010.
The most intensive use of pesticides in the CONFYM treatment was in 2007, when during the potato growth 2.4 kg ha−1 active ingredients in total were used: 300 g ha−1 herbicide Sencor (700 g kg−1 metribuzin), 0.3 L ha−1 insecticide Fastac (alpha-cypermethrin 50 g L−1), 2.5 kg ha−1 fungicide Ridomil Gold MZ 68 WG (metalaxyl 40 g kg−1, mancoceb 640 g kg−1), and three times 0.3 L ha−1 fungicide Shirlan (fluazinam 500 g L−1). In the following years the use of pesticides in CONFYM treatments was lower. In 2008 for oats two herbicides were used: 0.15 L ha−1 Sekator 375 OD (amidosulfuron 100 g L−1, jodosulfuron-metyl-natrium 25 g L−1) and 3 L ha−1 Roundup Gold (glyphosate 450 g L−1). In 2009 for barley the herbicide MCPA 0.9 kg ha−1 (MCPA 750 g L−1) and in 2010 for red clover the herbicide Agil 1 L ha−1 (propaquizafop100 g L−1) was used.
Weather conditions
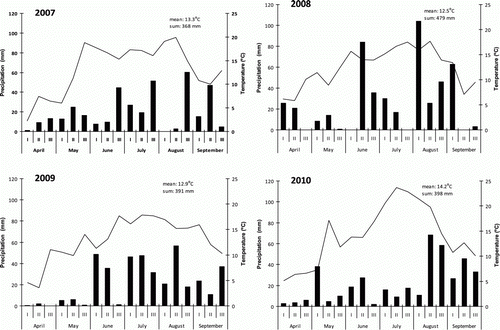
The weather conditions during the vegetation period in 2007–2010 were different (). In 2007 and 2010 the 6-month average temperature was 0.8–1.7 degrees warmer than in other years. The total amount of precipitation in 2007 was less than in 2008 and 2009. The average temperature in 2009 and 2010 on sampling time in April was similar, but April 2009 was extremely dry. The total amount of precipitation during the vegetation period was highest in 2008, when the total rainfall was 479 mm. Lowest total precipitation occurred in 2007 (368 mm). Also the distribution of precipitation differs by the year, such as in 2010, when the total precipitation of May, June, and July were only 143 mm but in August 137.6 mm and in September 105.2 mm. During our study the precipitation levels in September were similar, except in 2010, when the total amount of precipitation was 105.2 mm i.e. 22–24% higher than in 2007–2009 (67–71 mm).
Soil sampling
Soils were sampled in 2007–2010 in September, after harvesting and in 2009 and 2010 in April, before cultivation. Soil samples from each treatment in three replications were taken by a random method from the 0–20 cm soil layer (plough layer) with a 1 cm diameter auger. Soil samples were kept at 4 °C until they were analysed in the laboratory.
Soil chemistry
Soil pH (H2O) was measured according to the standard ISO 10390:Citation2005.
Microorganisms
All soil samples were examined microbiologically for total number of bacteria, molds, yeasts, mesophilic spore-forming bacteria, Fusarium spp., actinomycetes, azotobacteria, cellulose decomposers, denitrifying and nitrifying bacteria using the plate-count method. Decimal dilution series were prepared in accordance with EVS-EN ISO 6887-1:Citation2001. Microbiological counts were expressed as a number of colony-forming units (CFUs) g−1 of dry soil.
Plate Count Agar was used for isolation of total number of bacteria at 30 °C for 72 h (NMKL No 86, 3rd Edn, Citation1999 and ICC no. 125, Citation1978). For yeasts and molds the wort-agar medium were used at 25 °C for 5–7 days (ICC Standard no. 146, Citation1992). The total number of aerobic mesophilic spore-forming bacteria was estimated on the spore medium at 30 °C for 72 h (ICC no. 144 Citation1992). The number of Fusarium spp. was defined on Nash and Snyder culture medium (Booth Citation1971, Gerlach and Nirenberg Citation1982). To identify the azotobacteria the Ashby culture media were used. The cellulose decomposers were defined on Hutchinson culture medium and nitrifying bacteria on water agar. For denitrifying bacteria the Hiltay culture media was used (Viileberg Citation1966).
Data analyses
All results were based on three soil replicates. The data were analysed by ANOVA. The Tukey–Kramer honestly significant difference (HSD) test was used, and effect of treatment, sampling date and their interaction on CFU microbes and the correlation between soil pH (H2O) and different CFU microbes were tested, using the software JMP 5.0.1.2 (SAS Citation2002).
Results and discussion
Soil pH
Soil pH is one of the most influential factors in soil, and strongly influences the biomass, activity, and composition of the microbial community (Matthies et al. Citation1997, Blagodatskaya and Anderson Citation1998, Lauber et al. Citation2008, Jones et al. Citation2009).
At the beginning of 2007 the soil pH was higher in CONFYM (p<0.05, pH 7.06) and ORGFYM (pH 7.02) treatments than in ORGGRM treatment (pH 6.7, ). The results of 2007–2010 showed slightly higher soil pH in ORGFYM treatment (pH 6.89) and lower in ORGGRM and CONFYM treatments (pH 6.73 and 6.74). Mäder and his colleagues in their study (Citation2002) also found slightly higher soil pH in organic systems.
Table II. Mean values (CFU g−1 dry soil) of treatments and sampling dates as well as their interaction. Different letters behind the mean values indicate significant differences (p<0.05) in a category. Significance of model effects (p>F) is indicated. In the case of significant model effects a post hoc Tukey HSD test was performed to compare mean values.
During the test cycle, the pH was quite variable, which could be caused by the use of mineral fertilizer in CONFYM treatment as well as the weather conditions. Therefore although in 2007 the soil pH in CONFYM treatment was highest (pH 7.06), it showed a decreasing tendency year by year, while in other treatments the pH was more stable. In September 2010 in all treatments the pH was lowest (pH 6.5) of the test cycle. One reason could be the higher precipitation (105.2 mm) in September. During high rainfall the water passing through the soil leaches basic cations into drainage water. These basic cations are replaced by acidic cations such as aluminum (Al3 +) and hydrogen (H+). For this reason, soil pH decreases under high rainfall conditions (Hallik Citation1963). Another reason could be that in July 2010, the clover was cut and ploughed under the soil. In September while the samples were taken, the active clover residue decomposing process was taking place, which could decrease the soil pH. It has been found that the decay of green manures, especially in large quantities, tended to the formation of acid decomposition products and thus to an increase of soil acidity (Pieters Citation1927, Belachew and Abera Citation2011).
Soil pH is often correlated with important environmental factors influencing the microbial community, including nutrient availability (Fageria and Barbosa Citation2008). In our research the pH had a positive correlation with the denitrifying bacteria (p<0.01, r = 0.4174) and yeasts (r = 0.3901, ). Valera et al. (Citation1961) also found that the size of the denitrifying population was positively correlated with soil pH, and the denitrifying bacteria were more sensitive to acid environments than the bacterial microflora as a whole. Soil pH is a crucial abiotic factor having not only influence on the denitrification rate but, even more importantly, the proportion of the two major denitrification products, N2O and N2 (Šimek and Cooper Citation2002).
Table III. Correlation matrix (r) of pH(H2O) and CFU microbes at soil depth of 0–20 cm (n = 54, azotobacteria n = 36).
Conversely, it is well known that the optimum growth pH range for yeast is 3.0–5.0 and that they can also grow well at neutral pH range. Our research results showed the positive correlation between pH and yeasts, although Vreulink et al. (2007) found in their study on sandy soil that the correlation between soil pH and culturable soil yeast numbers is negative and Moawad et al. (Citation1986) observed no significant effect at all.
A positive correlation could be due to the fact that in 2009, when the acidity of the soil was higher than in the other years, in some of the soil replications no yeasts occurred. In April 2009 the weather was extremely dry; because of that it is possible that lower soil moisture levels may have a negative affect on the soil yeast populations.
The soil pH had negative correlation with cellulose decomposers (r = 0.4754, p<0.01) and with mesophilic spore-forming bacteria (r = 0.2937, p<0.05). The pH range for cellulose decomposers’ growth is rather narrow, approximately 6.5–8.5; in general, there is no good growth below pH of 6.6 or above pH 8.0 (Couke and Voets Citation1967). It is found that some of the cellulose decomposers will grow at a pH down to about 5.0 (Couke and Voets Citation1967). Although the soil pH ratio (pH 6.50–7.13) was good for cellulose decomposers’ growth the negative correlation can mean that in the test area there were more acidophilous cellulose decomposers in the soil than the neutrophil cellulose decomposers.
The influence of cropping system on CFU microbes
Total number of bacteria
The treatment and sampling date as well as their interaction had an impact on the total number of bacteria (p<0.0001, ). The total number of bacteria was higher in ORGFYM (8.17×106 CFUs) and lower in ORGGRM (5.87×106 CFUs) treatments.
The total number of bacteria had strong positive correlation with yeasts (r = 0.3609, p<0.01, ), and negative correlation with actinomycetes (r = 0.4159, p<0.01) and molds (r = 0.273, p<0.05).
In fall 2007, the abundance of total number of bacteria was higher (p<0.05) in ORGFYM treatment (9.24×106 CFUs, ). Their abundance were 35% lower in ORGGRM (5.97×106 CFUs) and 46% lower in CONFYM (5.02×106 CFUs) treatments. The main reason could be the intensive use of pesticide in CONFYM treatment. Although in 2007 the CONFYM as well as the ORGFYM treatment were fertilized with manure, during potato growth, the CONFYM treatment was treated once with herbicide and insecticide and four times with different fungicides (sum of active ingredients 2.4 kg ha−1).
In 2008, after potato in crop rotation the oats was cultivated. In that year in CONFYM treatment the herbicides and mineral fertilizer were used only once. The results showed a similar amount of total number of bacteria in ORGFYM (10.90×106 CFUs) and CONFYM (10.00×106 CFUs) treatments (). However, in the ORGGRM treatment without manure the total number of bacteria was lower (7.96×106 CFUs). April 2009 was extremely dry (). This could be the main reason that caused lower abundance of bacteria in all experimental treatments. In spring 2009, the total number of bacteria was higher in CONFYM (5.54×106 CFUs) treatment. In fall 2009 significant differences between treatments did not occur. In fall 2010, 33% higher abundance of bacteria was in ORGFYM (10.48×106 CFUs) compared with ORGGRM (8.07×106 CFUs).
The results of the study showed that fertilization with manure had positive direct- and after-effect on the total number of bacteria, even in CONFYM treatment. However in 2007, when in the conventional CONFYM treatment an excess amount of pesticide was used, the abundance of total number bacteria was lower than in other treatments. In 2008, in both treatments with manure (ORGFYM, CONFYM) the abundance was much higher than in the organic without manure treatment (ORGGRM).
Molds
It is known that the molds derive energy not through photosynthesis but from the organic matter in which they live. Typically, molds secrete hydrolytic enzymes, mainly from the hyphal tips. These enzymes degrade complex biopolymers such as starch, cellulose, and lignin into simpler substances which can be absorbed by the hyphae. In this way, molds play a major role in causing decomposition of organic material, enabling the recycling of nutrients throughout ecosystems (Madigan et al. Citation2003).
The statistical model showed that sampling date was the only factor that had effect on their abundance (). Although the abundance during the study in different treatments was similar and statistically significant differences did not occur, in 2007, there was a tendency that in CONFYM treatment (treated with pesticides i.e. with fungicides) the abundance of molds was 13–14% lower than in ORGFYM and ORGGRM treatments. Anderson (Citation1978) has pointed out that soil fungi and actinomycetes are not as susceptible to herbicides and insecticides as they are to fungicides.
In general, the abundance in all treatments was at its lowest measurement in fall 2008 (4.65–5.45×104 CFUs) and at the highest in fall 2009 (7.05–9.13×104 CFUs). This was caused by the fact that in September 2009, the amount and distribution of rainfall was uniform, and the daily average temperature was higher than in September 2008. It is well known that warm and humid weather is favorable for the growth of molds.
Their strong positive correlation (p<0.01, ) appeared with mesophilic spore-forming bacteria (r = 0.5842), Fusarium spp. (r = 0.6102) and actinomycetes (r = 0.6192). Also it had a positive correlation (p<0.05) with the nitrifying bacteria (r = 0.3364).
Yeasts
Yeasts are important members in many ecosystems and form a significant contribution to biodiversity (Fleet Citation1998). The soil is the ultimate repository for storage and even development of certain species of yeasts (Phaff and Starmer Citation1987). Most of the yeast species possess a wide spectrum of metabolic abilities, enabling them to utilize many of the hydrolytic products of plant materials generated by fungal and bacterial activities (Phaff and Starmer Citation1987).
The number of yeasts correlated positively with soil pH (p<0.01, r = 0.3901) and with total number of bacteria (p<0.01, r = 0.3609, ). All the analysed factors i.e. treatment, sampling date, and their interaction had significant impacts on the number of yeasts (). In 2007, as well as with the total number of bacteria their higher abundance was in ORGFYM treatment (6.05×103 CFUs). In 2007, the number of yeasts in CONFYM treatment was lower (1.69×103 CFUs). This suggests that the yeasts are very sensitive to fungicides, which were used four times in 2007. Dickinson (Citation1973) reported that some of fungicides reduced soil yeast populations. However, in fall 2008, the number of yeast populations in CONFYM treatment was already at the same level as in the other treatments. The reason could be that in 2008 in CONFYM treatment the herbicide was used only once and no other pesticides were used at all. In 2008 the abundance of yeasts was in all treatments highest in the test period. These could be a result of high precipitation (479 mm) during the vegetation period, although the Vreulink et al. (Citation2007) study found that the soil moisture content does not affect the soil yeast population size. The other reason could be that yeasts are particularly numerous on roots of certain plants such as cabbage, corn, sugar beet, and oats (Babeva and Belyanin Citation1966, Alexander Citation1977, Phaff et al. Citation1978). Thus it is possible that the oats growing in 2008 could increase the number of yeasts in the soil.
In the following years, their number declined, and significant differences between the treatments were no longer shown.
Mesophilic spore-forming bacteria
The treatment (p<0.0001), as well as sampling date (p<0.0001) and their interaction (p=0.016, ) impacted the abundance of mesophilic spore-forming bacteria. Higher numbers of mesophilic spore-forming bacteria were in CONFYM (4.95×105 CFUs) and ORGFYM (4.73×105 CFUs) treatments. The correlation with soil pH was negative (r = 0.2937, p<0.05, ). Mesophilic spore-forming bacteria correlated positively (p<0.01) with molds (r = 0.5842), Fusarium spp. (r = 0.5878), actinomycetes (r = 0.6363), nitrifying bacteria (r = 0.6282), and cellulose decomposers (r = 0.461).
In fall 2007, compared with ORGGRM treatment (2.52×105 CFUs, ) the average number of mesophilic spore-forming bacteria was higher in ORGFYM treatment (6.49×105 CFUs). In fall 2008, their greatest abundance as well as the abundance of yeasts, occurred in CONFYM treatment (5.34×105 CFUs, ). In fall 2010 in ORGFYM (5.74×105 CFUs) and CONFYM (7.10×105 CFUs) treatments the number of mesophilic spore-forming bacteria was 20% and 71% higher than in ORGGRM (2.03×105 CFUs) treatment.
Spore-forming bacteria are versatile microorganisms able to produce spores highly tolerant to adverse environmental conditions, e.g. high temperature and drought (Gorlach-Lira and Coutinho Citation2007) and intensive fertilizer and pesticide application (Bigelow et al. Citation2002). However, in fall 2007 when in CONFYM treatment (4.96×105 CFUs) the use of pesticides was most intensive, their abundance was 24% lower than in ORGFYM (6.49×105 CFUs) treatment. So we can conclude that the intensive use of pesticides still have a negative impact on their abundance.
Fusarium spp
Fusarium species are ubiquitous in soil and are important worldwide plant pathogens (Domsch and Gams Citation1970). Fusaria exist in soil as colonizers of living plants or plant residues within the soil or adjacent to the soil surface (Burgess Citation1981).
The factors that influenced the abundance of Fusarium spp. were the sampling date, and treatment and sampling date interaction ().
The lowest Fusarium spp. abundance occurred in 2007 and 2008, when the number of Fusarium spp. in trial treatments ranged from 0.99 to 2.32×103 (CFUs, ) and highest in fall 2009 (7.54 to 13.43×103 CFUs). The reason is that in September 2009 the distribution of rainfall was uniform and the daily average temperature was high (). Also McMullen et al. (Citation1997) states that for the Fusarium population frequent rainfall and high humidity are favorable.
In addition it should be noted, that Fusarium spp. had a strong positive correlation with molds, mesophilic spore-forming bacteria, actinomycetes, and cellulose decomposers (p<0.01, r = 0.3739, ).
Actinomycetes
The actinomycetes comprise more than 30% of the total population of microorganisms in soil; however, their biomass contribution is variable and much less than that of fungi (Kuster Citation1968, Gray and Williams Citation1971). In nature, they play an important role in the cycling of organic compounds and have also been associated with soil organic matter production.
Sampling date as well as its interaction with treatment had influence on the abundance of actinomycetes (). On their abundance, the treatment did not have any effect at all. In different treatments, over the entire period of study, the abundance of actinomycetes was similar and ranged from 0.77 to 3.27×106 (CFUs, ). Frey et al. (Citation1999) and Beare et al. (Citation1992) also found that the total actinomycete communities were affected only minimally by tillage regime and not at all by nitrogen fertilization. Soil organic matter content, pH, and moisture also failed to influence actinomycete communities in Western Australian soils (Keast and Tonkin Citation1983).
As with molds and Fusarium spp., their abundance was highest in fall 2009, and ranged from 2.08 to 3.27×106 (CFUs). This could be caused by the constant rainfall during the vegetation period and the slightly higher monthly daily temperature in September 2009. Although Keast and Tonkin (Citation1983) found in their study that soil moisture did not influence the actinomycete communities, we can conclude as well as in case of molds and Fusarium spp., that the humid and warm climate was favorable for the development of actinomycetes.
Actinomycetes are affected directly by the presence of available carbon sources and their number is especially high in land rich in organic matter. Amendment with organic nutrients such as crop residues and animal manure increases the abundance of actinomycetes (Alexander Citation1977). It should be the main reason why in fall 2008, in all treatment variants the abundance of actinomycetes was lower and ranged from 0.77 to 0.91×106 (CFUs), because after the crops which were growing in 2007 and 2008 (potato and oats) the content of organic matter was insufficient for development of actinomycetes.
In addition to the above-mentioned positive and negative correlations with the total number of bacteria, molds, and Fusarium spp., they also had a strong positive correlation with nitrifying bacteria (p<0.01, r = 0.5746).
Denitrifying bacteria
Denitrification is mainly sustained by denitrifying bacteria, although the ability of denitrification is also found in certain fungi (Zumft Citation1997). Denitrifying bacteria reduce nitrate (NO3−) to nitrous oxide (N2O) or to nitrogen gas (N2). With the ability to degrade organic matter, denitrifying bacteria play a crucial function in reducing organic carbon, thereby reducing nitrate in the wastewater and soils (Hallin and Pell Citation1998, Pai et al. Citation1999, Song et al. Citation2000). Factors regulating denitrification rates are low O2 partial pressure, available NO3 to serve as an oxidant, and organic C as an energy source for heterotrophic bacteria (Williams et al. Citation1992).
Factors that influenced the abundance of denitrifying bacteria in our study were treatment, sampling date, and their interaction (). The correlation matrix showed a strong positive correlation between the soil pH and denitrifying bacteria (p<0.01, r = 0.4174, ). Correlations between denitrifying bacteria and other microbe communities did not occur ().
In fall 2007, the tendency of denitrifying bacteria in CONFYM treatment showed 43% (2.82×105 CFUs) and in ORGGRM 77% (1.64×105 CFUs) lower abundance than in ORGFYM (4.95×105 CFUs, ) treatments. Enwall et al. (Citation2005) found in her study that the potential denitrifying and soil respiration rates were significantly higher in the field plots amended with organic fertilizers. The lower number of denitrifying bacteria in CONFYM treatment suggests that they are sensitive to pesticides. The denitrifying activity is often used for testing the effects of the pesticides because of their sensitivity to environmental toxicants. In following years, significant differences between treatments did not occur.
Nitrifying bacteria
Nitrifying bacteria are responsible for the biological oxidation of ammonia. These bacteria are chemolithotrophs, obtaining chemical energy from the oxidation process. This energy is used to elaborate organic compounds from carbon dioxide. Nitrifying bacteria usually occur in small numbers in upper layers of sediments as they are obligate aerobes (Kolwzan et al. Citation2006).
Treatment variant, sampling date (p<0.0001, ) as well as their interaction (p=0.009) had an impact on the abundance of nitrifying bacteria. Their number was higher in CONFYM (3.27×104 CFUs) and ORGFYM (2.89×104 CFUs) treatments, where the solid cattle manure was used and lower in ORGGRM treatment (1.90×104 CFUs), where only the green manure was used ().
The abundance of nitrifying bacteria was greatest in September 2009 (2.12 to 4.43×104 CFUs). This could also have been caused by the uniform rainfall and average 1.6–2.8 °C higher daily month temperature in September 2009, which was favorable on the development of nitrifying bacteria (, ). Nitrification is favored at moderate pH and in well-aerated soils, but declines as soils become very dry. The temperature response of nitrification is approximately with an optimum between 20 °C and 35 °C. The decline at higher temperatures may be partially due to increased biological O2 consumption (Prosser Citation1989, Grundmann et al. Citation1995, Parton et al. Citation2001, Avrahami et al. Citation2003).
The abundance of nitrifying bacteria was positively correlated (p<0.01, ) with actinomycetes (r = 0.5746), mesophilic spore-forming bacteria (r = 0.6282) and molds (r = 0.3364, p<0.05), as discussed above.
Azotobacteria
Azotobacter is a bacterium that can fix atmospheric nitrogen into the soil without the aid of a legume. It has beneficial effects on plant yields, due to the increase of fixed nitrogen content in soil (Mrkovački et al. Citation1996, Zahir and Arshad, Citation1996, Zahir et al. Citation1996, Pandey et al. Citation1998).
The abundance of azotobacteria was determined only in 2009 and 2010. Two-year data showed that none of the factors had an effect on the abundance of azotobacteria (). Fluctuations between the replications were extreme and significant differences between the treatments did not occur (). During the two-year study, their numbers ranged between 0.4 and 5.4×10 (CFUs).
However, at the same time the results of the analysis in spring 2009, spring 2010 and fall 2009 showed tendency of greater abundance in ORGGRM treatment where cattle manure, mineral fertilizers, and pesticides were not applied.
There were no statistically significant differences between abundance of actinomycetes and other microbes ().
Cellulose decomposers
The main cellulose-utilizing species are the aerobic and anaerobic hemophilic bacteria, filamentous fungi, basidiomycetes, thermophilic bacteria, and actinomycetes (Wright Citation2003).
The factors that influenced the number of cellulose decomposers in our research were treatment (p=0.0018), sampling date (p<0.0001) as well as their interaction (p=0.0249, ). Numbers of cellulose decomposers were higher in CONFYM (2.71×103 CFUs) and ORGFYM (2.92×103 CFUs) treatments and lower in ORGGRM (2.29×103 CFUs) treatment.
The abundance of cellulose decomposers was lowest in fall 2008. In September 2008, the daily temperature was the study period lowest and the distribution of precipitation was irregular (). This could affect the cellulose decomposers’ development in the soil. The rainfall in the beginning of September was high (63 mL), but in the middle and end of the month no or very small amount of precipitation occurred. Mendelssohn et al. (Citation1999) note that the soil moisture, temperature as well as fertility, oxygen, and pH are the important extrinsic abiotic variables affecting decomposition rate. Biotic factors such as plant residues quality and faunal activity are also important. Gillespie et al. (Citation1988) found that some fungi were effective cellulose degraders in fertile soil, whereas others were more effective in nutrient-poor soils. These studies support the conclusion of Park (Citation1976) who showed that the influence of nitrogen on the cellulolytic ability of soil micro-fungi varied from one species to another; some species respond better to low nitrogen levels, whereas others responded better to high levels. Although we did not measure the species of cellulose decomposers we could conclude that cellulose decomposers belonged to the species that responded better in high nitrogen levels, because their abundance was higher in the ORGFYM treatment where cattle manure was used and in CONFYM treatment where cattle manure and mineral fertilizers were used.
The 4-year test results showed that the application of cattle manure in organic treatment (ORGFYM) compared with organic treatment without manure (ORGGRM) increased the abundance of total number of bacteria, molds, yeasts, denitrifying bacteria and the soil pH. The use of solid cattle manure had a positive effect on the abundance of mesophilic spore-forming bacteria, nitrifying bacteria and cellulose decomposers, even in conventional (CONFYM) treatment. Therefore, although green manuring is considered an important management practice in organic cultivation, to maintain and increase the abundance of microbes in different microbial communities other organic fertilizers such as animal manure are important additions to green manure.
Acknowledgements
This work was funded by the Estonian Ministry of Agriculture. The authors would like to thank Helgi Laitamm for her great work in the laboratory.
References
- Alexander , M. 1977 . Introduction to Soil Microbiology , New York : John Wiley & Sons .
- Anderson , J. R. 1978 . “ Pesticide effects on non-target soil microorganisms ” . In Pesticide microbiology , Edited by: Hill , I. R. and Wright , S. J. L. 611 – 628 . London : Academic Press .
- Avrahami , S. , Liesack , W. and Conrad , R. 2003 . Effects of temperature and fertilizer on activity and community structure of soil ammonia oxidizers . Environmental Microbiology , 5 : 691 – 705 .
- Babeva , I. and Belyanin , A. I. 1966 . Yeasts of the rhizosphere . Mikrobiologiya , 35 : 712 – 720 .
- Beare , M. H. , Parmelee , R. W. , Hendrix , P. F. , Cheng , W. , Coleman , D. and Crossley , D. A. Jr . 1992 . Microbial and faunal interactions and effects on litter nitrogen and decomposition in agroecosystems . Ecological Monographs , 62 : 569 – 591 .
- Belachew , T. and Abera , Y. 2011 . Effect of green manuring in combination with nitrogen on soil fertility and yield of bread wheat (Triticum aestivum) under double cropping system of Sinana-dinsho, Southeast Ethiopia . Journal of Biodiversity and Environmental Sciences , 1 : 1 – 11 .
- Bigelow , C. A. , Bowman , D.C. and Wollum , A.G. II. 2002 . Characterization of microbial properties in newly constructed sand-based rootzones . Crop Science , 42 : 1611 – 1614 .
- Blagodatskaya , E. V. and Anderson , T. H. 1998 . Interactive effects of pH and substrate quality on the fungal-to-bacterial ratio and qCO2 of microbial communities in forest soils . Soil Biology and Biochemistry , 30 : 1269 – 1274 .
- Bolton , H. , Elliott , L. F. , Papendick , R. I. and Bezdicek , D. F. 1985 . Soil microbial biomass and selected soil enzyme activities: effect of fertilization and cropping practices . Soil Biology and Biochemistry , 17 : 297 – 302 .
- Booth , C. 1971 . The Genus Fusarium , Kew , , UK : Commonwealth Mycological Institute .
- Burgess , L. W. 1981 . “ General ecology of the Fusaria ” . In Fusarium diseases, biology, and taxonomy , Edited by: Nelson , P. E. , Toussoun , T. A. and Cook , R. J. 225 – 235 . University Park , PA : Pennsylvania State University Press .
- Coucke , P. and Voets , J. P. 1967 . The mineral requirement on Polyangium celluosum . Zeitschrift für allgemeine Mikrobiologie , 7 : 175 – 182 .
- Dickinson , C. H. 1973 . Interactions of fungicides and leaf saprophytes . Pesticide Science , 4 : 563 – 574 .
- Domsch , K. H. and Gams , W. 1970 . Fungi in agricultural soils , London : Longman Group .
- Enwall , K. , Philippot , L. and Hallin , S. 2005 . Activity and composition of the denitrifying bacterial community respond differently to long-term fertilization . Applied and Environmental Microbiology , 71 : 8335 – 8343 .
- Fageria , N. K. and Barbosa , M. P. 2008 . Influence of pH on productivity, nutrient use efficiency by dry bean, and soil phosphorus availability in a non-tillage system . Communications in Soil Science and Plant Analysis , 39 : 1016 – 1025 .
- FAO, ISSS, ISRIC 1998 . World reference base for soil resources . World Soil Resources Report . Rome .
- Fauci , M. F. and Dick , R. P. 1994 . Soil microbial dynamics – short-term and long-term effects of inorganic and organic nitrogen . Soil Science Society of America Journal , 58 : 801 – 806 .
- Fleet , G. H. 1998 . Yeasts in natural habitats . Food Technology and Biotechnology , 36 : 285 – 289 .
- Fliessbach , A. and Mäder , P. 2000 . Microbial biomass and size-density fractions differ between soils of organic and conventional agricultural system . Soil Biology and Biochemistry , 32 : 757 – 768 .
- Frey , S. D. , Elliott , E. T. and Paustian , K. 1999 . Bacterial and fungal abundance and biomass in conventional and no-till agroecosystems along two climactic gradients . Soil Biology and Biochemistry , 31 : 573 – 585 .
- Gerlach , W. & Nirenberg , H. 1982 . The genus of Fusarium – a pictorial atlas . Kommissionverlag Paul Parey . Berlin .
- Gillespie , J. , Latter , P. M. , & Widden , P. 1988 . Cellulolysis of cotton by fungi in three upland soils . In A. F. Harrison , P. M. Latter and D. W. H. Walton , Cotton strip assay: An index of decomposition in soils (Symposium No. 24) , pp. 60 – 67 . Institute of Terrestrial Ecology .
- Glick , B. 1995 . The enhancement of plant growth by free-living bacteria . Canadian Journal of Microbiology , 41 : 109 – 117 .
- Gorlach-Lira , K. and Coutinho , H. D. M. 2007 . Population dynamics and extracellular enzymes activity of mesophilic and thermophilic bacteria isolated from semi-arid soil of Northeastern Brazil . Brazilian Journal of Microbiology , 38 : 135 – 141 .
- Gray , T. R. G. and Williams , S. T. 1971 . Soil microorganisms , Edinburgh : Oliver & Boyd .
- Grundmann , G. L. , Renault , P. , Rosso , L. and Bardin , R. 1995 . Differential effects of soil water content and temperature on nitrification and aeration . Soil Science Society of America Journal , 59 : 1342 – 1349 .
- Hallik , O. 1963 . Agrokeemia . ( in Estonian ).
- Hallin , S. and Pell , M. 1998 . Metabolic properties of denitrifying bacteria adapting to methanol and ethanol in activated sludge – I. Stationary cultures . Water Research , 32 ( 1 ) : 13 – 18 .
- ICC Standard No. 125 1978 . Method of determining the count of aerobic mesophilic bacteria (plate count method) .
- ICC Standard No. 144 1992 . Enumeration of spores of mesophilic bacteria .
- ICC Standard No. 146 1992 . Enumeration of yeasts and molds (spatula method) .
- ISO 10390:2005 2005 . Soil quality – Determination of pH .
- ISO 6887-1:2001 2001 . Microbiology of food and animal feeding stuffs – Preparation of test samples . Initial suspension and decimal dilutions for microbiological examination – Part 1: General rules for the preparation of the initial suspension and decimal dilutions .
- Jones , R. T. , Robeson , M. S. , Lauber , C. L. , Hamady , M. , Knight , R. and Fierer , N. 2009 . A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses . ISME Journal , 3 : 442 – 453 .
- Kautz , T. , Wirth , S. and Ellmer , F. 2004 . Microbial activity in a sandy arable soil is governed by the fertilization regime . European Journal of Soil Biology , 40 : 87 – 94 .
- Keast , D. and Tonkin , C. 1983 . Antifungal activity of Western Australian soil actinomycetes against Phytopthora and Pythium species and a mycorrhizal fungus, Laccaria laccata . Australian Journal of Biological Sciences , 36 : 191 – 203 .
- Kirchner , M. J. , Wollum , A. G. and King , L. D. 1993 . Soil microbial populations and activities in reduced chemical input agroecosystems . Soil Science Society of America Journal , 57 : 1289 – 1295 .
- Kolwzan , B. , Adamiak , W. , Grabas , K. , & Pawe1czyk , A. 2006 . Introduction to environmental microbiology . Oficyna Wydawnicza Politechniki Wroc1awskiej, Wroc1aw .
- Kuster , E. 1968 . “ Taxonomy of soil actinomycetes and related organisms ” . In The ecology of soil bacteria , Edited by: Gray , T.R.G. and Parkinson , D. 322 – 336 . Toronto : University of Toronto Press .
- Lauber , C. L. , Strickland , M. S. , Bradford , M. A. and Fierer , N. 2008 . The influence of soil properties on the structure of bacterial and fungal communities across land-use types . Soil Biology and Biochemistry , 40 : 2407 – 2415 .
- Mäder , P. , Fliebbach , A. , Dubois , D. , Gunst , L. , Fried , P. and Niggli , U. 2002 . Soil fertility and biodiversity in organic farming . Science , 296 : 1694 – 1697 .
- Madigan , M. , Martinko , J. and Parker , J. 2003 . Brock biology of microorganisms , 10th edition , Upper Saddle River , NJ : Prentice Hall, Pearson Education, Inc. .
- Manici , L. M. , Caputo , F. and Babini , V. 2004 . Effect of green manure on Pythium spp. population and microbial communities in intensive cropping systems . Plant and Soil , 263 : 133 – 142 .
- Martens , D. A. , Johanson , J. B. and Frankeberger , W. T. Jr . 1992 . Production and persistence of soil enzymes with repeated additions of organic residues . Soil Science , 153 : 53 – 61 .
- Matthies , C. , Erhard , H. P. and Drake , H. L. 1997 . Effects of pH on the comparative culturability of fungi and bacteria from acidic and less acidic forest soils . Journal of Basic Microbiology , 37 : 335 – 343 .
- McMullen , M. , Jones , R. and Gallenberg , D. 1997 . Scab of wheat and barley: A re-emerging disease of developing impact . Plant Disease , 81 : 1340 – 1348 .
- Mendelssohn , I. A. , Sorrell , B. K. , Brix , H. , Schierup , H. H. , Lorenzen , B. and Maltby , E. 1999 . Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetlands in Denmark . Aquatic Botany , 64 : 381 – 398 .
- Moawad , H. , Salem , S. H. , Badr El-Din , S. M. S. , Khater , T. and Iskandar , M. 1986 . Yeasts in soils of Egypt . Zentralblatt Mikrobiologie , 141 : 431 – 435 .
- Mrkovački , N. , Mezei , S. and Kovačev , L. 1996 . Effect of Azotobacter inoculation on dry matter mass and nitrogen content in the hybrid varieties of sugar beet . A Periodical of Scientific Research on Field and Vegetable Crops , 25 : 107 – 113 .
- NMKL Method No. 86 . , 3rd Edn . 1999 .
- Novak , A. , Michalcewic , W. and Jakubiszyn , B. 1993 . Effect of fertilization with manure, straw and biohumus on numbers of bacteria, fungi, actinomycetes and microbial biomass in soil . Rzecz Nauki Polskiej /AR Szczecini , 57 : 101 – 113 .
- Omar , S. A. and Abdel-Sater , M. A. 2001 . Microbial populations and enzyme activities in soil treated with pesticides . Water Air Soil Pollution , 127 ( 1 ) : 49 – 63 .
- Pai , S.-L. , Chong , N.-M. and Chen , C.-H. 1999 . Potential applications of aerobic denitrifying bacteria as bioagents in wastewater treatment . Bioresource Technology , 68 ( 2 ) : 179 – 185 .
- Pandey , A. , Sharma , E. and Palni , L. 1998 . Influence of bacterial inoculation on maize in upland farming systems of the sikkim Himalaya . Soil Biology and Biochemistry , 3 : 379 – 384 .
- Pankhurst , C. E. , Hawke , B. G. , McDonald , H. J. , Kirkby , C. A. , Buckerfield , J. C. , Michelsen , P. , O'Brien , K. A. , Gupta , V. V. S. R. and Doube , B. M. 1995 . Evaluation of soil biological properties as potential bioindicators of soil health . Australian Journal of Experimental Agriculture , 35 : 1015 – 1028 .
- Park , D. 1976 . Nitrogen level and cellulose decomposition by fungi . International Biodeterioration Bulletin , 12 : 85 – 99 .
- Parton , W. J. , Holland , E. A. , Del Grosso , S. J. , Hartman , M. D. , Martin , R. E. , Mosier , A. R. , Ojima , D. S. and Schimel , D. S. 2001 . Generalized model for NOx and N2O emissions from soils . Journal of Geophysical Research , 106 : 403 – 419 .
- Perucci , P. , Bonciarelly , U. , Santiloechi , R. and Bianchi , A. A. 1997 . Effect of rotation, nitrogen and management of crop residues on some chemical. microbiological and biochemical properties of soil . Biology and Fertility of Soils , 24 : 311 – 316 .
- Phaff , H. J. , Miller , M. W. and Mark , E. M. 1978 . The life of yeasts , 2nd Edn , Cambridge , MA : Harvard University Press .
- Phaff , H. J. and Starmer , W. T. 1987 . “ Yeasts associated with plants, insects and soil ” . In The yeasts , Edited by: Rose , A. H. and Harrison , J. S. Vol. 1 , 123 – 180 . London : Academic Press .
- Pieters , A. J. 1927 . Green manuring: Principles and practices , New York : John Wiley & Sons .
- Prosser , J. I. 1989 . Autotrophic nitrification in bacteria . Advances in Microbial Physiology , 30 : 125 – 181 .
- SAS 2002 . JMP, Statistics and Graphics Guide, Version 5 . Cary , NC : SAS Institute .
- Shipton , P. J. 1977 . Monoculture and soilborne plant pathogens . Annual Review of Phytopathology , 15 : 387 – 407 .
- Singh , J. and Singh , D. K. 2005 . Bacterial, azotobacter, actinomycetes, and fungal population in soil after diazinon, imidacloprid, and lindane treatments in groundnut (Arachis hypogaea L.) fields . Journal of Environmental Science and Health, Part B , 40 ( 5 ) : 785 – 800 .
- Song , B. , Palleroni , N. J. and Häggblom , M. M. 2000 . Isolation and characterization of diverse halobenzoate-degrading denitrifying bacteria from soils and sediments . Applied and Environmental Microbiology , 66 ( 8 ) : 3446 – 3453 .
- Stolze , M. , Piorr , A. , Häring , A. and Dabbert , S. 2000 . “ The environmental impact of organic farming in Europe ” . In Organic farming in Europe: Economics and policy , Edited by: Dabbert , S. , Lampkin , N. , Michelsen , J. , Nieberg , H. and Zanoli , R. 1 – 125 . Stuttgart-Hohenheim : University of Hohenheim .
- Šimek , M. and Cooper , J. E. 2002 . The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years . European Journal of Soil Science , 53 : 345 – 354 .
- Valera, Concepcion , L. , & Alexander , M. 1961 . Nodulation factor for Rhizobium–legume symbiosis . Plant and Soil XV 15 ( 3 ), 268 – 280 .
- Vetemaa , A. & Mikk , M. 2011 . Organic Farming in Estonia 2010 . 10 18 Ministry of Agriculture, Republic of Estonia .
- Viileberg , L. 1966 . Mikrobioloogia praktikum . Tartu Riiklik Ülikool. (in Estonian)
- Vreulink , J. , Esterhuyse , A. , Jacobs , K. and Botha , A. 2007 . Note: soil properties that impact on yeast and actinomycete numbers in sandy low nutrient soils . Canadian Journal of Microbiology , 53 : 1369 – 1374 .
- Williams , E. J. , Hutchinson , G. L. and Fehsenfeld , F. C. 1992 . NOx and N2O emissions from soil . Global Biogeochemical Cycles , 6 : 351 – 388 .
- Wright , S. F. 2003 . The importance of soil microorganisms in aggregate stability . Proc. North Central Extension-Industry Soil Fertility Conference , 19 : 93 – 98 .
- Zahir , Z. A. and Arshad , M. 1996 . Effectiveness of Azotobacter inoculation for improving potato yield under fertilised conditions . Pakistan Journal of Agricultural Science , 33 : 1 – 5 .
- Zahir , Z. A. , Arshad , M. , Hussain , A. and Sarfraz , M. 1996 . Improving wheat yield by inoculation with Azotobacter under optimum fertiliser application . Pakistan Journal of Agricultural Science , 11 : 129 – 131 .
- Zumft , W. G. 1997 . Cell biology and molecular basis of denitrification . Microbiology and Molecular Biology Reviews , 61 : 533 – 616 .