Abstract
The productivity of white yam (Dioscorea rotundata Poir) must increase to sustainably meet the demand of the increasing populations in the developing world where this is a staple crop. Although this could be achieved through the use of mineral fertilizers, reports indicate limited effects of these inputs on tuber yield. We hypothesized (i) that D. rotundata has a small and shallow root system and (ii) that this root system does not respond to mineral fertilizer application. Two field experiments were conducted in Côte d'Ivoire in year 1 and in year 2 to test these hypotheses. In the first field experiment we measured biomass production, as well as root density during plant growth in fertilized and unfertilized plots while in the second experiment, we analysed the distribution pattern of roots in horizontal and vertical root profiles in fertilized plots. The root system of D. rotundata consisted of seminal, adventitious and tubercular roots. Only the adventitious roots remained alive until the end of the growth cycle. The root length density was very low with a maximum of 0.25 cm cm−3. No roots were observed in the 15–30 cm horizon at 50 cm from the plant's crown. The horizontal and vertical root maps revealed that roots were mostly distributed in clumps, and there was a good correlation between the two methods of root sampling for this species. Fertilizer application had no significant effect on plant biomass, fresh tuber yield or on root growth. However, thinner and longer roots and higher tuber yields were observed in year 1 than in year 2. The different weather conditions and more specifically the higher soil temperature might explain the results obtained in year 1. Therefore, it could be recommended to farmers not to fertilize D. rotundata.
Introduction
The demand for yam (Dioscorea spp.) tuber is steadily increasing in west Africa, Oceania, south Asia and the West Indies due to population growth (Asiedu and Sartie Citation2010). In Africa, in the last decades, the increased demand for tubers has been met mostly by increasing the cultivated area while average fresh tuber yields remained stable around 10 t ha−1 year−1 (FAO Citation2011). Increasing the cultivated area to produce relatively low yields, however, is not a sustainable practice because ecosystem services provided by non-cultivated areas should be conserved (Paillard et al. Citation2011). One way to increase yam yields would be to apply mineral fertilizers to satisfy the high N and K demand of yam (Degras Citation1993). However, yam tuber yield does not systematically increase upon mineral fertilizer application (Asadu et al. Citation1996, Sotomayor-Ramirez et al. Citation2003, O'Sullivan and Ernest Citation2008, Srivastava et al. Citation2010, Diby et al. Citation2011). Root morphology affects plant growth through nutrient and water uptake. Lynch (Citation2007) showed that substantial yield gains had been obtained in low-fertility soils by using bean and soybean cultivars with adapted root morphologies. Lynch (Citation2007) concluded that breeding for crops with adequate root architecture would increase crop production in developing countries where soil fertility is often low and fertilizers are scarcely accessible.
Root distribution patterns of yams have not been studied extensively. O'Sullivan (Citation2008) showed that the roots of D. alata (water yam) could grow horizontally as far as 5 m from the crown of the plant and could penetrate as deep as 40 cm. Recently, Hgaza et al. (Citation2011) showed that D. alata planted in 50-cm high mounds had a low root length density (RLD) and a low root mass density (RMD) when compared with other crops. No roots were observed below a depth of 15 cm at a distance of 50 cm from the crown (i.e. outside the mound) by Hgaza et al. (Citation2011). While shoot and tuber production were significantly increased by mineral fertilizer application, these authors did not observe any significant effect of the applied fertilizer on root growth and distribution. Less information is available on D. rotundata (white yam) which is the most important yam species grown in Africa (Lebot Citation2009). Njoku et al. (Citation1984) showed that the roots of D. rotundata could reach 2.5 m in length in the field and Charles-Dominique et al. (Citation2009) made an architectural analysis of the growth of D. rotundata under glasshouse conditions. These studies, however, did not discuss parameters such as root length density and root mass density for D. rotundata, whereas these are important parameters for nutrient and water uptake.
The aim of the present study was to determine whether mineral fertilizer providing sufficient nutrients for optimal tuber production would affect the root growth of D. rotundata and tuber yield in a low-fertility savannah soil. The hypotheses of this paper are (i) that D. rotundata has a small and shallow root system and (ii) that it does not respond to mineral fertilizer application. These hypotheses were tested in two field experiments conducted in 2006 and 2007 in the centre of Côte d'Ivoire. In the first field experiment shoot, root and tuber biomass, as well as root length density, root mass density and root diameter were measured during plant growth in fertilized and non-fertilized plots while in the second experiment the distribution of roots in horizontal and vertical root profiles were determined.
Materials and methods
Plant material and experimental conditions
The experiments were carried out at a savannah site (60° 40′ N 5° 09′ W) located close to the outpost of the Centre Suisse de Recherches Scientifiques (CSRS, Abidjan, Côte d'Ivoire) in Bringakro in the centre of Côte d'Ivoire, in 2006 (year 1) and in 2007 (year 2). The site had been left fallow for over 20 years.
The soil was acidic, sandy and shallow with a depth less than 30 cm. Selected soil properties for this site are given in Hgaza et al. (Citation2010). The total rainfall recorded during the growing season (i.e. from May to November) was 750 and 1010 mm in year 1 and year 2, respectively. The average soil temperatures recorded at 20-cm depth throughout the growing seasons was 30 °C in year 1 and 25 °C in year 2. The cumulative solar radiation observed during the growing seasons were 2363 and 1968 MJ m−2 in year 1 and year 2, respectively.
A modern cultivar of D. rotundata, TDr 89/02461, bred by the International Institute of Tropical Agriculture, Nigeria, was planted due to its high yield, resistance to major pests and diseases, and suitability for cultivation without stakes (Ettien and Tschannen Citation2003). The head part of the tuber (mother sett), weighing 100 g fresh weight, was used as planting material to reduce sprouting heterogeneity and variability in time of emergence. The setts were soaked in 15 L of a watery mixture containing 600 g diazinon L−1 (insecticide), 240 g oxamyl L−1 (nematicide) and mancozebe 80% (fungicide) and air-dried for a day before planting. In year 2, the soil was treated against nematodes using 240 g oxamyl L−1 ha−1 2 weeks before planting.
The site was cleared in year 1 without burning and the debris removed. The soil surface was prepared with hoes and mounds of approximately 50 cm were built at a density of one per square metre. One mother sett was planted in each mound at a depth of 10 cm. In both years 90% of the plants had germinated at 2 weeks after planting. The plots were kept free of weeds by regular manual weeding. The growing season of D. rotundata spanned from May to late November, including a short dry season (July to August) and the beginning of the long dry season (November). To prevent water stress during plant growth, a drip irrigation system was installed. In year 1, 420 mm of water was provided by irrigation from July until the first half of October. In contrast, the rain was well distributed during the growth period in year 2, and irrigation was not required. The total amount of water (irrigation and rain) that the crop received was similar in the two years (1170 mm in year 1 and 1010 mm in year 2).
Experimental design and measurements
In both years, the first experiment was set up to assess the response of root morphology and plant growth to the application of mineral fertilizer. The second experiment aimed to map the distribution of roots in horizontal and vertical profiles. The second experiment was performed only on yams that had been fertilized.
The first experiment was arranged in a randomized complete block design with two fertilization treatments (non-fertilized (R0) and fertilized (R1)) which were replicated four times in individual plots of 9 m×4 m. The non-fertilized crop received no additional nutrients, while the fertilized treatment received 120-10-130-60 kg ha−1 of N-P-K-Ca, respectively, in the form of NH4NO3, K2SO4, Ca(H2PO4)2 and dolomite. This fertilizer rate would cover, according to the results obtained by Diby (Citation2005) on the same yam cultivar, the export of nutrients by 25 t of fresh tuber. The fertilizer was broadcasted in two equal splits at the time of the maximum growth of the above-ground organs and during tuber bulking at 70 and 110 days after planting (DAP), respectively, to maximize uptake and minimize nutrient losses. Two plants per replicate were sampled during the vegetative growth phase (60 DAP), at tuber initiation (90 DAP), during the tuber bulking (120 DAP), during the tuber maturation phase (150 DAP), while six plants were sampled per replicate at the final harvest (190 DAP) to reduce variability. The sampled plants were taken from the central part of the plot. They were surrounded by 14 plants subjected to the same treatment but that were not sampled. At the first four harvests both root morphological characteristics and the biomass of the plant organs were measured. At the final harvest, only the biomass of the plant organs was measured i.e. no morphological characteristics were measured on the roots.
Soil cores were taken with an Eijelkamp root auger (length 15 cm, diameter 8 cm) in the vicinity of two plants per plot at 60, 90, 120 and 150 DAP to assess the variations in root morphology in space and time. The soil cores were collected on opposite sides of each plant, first at a distance of 25 cm from the crown of the plant (i.e. within the mound) at 2 depths from 0 to 15 cm (H1) and from 15 to 30 cm (H2), and then at a distance of 50 cm from the crown of the plant (i.e. outside of the mound) at 2 depths from 0 to 15 cm (H3) and from 15 to 30 cm (H4). Soon after obtaining the soil cores, the entire plant was removed, the different organs (shoots, roots and tuber) were separated, and the biomass measured by drying the plant samples at 70 °C for 72 h (constant weight). The soil cores were taken to the laboratory, and the roots were separated from the soil by elutriation in water (Smucker et al. Citation1982). Debris and dead roots were manually removed from the living roots based on their colour and flexibility. Thereafter, the roots were gently rinsed in running water and carefully extended in a thin layer of water (2–3 mm) on a transparent tray and scanned. The total root length and the average root diameter (ARD, mm) were directly determined using WinRhizo V. 2003b as explained by Himmelbauer et al. (Citation2004). The root length density (RLD, cm cm−3) was expressed as the total root length per unit of sampled soil volume. The root mass density (RMD, mg cm−3) was expressed as the total root biomass per unit of sampled soil volume. The specific root length (SRL, cm mg−1) was determined as the root length per unit of root dry weight. Finally, the plant biomass including the roots removed from the tray was determined after oven drying at 70 °C for 72 h.
The second experiment was laid out in a randomized complete block design with four replicates. Each plot measured 5 m×4 m. As in the experiment described above, the plants were sampled from the central part of the plot which was surrounded by border plants treated identically but not sampled. All the plots received the fertilization regime described above. In this experiment, two plants per plot were destructively sampled during the vegetative growth phase (60 DAP) and at tuber bulking (130 DAP) to map the root system on both horizontal and vertical profiles. At both sampling dates, one plant was used for horizontal root mapping at three positions: 5 cm below the Primary Nodal Complex (PNC, D1), 25 cm below the PNC (D2) and 50 cm below the PNC (D3). The aerial parts of the plant were removed, a pit was dug until the target depth was reached and the soil carefully removed with a knife, leaving the roots in situ. The other plant in the same plot was used for the vertical root mapping. Trenches were dug at different distances from the centre of the mound: the first trench was dug at the level of the crown of the plant (0 cm, V1), the second at 25 cm from the crown of the plant (V2) and the third at a distance of 50 cm from the crown of the plant (V3). The wall trench profile method (Pagès and Pellerin Citation1996) was used to map the root distribution in horizontal and vertical profiles. On the vertical maps, the PNC was considered as the reference point (0, 0) and the negative Y-axis represented the depth. When all the root impacts had been marked on the plastic sheets, the sheets were brought to the laboratory and photographed. The photos were digitalized using Scion image software (http://www.scioncorp.com) to determine the coordinates (X, Y) of each root impact.
As none of the methods used in this study is perfect for assessing the root distribution (Logsdon and Allmaras Citation1991), the results from the soil core method were correlated to those given by the vertical profile. This correlation considered the sum of the root lengths (or root masses) measured in H1 and H2taken at 60 and 120 DAP, and the number of root impacts in V2 measured at 60 and 130 DAP as well as the root length (or root mass) measured in H3 (there were no roots in H4) and the number of root impacts in V3 for the same sampling times.
Statistical analysis
Analyses of variance were carried out (GLM Model, SAS v. 9.1, SAS Institute) to detect the effect of fertilization (R0, R1), sampling position (H1, H2, H3 and H4) and year (2006, 2007) on root growth parameters (RLD, RMD, SRL and ARD), and on dry matter in plant organs. A statistical significant effect was detected at p < 0.05. Standard errors (S.E.) were calculated for each mean value. Linear and non-linear regressions were performed using SAS v. 9.1 (SAS Institute). The validity of these regressions was evaluated by comparing the predicted and experimental values and by examining the residuals. Graphs were plotted using the SigmaPlot program.
A nearest neighbour analysis (Clark and Evans Citation1954) was used to test the root map patterns. This technique allows testing the distribution of data points, i.e. expected mean distance (rE) between roots against a Complete Spatial Randomness (CSR) distribution. The null hypothesis of CSR assumes that data points are distributed randomly. The nearest neighbour index value (R) is the ratio between rA and rE in the study area, where rA is the mean nearest neighbour distance which is defined as the closest distance between two data points. The nearest neighbour index values range from 0 for perfectly clumped points, to R = 1 for randomly distributed points to a maximum value of R = 2.15 for uniformly distributed points. The nearest neighbour analysis was performed using Surfer 8.0 software (Golden, CO, USA). The root maps were characterized by the root impacts number (RIN), the nearest neighbour distance (rA) and the root distribution pattern derived from R.
Results
Dry matter production during plant growth and fresh tuber yield at final harvest
Mineral fertilizer inputs had not significantly affected the root, shoot and tuber production in both years (). In contrast a significant effect of year was observed on shoot dry matter at 90, 150 and 190 DAP in the unfertilized treatment, and at 60, 90 and 150 DAP in the fertilized treatment. The final tuber dry matter and fresh tuber yield were not significantly affected by the year in the absence of fertilizer application whereas they were significantly higher (p≤0.05) in year 1 than in year 2 due to fertilizer application.
Table I. Effect of year on dry matter partitioning to shoots (leaves + vines), roots and tubers, and final fresh tuber yield of D. rotundata grown with and without fertilizers. Data are presented as means (standard error) of four replicates at each sampling date.
Root growth parameters
The root system of D. rotundata consisted of seminal roots observed on the mother sett, adventitious roots derived from the PNC and tubercular roots that grew from the new tuber. Only the adventitious roots remained alive until the harvest. The seminal roots disappeared as the mother sett was rotting, and the tubercular roots started to show necrosis after 150 DAP. Unfortunately, it was not possible to distinguish the different root types in the soil cores; therefore all the roots sorted from the soil cores were used to estimate root growth parameters. No roots were observed at H4.
A significant effect of year (p≤0.05) was observed on root length density (RLD) at 60, 90 and 160 DAP but not at 120 DAP. Except at 90 DAP, no significant effect of the year was observed in the root mass density (RMD). In contrast, the year significantly (p≤0.05) affected specific root lengths (SRL) and the average root diameter (ARD) except at 160 DAP for ARD (result not shown). The sampling position significantly affected (p≤0.05) the RLD, RMD and SRL at each sampling date except at 160 DAP for the SRL. Mineral fertilizer inputs had no significant impact on any of the root parameters measured in both years. Therefore, the results presented hereafter refer to the average values of the fertilized and unfertilized plants.
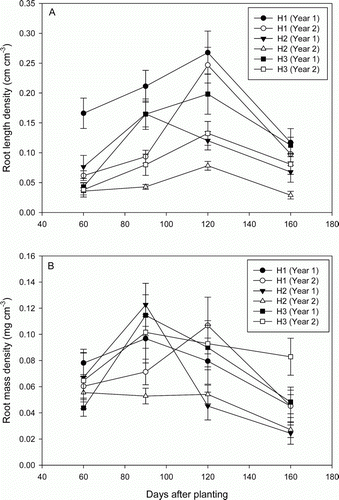
The root length density (RLD) increased over time, reached a peak and then declined until 150 DAP (A). In both years, RLD was highest at H1, lowest at H2 and intermediate at H3. The maximum RLD was observed at 120 DAP at H1 and H3. The maximum value of the RLD at H1 was not different between year 1 and year 2. In contrast, the maximum RLD at H3 was significantly higher (p≤0.05) in year 1 than in year 2. At H2, the maximum RLD was twice as high (p≤0.05) in year 1 as in year 2. The maximum root mass density (RMD) was observed at the beginning of tuber growth (90 DAP) except at H1 in year 1, while the maximum RMD was reached one month later in year 2 (B). The maximum RMD was not significantly different between the sampling positions in year 1 but was twice as high (p≤0.05) at H1 and H3, which was also seen at H2 in year 2.
Figure 1. Effect of sampling position and year on (A) root length densities and (B) root mass densities of D. rotundata. Data presented as mean values from fertilized and unfertilized treatments (n = 8). Vertical bars are standard errors. H1, H2 and H3 represents sampling positions.
In both years in all sampling positions, the average root diameter (ARD) decreased over time to reach a minimum at 120 DAP and remained stable until 150 DAP (A). Except at H1, the ARD in year 1 was significantly lower (p≤0.05) than in year 2. The maximum values for ARD were observed at H3 at 60 DAP in both years.
Figure 2. Effect of sampling position and year on (A) average root diameter and (B) specific root length of D. rotundata. Data presented as mean values from fertilized and unfertilized treatments (n = 8). Vertical bars are standard errors. H1, H2 and H3 represent sampling positions.
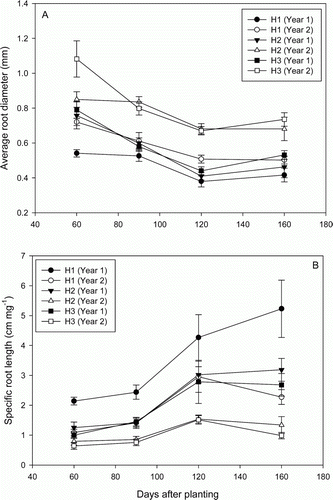
The variation pattern of the specific root length (SRL) over time presented three phases in both years (B). It remained constant between 60 and 90 DAP, and increased until 120 DAP, when it reached a plateau, except at H1 in year 1 where the SRL continued to increase. In both years, the SRL was not significantly different between H2 and H3, but both were significantly lower (p < 0.05) than at H1. At each sampling position, the SRL was higher in year 1 than in year 2.
Relationship between root biomass, root impact numbers and root density
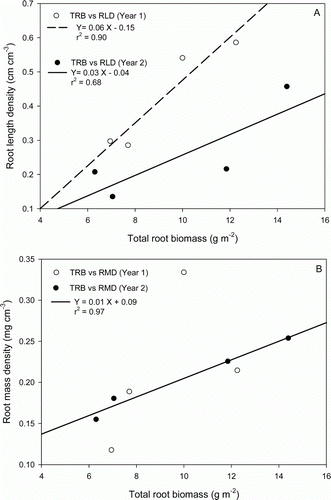
The root biomass of the excavated plants was significantly (p≤0.05) correlated to the sum of the RLD measured in H1, H2 and H3 (A). However, the correlation equation obtained in year 1 was different from the equation observed in year 2. A significant correlation was observed between the root biomass and the RMD measured in the three horizons in year 2 (B), but this correlation was not valid in year 1 because of the high RMD observed at 90 DAP. The coefficient of variation of the RLD and RMD was significantly (p≤0.05) correlated to the coefficient of variation of the shoot dry matter in year 2 but not in year 1 (results not shown).
Figure 3. Relationship between the total root biomass (TRB) measured in excavated plants and the sum of the root length density (RLD) (A) or the sum of the root mass density (RMD) (B) for sampling positions H1, H2 and H3 in Year 1 (dashed line, white symbols) and Year 2 (solid line, black symbols).
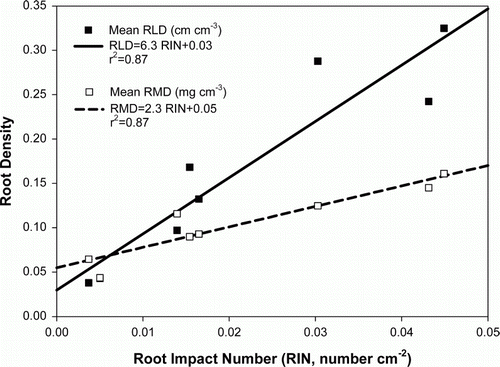
The root impact numbers (RINs) counted on vertical maps observed at 25 and 50 cm (V2 and V3) from the crown of the plant, after 60 and 130 DAP ranged from 0.005 to 0.05 cm−2 (). There were significant linear relationships (p≤0.05) between RLD and RMD measured at H1+H2 and at H3 and the RINs measured at V2 and V3 when combining all the samples for both years.
Figure 4. Relationship between root length density (▪), root mass density (□) and root impact numbers of D. rotundata. Data are presented as the mean value observed at 25 and 50 cm from the yam plant after 60 and 130 days after planting of all treatments (fertilized and unfertilized), sampling dates and positions and years.
Root system mapping
Horizontal maps at 60 DAP indicate that the root distribution patterns evolved in both years from a random distribution just below the PNC (D1) to a clumped distribution at D2 (). At 60 DAP the root distribution at D3 was random in year 1 and clumped in year 2. Horizontal maps observed at 130 DAP show that the roots were distributed as clumps in both years.
Table II. Root map patterns observed at each sampling position at 60 and 130 DAP in year 1 (2006) and year 2 (2007) using the nearest neighbour test for the null hypothesis of complete spatial randomness. The ratio between parentheses represents the frequency of observation of the pattern relative to the number of observed maps in the treatment at each sampling position and at each observation date.
Vertical maps at 60 DAP indicate that root distribution patterns evolved from clumps inside the mound (V1 and V2) towards uniform or random patterns in the space between mounds (V3) in year 1, while the pattern evolved from clumps at V1 to random at V2 and uniform at V3 in year 2. Vertical maps observed at 130 DAP show that the roots were distributed as clumps in both years.
Discussion
The growth of the root system of D. rotundata
During vegetative growth (at 60 DAP), very few roots were found below 15 cm in the mound and outside the mound. The roots of D. rotundata had a large diameter, and a low SLR, and the root system was composed of seminal and adventitious roots. Over time, roots became longer and thinner and were able to explore the 15–30 cm horizon within the mound and the first 15 cm of the space between the mounds. This development can be attributed to the growth of adventitious roots after tuber initiation and during tuber filling. The peak in the root mass density observed at 90 DAP in year 1 and at 120 DAP in year 2 can be related to the formation of thicker roots derived from the new tubers. The adventitious roots stayed alive after 150 DAP, confirming the description given by Charles-Dominique et al. (Citation2009).
As observed in potatoes (Vos and Groenwold Citation1987) and maize (Logsdon and Allmaras Citation1991), the root densities varied widely from sample to sample in D. rotundata, resulting in high coefficients of variation (64% both for RLD and RMD). D. rotundata root reached a maximum RLD of 0.25 cm cm−3 at 25 cm from the plant's crown during the period of rapid tuber growth. The maximum RLD of 0.26 cm cm−3 observed in D. rotundata was smaller than the maximum RLD of 11.3 and 1.5 cm cm−3, reported for potatoes (Ahmadi et al. Citation2011) and cassava (Lose et al. Citation2003), respectively. The root spatial distribution pattern observed in this study showed that D. rotundata is also a shallow-rooting species, similar to D. esculenta (Melteras et al. Citation2008), D. alata (Hgaza et al. Citation2011), cassava (Lose et al. Citation2003) and potatoes (Vos and Groenwold Citation1986, Ahmadi et al. Citation2011).
The significant (p≤0.05) correlation in RLD and RMD between the soil coring method and the vertical wall trench method shows that the wall trench method provides a representative characterization of the root system. The results using the wall trench method showed that the roots of D. rotundata were mostly distributed as clumps. A clumped distribution may allow roots to better explore a given soil volume, or it may be a response of the root system to soil physical heterogeneities (Logsdon and Allmaras Citation1991, Pellerin and Pagès Citation1996). In our study, because the soil was very sandy and loosened by mounding before planting, strong differences in physical properties within the mound were not expected. This clumpy distribution of roots in the mound may also be due to a response of the root system to exploit nutrients released from soil organic matter patches. The clumpy distribution observed outside the mound (space between mounds), however, may also be due to increased soil mechanical impedance.
The different relationships obtained between RLD, RMD and root biomass suggest that total root biomass is not a reliable indicator of root length and root length should be measured directly to properly characterize the uptake capacity of the root system (van Noordwijk and Brouwer Citation1991).
Effect of fertilization and environmental factors on the root system and the yield of D. rotundata
The longer and thinner root observed in year 1 in comparison to year 2 can be explained by the different weather conditions observed during these two seasons. Pardales et al. (Citation1999) and Qin et al. (Citation2006) observed higher RLDs in sweet potato and maize respectively when these plants were subjected to higher soil temperatures. This reaction observed in D. rotundata might be either a direct response of roots to high temperature stress or a response to another parameter such as organic matter mineralization, which increases with temperature. The higher temperatures observed in year 1 coupled with irrigation might have enhanced organic matter mineralization of the soil organic matter accumulated during this long-term fallow. The effect of temperature on mineralization may explain the longer and finer roots observed in year 1 as increased nitrate concentration in the soil solution stimulates lateral root elongation (Forde Citation2002). In year 2, a large fraction of the native soil organic matter would have been already mineralized while the higher rainfall regime might have led to higher N leaching. The absence of effect of fertilizer input on root morphological characteristics suggests either that the amount of nitrate released from the fertilizer application was not sufficient or that it was not available for the roots.
The study demonstrated that D. rotundata has a small, clumped and shallow root system that did not show a response to mineral fertilizer application and the tuber yield was not different between unfertilized and fertilized treatments. Therefore, it could be recommended to farmers not to fertilize D. rotundata. Fresh tuber yield in year 1 was high (22 t ha−1), comparable to the yield reported by Law-Ogbomo and Remison (Citation2008) (24 t ha−1), whereas in year 2 D. rotundata tuber yield was only 14 t ha−1 which was comparable to the yield reported by Diby et al. (Citation2011). This suggests that the root system observed in year 1 in this study was not limiting for a high tuber yield. The development of long and fine roots in D. rotundata might be related to high soil temperature, but also to high rates of organic matter mineralization which might be influenced by management. The variability of root growth and distribution also should be explored in different cultivars of D. rotundata to promote the breeding of improved cultivars with larger and extensive root systems.
Acknowledgements
This project was supported by the Swiss Development and Cooperation Agency through the Research Fellowship Partnership Program managed by the North-South Centre of the ETH Zurich, they are all acknowledged. Thanks are extended to the whole research crew based in Bringakro (CSRS) for field and laboratory assistance.
References
- Ahmadi , S. H. , Plauborg , F. , Andersen , M. N. , Sepaskhah , A. R. , Jensen , C. R. and Hansen , S. 2011 . Effects of irrigation strategies and soils on field grown potatoes: Root distribution . Agricultural Water Management , 98 : 1280 – 1290 .
- Asadu , C. L. A. , Akamigbo , F. O. R. , Nweke , F. I. and Ezumah , H. 1996 . Evaluation of six cultivars of white yam (Dioscorea rotundata) across three yam-growing areas in southeastern Nigeria . Journal of Agricultural Science, Cambridge , 127 : 463 – 468 .
- Asiedu , R. and Sartie , A. 2010 . Crops that feed the world. 1. Yams for income and food security . Food Security , 2 : 305 – 315 .
- Charles-Dominique , T. , Mangenet , T. , Rey , H. , Jourdan , C. and Edelin , C. 2009 . Architectural analysis of root system of sexually vs. vegetatively propagated yam (Dioscorea rotundata Poir.), a tuber monocot . Plant and Soil , 317 : 61 – 77 .
- Clark , P. J. and Evans , F. C. 1954 . Distance to nearest neighbor as a measure of spatial relationships in populations . Ecology , 35 : 445 – 453 .
- Degras , L. 1993 . The yam: A tropical root crop , London : MacMillan Press .
- Diby , L. N. 2005 . Etude de l’élaboration du rendement chez deux espèces d'igname (Dioscorea spp.) . Thèse Unique de Doctorat. UFR des sciences de la terre et des ressources minières. Université de Cocody – Abidjan, Côte d'Ivoire . 155 p.
- Diby , L. N. G. , Tié , B. T. , Girardin , O. , Sangakkara , R. , & Frossard , E. 2011 . Growth and nutrient use efficiencies of yam (Dioscorea spp.) grown in two contrasting soils of West Africa . International Journal of Agronomy , Article ID 175958 , doi: 1155/2011/175958 .
- Ettien , J. B. & Tschannen , A. 2003 . Evaluation de nouvelles variétés d'igname en Côte d'Ivoire. Bilan de trois ans d'expérience avec des génotypes améliorés par l'IITA . In J. Y. Jamin , B. L. Seiny , and C. Floret , Actes du colloque Savanes africaines: des espaces en mutation, des acteurs face à de nouveaux défis , mai 2002, Garoua, Cameroun. Prasac, N'Djamena, Tchad – Cirad, Montpellier, France .
- FAO 2011 . Statistical databases . Available at: http://faostat.fao.org/ (Accessed 22 August 2011) .
- Forde , B. G. 2002 . Local and long-range signaling pathways regulating plant responses to nitrate . Annual Review of Plant Biology , 53 : 203 – 224 .
- Hgaza , V. K. , Diby , L. N. , Assa , A. and Aké , S. 2010 . How fertilization affects yam (Dioscorea alata L.) growth and tuber yield across the years . African Journal of Plant Science , 4 : 53 – 60 .
- Hgaza , V. K. , Diby , L. N. , Tié , T. B. , Tschannen , A. , Aké , S. , Assa , A. and Frossard , E. 2011 . Growth and distribution of roots of Dioscorea alata L. do not respond to mineral fertilizer application . Open Plant Science Journal , 5 : 14 – 22 .
- Himmelbauer , M. L. , Loiskandl , W. and Kastanek , F. 2004 . Estimating length, average diameter and surface area of roots using two different image analyses systems . Plant and Soil , 260 : 111 – 120 .
- Law-Ogbomo , L. E. and Remison , S. U. 2008 . Growth and yield of white guinea yam (Dioscorea rotundata Poir.) influenced by NPK fertilization on a forest site in Nigeria . Journal of Tropical Agriculture , 46 : 21 – 24 .
- Lebot , V. 2009 . Tropical root and tuber crops , Wallingford : CABI Publishing .
- Logsdon , S. D. and Allmaras , R. R. 1991 . Maize and soybean root clustering as indicated by root mapping . Plant and Soil , 131 : 169 – 176 .
- Lose , S. J. , Hilger , T. H. , Leihner , D. E. and Kroschel , J. 2003 . Cassava, maize and tree root development as affected various agroforestery and cropping systems in Benin, West Africa . Agriculture, Ecosystems and Environment , 100 : 137 – 151 .
- Lynch , J. P. 2007 . Roots of the second green revolution . Australian Journal of Botany , 55 : 493 – 512 .
- Melteras , M. , Lebot , V. , Asher , C. J. and O'sullivan , J. N. 2008 . Crop development and root distribution in lesser yam (Dioscorea esculenta): Implications for fertilization . Experimental Agriculture , 44 : 209 – 221 .
- Njoku , E. , Nwoke , F. I. O. , Okonkwo , S. N. C. and Oyolu , C. 1984 . Pattern of growth and development in Dioscorea rotundata Poir . Tropical Agriculture (Trinidad) , 61 : 17 – 19 .
- O'Sullivan , J. N. 2008 . Root distribution of yam (Dioscorea alata) determined by strontium tracer . Experimental Agriculture , 44 : 223 – 233 .
- O'Sullivan , J. N. and Ernest , J. 2008 . Yam nutrition and soil fertility management in the Pacific , Brisbane : Australian Centre for International Agricultural Research .
- Pagès , L. and Pellerin , S. 1996 . Study of differences between vertical root maps observed in maize crop and stimulated maps obtained using a model for the three-dimensional architecture of the root system . Plant and Soil , 182 : 329 – 337 .
- Paillard , S. , Treyer , S. , & Dorin , B. 2011 . Agrimonde: Scenarios and challenges for feeding the world in 2050 . Versailles : Ed. Quae .
- Pardales , J. R. , Banoc , M. D. , Yamauchi , A. , Lijima , M. and Kono , Y. 1999 . Root system development of cassava and sweet potato during early growth stage as affected by high root zone temperature . Plant Production Science , 2 : 247 – 251 .
- Pellerin , S. and Pagès , L. 1996 . Evaluation in field conditions of a three-dimensional architecture model of the maize root system: comparison of stimulated and observed horizontal root maps . Plant and Soil , 178 : 101 – 112 .
- Qin , R. , Stamp , P. and Richner , W. 2006 . Impact of tillage on maize rooting in a Cambisol and Luvisol in Switzerland . Soil and Tillage Research , 85 : 50 – 61 .
- Smucker , A. J. M. , Srivastava , A. K. and McBurney , S. L. 1982 . Quantitative separation of roots from compacted soil profiles by the hydropneumatic elutriation system . Agronomy Journal , 74 : 500 – 503 .
- Sotomayor-Ramirez , D. , Gonzales-Vélez , A. and Roman-Paoli , E. 2003 . Yam (Dioscorea spp.) response to fertilization in soils of semiarid southern coast of Puerto Rico . Journal of Agriculture of University of Puerto Rico , 87 : 91 – 103 .
- Srivastava , A. , Dagbenonbakin , G. D. and Gaiser , T. 2010 . Effect of fertilization on yam (Dioscorea rotundata) biomass production . Journal of Plant Nutrition , 33 : 1056 – 1065 .
- van Noordwijk , M. and Brouwer , G. 1991 . “ Review of quantitative root length data in agriculture ” . In Plant roots and their environment , Edited by: McMichael , B. L. and Persson , H. 402 – 409 . Amsterdam : Elsevier .
- Vos , J. and Groenwold , J. 1986 . Root growth of potato crops on a marine-clay soil . Plant and Soil , 94 : 17 – 33 .
- Vos , J. and Groenwold , J. 1987 . “ The relation between root growth along observation tubes and in bulk soil ” . In Minirhizotron observation tubes: Methods and applications for measuring rhizosphere dynamics , Edited by: Taylor , H. M. 39 – 49 . Madison , WI : ASA .