Abstract
Turfgrasses are continuously exposed to a wide range of detrimental stresses, such as mowing, traffic, low or high temperatures, salinity, drought, UV, hypoxia etc. Plant responses to stimuli involve nearly every aspect of plant physiology and metabolism. Carbohydrates, primary sucrose and starch, as principal metabolic products of photosynthesis, are stored in bermudagrass (Cynodon spp.) in stolons and rhizomes. Total nonstructural carbohydrates (TNC) levels have been used as indicators of health and physiological status in bermudagrass. TNC levels vary during the year and are weakly affected by N source. Establishment is a critical phase of bermudagrass management. The objectives of this research were to assess and characterize stolon carbohydrate content in nine bermudagrass cultivars during the full establishment phase in relation to establishment rates. Morphological and growth analyses showed distinct properties among the cultivars selected for this study. Significant differences in turf coverage among cultivars on each rating date were present starting from early growth stages. Carbohydrates localization was used to differentiate bermudagrass cultivars. The relationships between different carbohydrates and their role on growth were also explored. Gradients along the stolon from tip to older internodes were evident for starch. Our study provides further insight into bermudagrass physiology, with cultivar differences in carbohydrates production and localization providing valuable selection information for turfgrass breeders and practitioners, especially during the first year. Our data indicate that TNC levels were affected by cultivar and stolon maturity level. TNC content was found to correlate with establishment rate.
Introduction
Bermudagrass (Cynodon spp.), belonging to the Chlorideae tribe, is the prevailing warm-season turfgrass used on highly maintained areas in warm and transition zones of the USA, such as golf courses and athletic fields. Recently it has also became an increasingly popular species in the Mediterranean Basin (Volterrani et al. Citation1997, Croce et al. Citation2001, Citation2004) as it provides a uniform, fast-growing, high-quality surface in full sun. Compared with cool-season grasses, its positive attributes include good heat and drought tolerance, pest resistance, traffic tolerance, and tolerance to a wide range of soil types and water quality (Beard Citation1973). Although in the transition zone active growth is not achieved all year round, bermudagrass provides a functional surface in low-budget facilities where overseeding is normally not carried out (Anderson et al. Citation2007, Patton et al. Citation2008).
The genus Cynodon is comprised of nine species and ten varieties. The principal taxa used as turf are C. dactylon var. dactylon, C. incompletus, C. transvaalensis and C. magennisii. Cynodon spp. has a wide distribution, genetic and cytotaxonomic diversity, containing agamic and gamic types as well as sterile triploid hybrids obtained by interspecific hybridization of C. dactylon var. dactylon×C. transvaalensis. Furthermore, natural and induced mutations from hybrid cultivars have also been the source material to select putting green genotypes with unique morphological traits and growth patterns (Taliaferro Citation1995).
Establishment is a critical phase of bermudagrass management. A rapid establishment is a desirable character as this exposes bare soil for a shorter period, thus limiting weed competition and allowing earlier harvest in sod production. Richardson et al. (Citation2004) pointed out that seeded bermudagrass produces only stolons during the establishment year. High lateral growth rate is one of the most desirable traits for turfgrass breeders and growers. Establishment rate and the ability to generate a uniform and dense sward are related to the lateral growth stems.
Turfgrasses are continuously exposed to a wide range of detrimental stresses, such as mowing, traffic, low or high temperatures, salinity, drought, UV, anoxia etc. Plant responses to stimuli involve nearly every aspect of plant physiology and metabolism. Carbohydrates, primarily sucrose and starch, as principal metabolic products of photosynthesis, are stored in bermudagrass in specialized storage tissues such as stolons and rhizomes (Beard Citation1973, Hull Citation1992, Shepard Citation1992).
Total nonstructural carbohydrates (TNC) levels have been used to determine health and physiological status in turfgrass. In bermudagrass, TNC levels vary during the year and are weakly affected by N source (Goatley et al. Citation1998). It is known that agronomic management and environment affect turfgrass carbohydrates concentrations such as mowing frequency and height (White Citation1973, Sheffer et al. Citation1979, Damiani et al. Citation2004), as well as shade (Bunnell et al. Citation2005). Nonstructural carbohydrates, like starch, glucose, sucrose, and fructose are involved during dormancy recovery, regrowth and recuperative capacity from spring dead spots and cold stress (Dunn et al. Citation1980, White and Schmidt Citation1990, Munshaw et al. Citation2006). TNC levels increase as bermudagrass acclimates to colder weather (Dunn and Nelson Citation1974) because soluble sugars such as sucrose also function as cryoprotectants. Monitoring of TNC concentrations has been used to determine physiological status in turfgrasses (Sheffer et al. Citation1979, Hull Citation1992). TNC levels were monitored for different nutritional treatments, water regimes, plant growth regulator treatments, temperature differences, and cultural practices associated with maintaining high-quality turfgrass. A reason to investigate carbohydrates partitioning is to predict turfgrass health or recuperative potential based on environmental influences and cultural practices (Macolino et al. Citation2010, Lulli et al. Citation2012).
The objectives of this research were to assess and characterize stolon carbohydrate content in nine bermudagrass cultivars during the establishment phase, and to assess the relationship between carbohydrates content and establishment rate. This information would provide further insight into bermudagrass physiology and investigations on the physiological bases of cultivar differences in carbohydrates production and localization would provide valuable selection information for turfgrass breeders and practitioners, especially during the first year.
Materials and methods
Experimental conditions
Based on preliminary results from a field study (data not shown), nine bermudagrass cultivars with contrasting establishment rate (three slow-growing, three average and three fast-growing), and grouped as seeded, vegetative and putting green types, were selected for the present study (). The bermudagrass cultivars were evaluated at the experimental station of Centre for Research on Turfgrass for Environment and Sports (CeRTES), University of Pisa, Italy (43° 40′ N, 10° 19′ E; 6 m a.s.l.), during the summer of 2010. On 7 April, stolons of vegetatively propagated cultivars were planted into 120-hole seed trays filled with a peat-based mix, and seeded cultivars were seeded in seed trays as per stolons. Plants were raised in the greenhouse (24±5 °C) and were fertilized on 13 May with 30 kg ha−1 N, 10 kg ha−1 P and 10 kg ha−1 K using a soluble fertilizer.
Table I. Bermudagrass (Cynodon spp.) cultivar, species and type of establishment commonly used.
On 31 May, both vegetative and seeded cultivars plants were transplanted in the field as 5 cm3 plugs with a single plant. Plugs were manually transplanted in the centre of the 1.5×1.5 m experimental plots arranged in a randomized complete-block design with four replications. Following establishment, the fertilization programme included a monthly application of 50 kg ha−1 N, 10 kg ha−1 P and 40 kg ha−1 K from June to September. Irrigation was applied three times daily during the first 10 days to promote establishment, and then as needed to prevent wilting and heat stress. The study was conducted on a typical Xerofluvent soil (silt-loam type with 28% sand, 55% silt and 17% clay, pH 7.8, and 18 g kg−1 organic matter). In order to minimize weed competition the experimental area was treated twice a year with 2.88 kg ha−1 glyphosate [N-(phosphonomethyl)glycine] starting 3 years before establishment and with 3.36 kg ha−1 oxadiazon [3-[2,4-dichloro-5-(1-methylethoxy)phenyl]-5-(1,1-dimethyl)-1,3,4-oxadiazol-2-(3H)-one] before transplanting. No pesticides were applied and competing weeds were manually removed during establishment. In addition, to avoid cultivars by mowing interaction, the plots were not mowed (Higgins Citation1998, Unruh et al. Citation2006).
Establishment rate and biometric traits
For each plot, from 28 to 56 d after planting (DAP), two stolons longer than 3.5 cm were selected and weekly measurements of lateral spread were collected, marking the tip with coloured tailor pins, until each stolon reached the border of its plot. Total stolon length 28 DAP, and the stolon growth rate, by dividing the growth (length) from 28 DAP to 56 DAP of each stolon by number of days, were determined. Stolon traits were measured starting from 3.5 cm from the vegetative plug.
Bermudagrass coverage was determined with digital images analysis (DIA) (Richardson et al. Citation2001). Digital images of each plot were obtained weekly, in full sunlight between 1200 and 1400 h, with a Sony DSC-T70 Cyber-shot® (Sony Corporation, Tokyo, Japan) digital camera mounted on a tripod to ensure a consistent distance of the lens from the soil surface (l.02 m). Images, taken weekly from 14 to 56 DAP, were saved in JPEG (joint photographic experts group,.jpg) format with a size of 2592×1944 pixels, and were analysed with an automated procedure available as web-based software (www.imaging-crops.dk). Graduated tapes were used to determine the actual surface area covered by the images and raw pixel coordinates were converted into a specified measurement unit – cm2. Scatter plots of the per cent of green cover (growth pattern) data versus days after plugging indicated a non-linear relationship, therefore natural logarithm transformation of data was use to obtain a linear relationship. Establishment rate for each cultivar was determined by fitting coverage data across time to the linear model [Coverage=(K×DAP) + I], where K is the rate of increase (establishment rate, loge coverage d−1), DAP is days after planting, and I was equal to the natural logarithm of 1.5 cm2, which was the starting coverage for all plots.
Carbohydrates analysis
At 56 DAP, from each plot, two non-branched primary stolons were harvested and, regardless of their length, the first, third, middle and basal nodes from the stolon tip downward were selectively collected. These criteria were selected to highlight the most general and comparable development carbohydrates characteristics.
Immediately after sampling and washing off soil with water and removing roots and shoots, all samples were frozen in liquid N2. Samples were stored at −70 °C until carbohydrates analysis.
Soluble carbohydrates
Samples (0.5 g fresh weight) were ground to a powder and extracted as described by Tobias et al. (Citation1992). Samples were assayed by coupled enzymatic assay methods (Guglielminetti et al. Citation1995) measuring the increase in A340. The accuracy of the method was tested using standards with known amounts of carbohydrates. Incubations of samples and standards were carried out at 37 °C for 30 min. The reaction mixtures (1 mL) were as follows. Glucose: 100 mM Tris-HCl, pH 7.6, 3 mM MgCl2, 2 mM ATP, 0.6 mM NADP, 1 unit Glc6P dehydrogenase; fructose was assayed as described for glucose plus the addition of two units of PGI; the increase in A340 was recorded. Sucrose was first broken down using 85 units of invertase (in 15 mM Na-acetate, pH 4.6) and the resulting glucose and fructose were assayed as described above.
Recovery experiments evaluated losses taking place during the extraction procedures. Two tests were done for each metabolite by adding known amount of authentic standards to the samples prior to the extraction. The concentrations of the standards added were similar to those estimated to be present in the tissues in preliminary experiments. The percentage of recovery ranged between 96 and 108% depending on the sugar. Data were corrected on the basis of the recovery percentages obtained for each sample, and expressed as µmoles hexoses equivalent g−1 FW.
Starch analysis
Samples (100 mg FW) were ground in a mortar, resuspended in 100 mL of 10 mM KOH and boiled for 1 min; 1 mL of 1 N HCl was then added to each sample. The starch standard solution was prepared using 100 mg of potato soluble starch dissolved in 100 mL dH2O and boiled 1 min. To both samples (50 µL) and standards (from 0 to 100 µL), adjusted to 150 µL with dH2O, 1 mL of fresh iodine solution was added (0.13% K2 and 0.3% KI, dissolved in dH2O) and the absorbance was read at 595 nm immediately. Starch concentration was expressed as mg starch g−1 FW.
Statistical analysis
The statistical analyses of biometric and growth traits were performed using one-way analysis of variance (ANOVA) to determine whether a significant difference among cultivars and groups existed. When significant differences were found, the means were compared using the least significant difference (LSD) test. Significant differences for all statistical tests were evaluated at the level of p=0.05. Bermudagrass coverage was related to a number of explanatory variables by multiple regression modelling. The relative explanatory power of each variable was assessed on its own and in combination with all others. Non-significant main effect variables (factors) were removed only when their interactions were not significant. P-values above 0.05 were used as a cutoff point in the removal of explanatory variables.
All computations were performed with R 2.14.0 (R Development Core Team Citation2011) and R package ggplot2 (Wickham Citation2009). For the carbohydrates data, the Student–Newman–Keuls (SNK) test was used for a posteriori multiple comparison of means.
Results and discussion
Morphological and growth analyses () revealed distinct properties among the nine cultivars used in this study. Significant differences in turf coverage among cultivars on each rating date were present starting from early growth stages. At 56 DAP (26 July), coverage was greatest for ‘Tifsport’, ‘Riviera’ and ‘Santa Ana’ (3694, 2212 and 1514 cm2, respectively), while the dwarf entries, ‘Champion’, ‘Tifdwarf’ and ‘Miniverde’, produced a lower turf cover.
Table II. Bermudagrass (Cynodon spp.) coverage, establishment rate, stolon number, stolon length and stolon growth rate by cultivar.
Establishment rate (K) was determined by linear regression with R 2 values ranging from 0.94 to 0.99. Establishment rate ranged from 0.047 to 0.140 loge (coverage) d−1 for ‘Miniverde’ and ‘Tifsport’, respectively, with a mean value of 0.102. Among the seeded entries, ‘Riviera’ had the greatest coverage but was not significantly greater than ‘Princess 77’. ‘Tifway’, a 27-chromosome sterile interspecific hybrid (Taliaferro Citation1995) for tee, fairways, athletic fields and lawns, is considered an industry standard because, since its release in 1960, it has been widely used by turfgrass practitioners and researchers all around the warm temperate and transition zone. In our study, ‘Tifway’ produced significantly lower coverage 56 DAP (430 cm2) than average and it exhibited an establishment rate significantly lower (p<0.001) than seeded entries. In this study, differences between vegetative and seeded entries were not significant, but the dwarf bermudagrasses had a lower establishment rate (0.062, p<0.001). ‘Tifdwarf’, a sterile triploid released in 1965 that is similar to another dwarf cultivar (‘Tifgreen’) but with smaller biometric characteristics and darker green colour due to its purple anthocyanin content, had the highest establishment rates among the dwarf types (Burton Citation1991).
Significant differences (p<0.001) were observed in stolon number per plant, total stolon length and stolon growth rate among cultivars (), with ‘Tifsport’ being the most aggressive cultivar as to lateral spread. Stolon number per plant at 28 DAP ranged from 0 to 11.7 (‘Miniverde’ did not produce stolons longer than 3.5 cm) and total stolon length ranged from 0 to 208.8 cm. Large differences were found in stolon growth rate, that ranged from 4.9 to 38.2 mm per day (by both the dwarf entries and ‘Tifsport’, respectively). Concerning stolon number per plant and stolon growth rate, dwarf entries had significantly lower values compared with seeded and vegetative entries (p<0.001 for both parameters), while no differences were detected between the two groups in all stolon growth performance. This confirms that improved seeded cultivars may provide a quick, easy and economical way to establish a quality bermudagrass turf, with similar performance as sterile interspecific hybrids. Regression analysis revealed that total stolon length is a significant predictor of coverage (R 2 =0.75, p<0.0001), better than number of stolons per plant (R 2 =0.43, p<0.0001).
Analysing the stolon growth traits, differences in surface colonization patterns were found between the entries. For example, ‘Santa Ana’ had the largest stolon number (11.7), but it was penalized by a shorter stolon length.
The stepwise multiple regression analyses associated coverage as response variable with stolon numbers and total stolon length of bermudagrass as explanatory variables, with the following model:
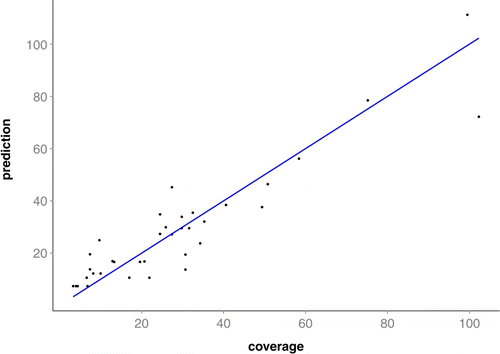
where cov28 = coverage at 28 DAP (cm2), nst =number of stolons per plant at 28 DAP, and lcum = total stolon length at 28 DAP. For the multiple regression equation, residuals from regression were plotted in relation to predicted and observed variables to ensure that error variances were homogeneous and that no evidence existed for lack of fit of data to the regression equation, as well as whether there is systematic over- or under-prediction in specific ranges of the data (). The final model explained 85.5% of the coverage variability.
Figure 1. Plot prediction of coverage against true response. Multiple regression analyses associated bermudagrass coverage with stolon number and stolon total length, with the following model: cov28 = nst + lcum2+(lcum×nst) where cov28 = coverage at 28 DAP (cm2), nst = number of stolons per plant at 28 DAP, and lcum = total stolon length at 28 DAP.
Starch reserves were consistently lower in the younger tissues (A, 2B) than in the middle (C) and basal node (D), except in ‘Tifway’, ‘Champion’ and ‘Princess 77’, that showed a constant level of this reserve carbohydrate. Starch increased considerably in older tissues, with concentrations averaging 4.8 mg g−1 fresh wt in apical and third nodes and 29.8–52.0 mg g−1 fresh wt in the middle and basal node, respectively. Starch concentrations decreased by an average of 43% from basal to the middle node. Concentrations ranged from 4.1 to 60.3 mg g−1 fresh wt in the middle and from 4.3 to 95.5 mg g−1 fresh wt in the basal node. Additionally, there was a close positive correlation (r=0.91, p<0.0001) between middle and basal starch concentrations. It is notable that synthesis of starch had similar pattern in two dwarf cultivars, reaching at the basal node 59.1 and 57.6 mg starch g−1 fresh weight, respectively for ‘Tifdwarf’ and ‘Miniverde’. Starch concentration in the middle node of stolons was positively correlated with stolon growth rate (r=0.77, p<0.0001).
Figure 2. Concentration of starch in bermudagrass (A) apical, (B) third node, (C) middle and (D) basal stolon node observed 56 DAP for nine cultivars, sorted according to increasing establishment rate. Error bars represent one standard error of the mean (n = 3).
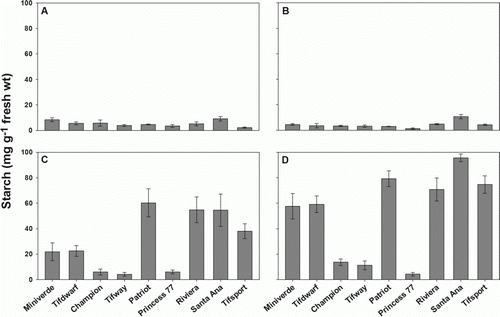
Furthermore, our results showed that, with the exception of the seeded cultivar ‘Princess 77’, all the quickest cultivars, with establishment rates significantly greater than the mean value of 0.0102 loge (coverage) d−1, accumulated high concentrations of starch in the middle node and even higher concentration in the older tissue. Contrary to this evidence, a high starch accumulation was observed in ‘Tifdwarf’ and ‘Miniverde’ dwarf types, in spite of the slower establishment. Carbohydrate accumulation in stolons during active growth is a direct result of higher photosynthetic rates than respiration rates (Levitt Citation1980). Our results indicate that in fast-growing cultivars, starch content is probably related to a more efficient photosynthetic pathway that permits a higher starch production rate, consumption and accumulation in the reserve organs. This trend was not observed in ‘Princess 77’, ‘Tifway’ and ‘Champion’, where no starch accumulation was evident among the node analysed. The lower starch content may be the result of carbohydrate respiration to sustain active growth. Additionally, on dwarf bermudagrasses an efficient photosynthesis is deemed to take place but the slower metabolism generates a high starch accumulation, similar to the faster cultivars.
Total soluble sugars (TSS) data analysis shows consistently low concentrations in younger stolon tissues, where the mean value ranged from 37.7 to 25.0 µmol g−1 FW for the apical and third node, respectively. TSS content increased considerably with stolon age, with concentrations averaging 56.3 and 59.3 µmol g−1 FW, respectively in the middle and basal stolons ().
Figure 3. Concentration of glucose, fructose and sucrose (as hexose equivalent) in bermudagrass (A) apical, (B) third node, (C) middle and (D) basal stolon node observed 56 DAP for nine cultivars, sorted according to increasing establishment rate. Error bars represent one standard error of the mean (n = 3).
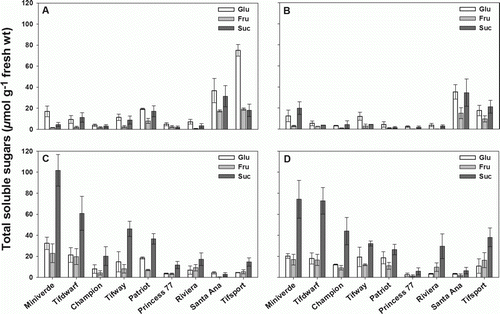
Sucrose comprised the majority of the total sugar concentration in bermudagrass stolons, which is consistent with researches conducted on other C4 species (Patton et al. Citation2007, Lulli et al. Citation2011). Glucose comprised much of the total sugar concentration in the apical stolon node. Apical glucose was positively correlated to coverage (r=0.80, p<0.05). Additionally, there were close negative correlations between glucose, sucrose, TSS in the middle tissues and bermudagrass establishment rate (r=−0.85, −0.88, −0.87, respectively, p<0.01 for all). Basal sucrose and basal TSS were found to be negatively correlated with establishment rate (r=−0.77, −0.75, respectively, p<0.05 for both).
As expected, our study confirmed considerable differences in bermudagrass growth and establishment rate between cultivars. These differences were reflected in starch and total nonstructural carbohydrate accumulation, as the result of the balance between carbohydrate production and consumption. These traits may be targeted specifically for identification of new bermudagrass cultivars during selection, and can be implemented for this process. These traits may be targeted specifically for breeding of new bermudagrass cultivars using phenotypic or marker-assisted selection procedures.
References
- Anderson , J. A. , Taliaferro , C. M. , & Wu , Y. Q. 2007 . Freeze tolerance of seed- and vegetatively-propagated bermudagrasses compared with standard cultivars . Applied Turfgrass Science , May , 1–7 .
- Beard , J. B. 1973 . Turfgrass: Science and culture , Englewood Cliffs , NJ : Prentice-Hall .
- Bunnell , B. T. , McCarty , L. B. , Faust , J. E. , Bridges , W. C. and Rajapakse , N. C. 2005 . Quantifying a daily light integral requirement of a ‘Tifeagle’ bermudagrass golf green . Crop Science , 45 : 569 – 574 .
- Burton , G. W. 1991 . A history of turf research at Tifton . USGA Green Section Record , 29 : 12 – 14 .
- Croce , P. , De Luca , A. , Mocioni , M. , Volterrani , M. and Beard , J. B. 2001 . Warm-season turfgrass species and cultivar characterizations for a mediterranean climate . International Turfgrass Society Research Journal , 9 : 855 – 859 .
- Croce , P. , De Luca , A. , Mocioni , M. , Volterrani , M. and Beard , J. B. 2004 . Adaptability of warmseason turfgrass species and cultivars in a mediterranean climate . Acta Horticulturae , 661 : 365 – 368 .
- Damiani , C. R. , Volterrani , M. , Lercari , S. , Stefanini , S. , Alpi , A. and Guglielminetti , L. 2004 . Comparative study on fructan accumulation ability in seventeen tall fescue varieties . Acta Horticulturae , 661 : 217 – 225 .
- Dunn , J. H. and Nelson , C. J. 1974 . Chemical changes occurring in three bermudagrass turf cultivars in relation to cold hardiness . Agronomy Journal , 66 : 28 – 31 .
- Dunn , J. H. , Nelson , C. J. and Sebaugh , J. L. 1980 . Characterization of thatch, rhizomes, carbohydrates, and spring deadspot in twenty cultivars of bermudagrass . Journal of the American Society for Horticultural Science , 105 : 653 – 657 .
- Goatley , J. M. Jr , Maddox , V. L. and Hensler , K. L. 1998 . Late-season applications of various nitrogen sources affect color and carbohydrate content of ‘Tiflawn’ and Arizona common bermudagrass . HortScience , 33 : 692 – 695 .
- Guglielminetti , L. , Perata , P. and Alpi , A. 1995 . Effect of anoxia on carbohydrate-metabolism in rice seedlings . Plant Physiology , 108 : 735 – 741 .
- Higgins , J. 1998 . Zoysiagrass lawns . In Alabama Cooperative Extension System. Anr-1129 . Auburn , AL : Alabama A&M and Auburn University .
- Hull , R. J. 1992 . “ Energy relations and carbohydrate partitioning in turfgrasses ” . In Turfgrass , Edited by: Waddington , D. V. , Carrow , R. N. and Shearman , R. C. 175 – 205 . Madison , WI : ASA, CSSA, & SSSA .
- Levitt , J. 1980 . Responses of plants to environmental stress , 2nd edn Vol. 1: Chilling, freezing, and high temperature stresses . New York : Academic Press .
- Lulli , F. , Guglielminetti , L. , Grossi , N. , Armeni , R. , Stefanini , S. and Volterrani , M. 2011 . Physiological and morphological factors influencing leaf, rhizome and stolon tensile strength in C4 turfgrass species . Functional Plant Biology , 38 : 919 – 926 .
- Lulli , F. , Volterrani , M. , Grossi , N. , Armeni , R. , Stefanini , S. , & Guglielminetti , L. 2012 . Physiological and morphological factors influencing wear resistance and recovery in C3 and C4 turfgrass species . Functional Plant Biology , in press .
- Macolino , S. , Serena , M. , Leinauer , B. and Ziliotto , U. 2010 . Preliminary findings on the correlation between water-soluble carbohydrate content in stolons and first year green-up of seeded bermudagrass cultivars . HortTechnology , 20 : 758 – 763 .
- Munshaw , G. C. , Ervin , E. H. , Shang , C. , Askew , S. D. , Zhang , X. and Lemus , R. W. 2006 . Influence of late-season iron, nitrogen, and seaweed extract on fall color retention and cold tolerance of four bermudagrass cultivars . Crop Science , 46 : 273 – 283 .
- Patton , A. J. , Cunningham , S. M. , Volenec , J. J. and Reicher , Z. J. 2007 . Differences in freeze tolerance of zoysiagrasses: II. Carbohydrate and proline accumulation . Crop Science , 47 : 2170 – 2181 .
- Patton , A. J. , Richardson , M. D. , Karcher , D. E. , Boyd , J. W. , Reicher , Z. J. , Fry , J. D. , McElroy S. J. , & Munshaw , G. C. 2008 . A guide to establishing seeded bermudagrass in the transition zone . Applied Turfgrass Science , January , 1–20 .
- R Development Core Team 2011 . R: A language and environment for statistical computing . Vienna , , Austria : R Foundation for Statistical Computing . http://www.R-project.org
- Richardson , M. D. , Karcher , D. E. and Purcell , L. C. 2001 . Quantifying turfgrass cover using digital image analysis . Crop Science , 41 : 1884 – 1888 .
- Richardson , M. D. , Karcher , D. E. , Berger , P. and Boyd , J. W. 2004 . Utilizing improved seeded bermudagrasses on transition-zone sports fields . Acta Horticulturae , 661 : 369 – 374 .
- Sheffer , K. M. , Watschke , T. L. and Duich , J. M. 1979 . Carbohydrate sampling in kentucky bluegrass turf . Agronomy Journal , 71 : 301 – 304 .
- Shepard , D. P. 1992 . Effects of four plant growth regulators on carbohydrate reserves and cold tolerance of warm-season turf . North Carolina State University .
- Taliaferro , C. M. 1995 . Diversity and vulnerability of bermuda turfgrass species . Crop Science , 35 : 327 – 332 .
- Tobias , R. B. , Boyer , C. D. and Shannon , J. C. 1992 . Alterations in carbohydrate intermediates in the endosperm of starch-deficient maize (Zea mays L) genotypes . Plant Physiology , 99 : 146 – 152 .
- Unruh , J. B. , Trenholm , L. E. , & Cisar , J. L. 2006 . Zoysiagrass in Florida . Enh11., ed. Service, FCE, [1–7]. Gainesville, FL: Florida Cooperative Extension Service, Gainesville, FL .
- Volterrani , M. , Grossi , N. , Pardini , G. , Miele , S. and Gaetani , M. 1997 . Warm season turfgrass adaptation in Italy . International Turfgrass Society Research Journal , 8 : 1344 – 1354 .
- White , L. M. 1973 . Carbohydrate reserves of grasses: A review . Journal of Range Management , 26 : 13 – 18 .
- White , R. H. and Schmidt , R. E. 1990 . Fall performance and post-dormancy growth of ‘midiron’ bermudagrass in response to nitrogen, iron, and benzyladenine . Journal of the American Society for Horticultural Science , 115 : 57 – 61 .
- Wickham , H. 2009 . Ggplot2: Elegant graphics for data analysis. Use r! New York : Springer . http://had.co.nz/ggplot2/book