Abstract
Competition is one of the main forms of interaction between cultivated crops and their neighboring plants. Allelopathy is a chemical mechanism that gives plants an advantage in competing for limited resources. Switchgrass (Panicum virgatum L.) has recently been introduced to China's Loess Plateau. As a non-native species, the competitive or allelopathic effects of switchgrass plants or switchgrass residue could have an important effect on weed growth in the switchgrass stand. In this field experiment, we investigated the effect of eight switchgrass cultivars (Blackwell, Cave-in-Rock, Dakota, Forestberg, Illinois USA, Nebraska 28, Pathfinder, and Sunburst) on associated weed growth. Weed density and biomass under each switchgrass cultivar were measured on four dates during the growing season. The effect of switchgrass residue on associated weed growth was also studied. Almost all of the switchgrass cultivars suppressed weed growth early in the growing season; however, Cave-in-Rock was the only switchgrass cultivar that significantly suppressed weed growth throughout the entire growing season. There was a significant negative relationship between switchgrass biomass and weed biomass during the middle part of the growing season (i.e., 28 July and 30 August). This indicated that the competitive effects of switchgrass had the greatest effect on weed growth during this stage. The residue of Blackwell, Illinois USA, and Pathfinder suppressed weed growth more than the growing switchgrass plants did. These results have implications for weed management strategies in agroecosystems and provide important information for the introduction of switchgrass to new ecosystems.
Introduction
Switchgrass (Panicum virgatum L.) is a highly productive warm-season grass with excellent potential as a bioenergy crop. Switchgrass ploidy varies from dodecaploid (2n = 12x = 108) to tetraploid (2n = 2x = 18) (Hulquist et al., Citation1996). Switchgrass is divided into two ecotypes: upland switchgrass and lowland switchgrass. Upland switchgrass ecotypes are generally sextuploids and octaploids. These ecotypes are adaptable to arid regions and generally grow to a height of 1.5–2.0 m. Lowland ecotypes are generally tetraploids (Brunken & Ester, Citation1975; Hulquist et al., Citation1996, Citation1997). Lowland switchgrass is more adapted for growth in wet conditions, where it reaches 3–4 m height (Moser & Vogel, Citation1995).
Because of its adaptability to harsh environments, Chinese scientists have recently introduced switchgrass to China's Loess Plateau (Ma et al., Citation2011). There is always concern that a non-native species may disrupt the natural ecosystem by out-competing native plants for light, nutrients, and water (Peter & Allison, Citation1998; Thomas & Peter, Citation1998; Mack et al., Citation2000; Reichard & White, Citation2001). However, switchgrass does not seem to have these negative effects in the Loess Plateau.
Effective weed control is an important component in agricultural management. The detrimental effect of weeds is most often due to their competition for resources (Radosevich, Citation1997). Cover crops are commonly used by vegetable growers (Beveridge & Naylor, Citation1999) to control weeds, to improve soil fertility and structure, and to conserve soil and water (Teasdale, Citation1996; Sainju & Singh, Citation1997; Reddy, Citation2003). Early crop vigor (Acciaresi et al., Citation2001; Bertholdsson, Citation2005), early season crop ground vegetation cover (Huel & Hucl, Citation1996; Lemerle et al., Citation1996; Cousens et al., Citation2003), and plant height (Didon & Hansson, Citation2002) are widely reported as important characteristics for enhancing crop competitiveness. A number of studies have been done about the effect of cover crops on weed suppression in vegetable production systems (Teasdale & Daughtry, Citation1993; Fisk et al., Citation2001; Reddy, Citation2003; Summers et al., Citation2009).
Some gramineous plants inhibit the growth of associated plants (Creamer et al., Citation1996; Doohan et al., Citation2000; Widmer & Abawi, Citation2000). One explanation is that compounds produced during the decomposition of gramineous plant residue in soil may be toxic to associated plants (Davis et al., Citation1996). Alternatively, the gramineous plants may produce secondary metabolites (i.e., allelochemicals) that are unnecessary for basic metabolism but confer ecological advantages by killing, suppressing, weakening, or repelling nearby competitors for nutrients, space, or other resources (Del Moral, Citation1975; Ben-Hammouda et al., Citation1995; Weston, Citation2005). Allelopathy is considered to be a ‘novel weapon’ against invasion by exotic plants (Callaway & Ridenour, Citation2004; Wu & Peng, Citation2005; Chen et al., Citation2009). Allelopathy is one of the main mechanisms for balancing plant populations in a stable ecosystem.
Allelopathic effect is strongly coupled with stresses in the crop environment, including temperature extremes, nutrient deficiency, and moisture variables. These stress conditions often enhance the production of allelochemicals, thus increasing the potential for allelopathic interference. In the paradigm of interactions, it seems that the crops are more sensitive to allelopathy when moisture, temperature, or nutrient conditions are less than optimal.
Weed control should be considered as one of the main criteria for the selection of switchgrass cultivars which are adaptable to arid or semiarid regions. The objectives of this study were (1) to compare the effect of different switchgrass cultivars on weed growth; (2) to quantify changes in the suppressive effect of switchgrass across time; and (3) to determine the effect of switchgrass residue on weed growth.
Materials and method
Seeds of eight switchgrass cultivars were provided by Professor Nobumasa Ichizen, Utsunomiya University, Japan (). The cultivars were Blackwell, Cave-in-Rock, Dakota, Forestberg, Illinois USA, Nebraska 28, Pathfinder, and Sunburst. The cultivars were planted separately in 2 m×5 m plots at an experiment site in Dingbian County, Shaanxi Province (36°49′–37°53′N, 107°15′–108°22′E) in May 2009. Dingbian County is in the transition zone between the Loess Plateau and the Ordos Basin. The region has a continental, semiarid climate. The average annual temperature is 7.9°C and the annual precipitation is 317 mm. The plots were cultivated to a depth of 30 cm before sowing switchgrass. The switchgrass was seeded 3–4 cm deep with a 40-cm row spacing. Two adjoining plots were cultivated at the same time but left unseeded as controls. The switchgrass seeds germinated after nine days. Each plot (including the controls) was mulched with wheat straw to conserve soil moisture and watered daily during the first growing season.
Table I. Ecotypes and chromosome ploidy of the switchgrass cultivars used in this study.
Effect of growing switchgrass plants on weed biomass and density
In the spring of 2010 (i.e., one year after the switchgrass was planted), we randomly established three 1 m2 subplots within each switchgrass stand. The switchgrass and weeds in each subplot were cut at ground level on 30 June, 28 July, 30 August, and 13 October. The switchgrass and weeds were immediately separated and weighed. The samples were then dried at 80°C and weighed again. Weed density was also counted in each subplot. The three subplots were considered as replications.
Effect of switchgrass residue on weed biomass
The switchgrass remained in the field throughout the winter of 2010–2011. In March 2011, we cut the switchgrass in each plot at ground level and then spread the residue evenly across the soil surface. The growing switchgrass, the switchgrass residue, and the weeds were collected from five 1 m2 subplots within each plot on 20 June 2011. The fresh weight of the growing switchgrass and the weeds was determined immediately. The samples were then dried at 80°C and the dry weight of the switchgrass, switchgrass residue, and weeds was measured. A plot with no switchgrass or switchgrass residue was used as a control.
Data analysis
Analysis of variance was conducted using JMP v6.0 software (SAS Institute Inc., Cary, NC, USA). Means were compared using the least significant difference (LSD) method at P≤0.05.
Results
Growing switchgrass plants generally inhibited weed growth (). Weed growth in the plots generally increased across time. Only Cave-in-Rock suppressed weed growth throughout the entire growing season. Blackwell suppressed the weed growth in the early and middle parts of growing season but had less effect on weed growth at the end of the growing season. Forestberg, Dakota, Nebraska 28, Pathfinder, and Sunburst suppressed weed growth early in the growing season but not late in the growing season. Illinois USA suppressed weed growth mostly during the middle of the growing season but had less effect during the early and late parts of the growing season.
Table II. The effects of eight switchgrass cultivars on weed fresh weight and dry weight at four sampling times during the growing season. Means within a column followed by a different lowercase letter are significantly different at the 5% level.
Switchgrass suppressed not only total weed biomass but also weed density and individual weed biomass (). Cave-in-Rock suppressed weed density more significantly than the other cultivars did. During the early part of the growing season (i.e., 30 June), weed density was highest in the Dakota and Pathfinder stands. Weed density was less in the switchgrass plots than in the control plots during the middle part of the growing season (i.e., 28 July and 30 August); however, by the end of the growing season, the suppressive effects of Dakota, Forestberg, Illinois, Nebraska 28, and Pathfinder switchgrass had disappeared. Only Cave-in-Rock suppressed individual weed biomass at the end of growing season (i.e., 13 October).
Table III. The effects of eight switchgrass cultivars on weed density and individual weed biomass at four sampling times during the growing season. Means within a column followed by a different lowercase letter are significantly different at the 5% level.
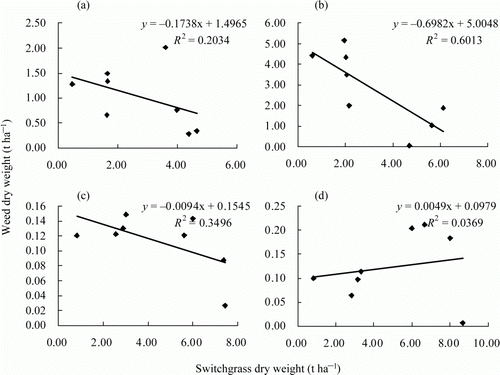
There was a significant negative relationship between switchgrass biomass and weed biomass on 28 July (b, R 2=0.6013) and on 30 August (c, R 2=0.3496). In contrast, there was no significant correlation between switchgrass biomass and weed biomass on 30 June (a, R 2=0.2034) or on 13 October (d, R 2=0.0369).
Figure 1. Linear regressions between weed dry weight and switchgrass dry weight at four sampling times during the growing season. Samples were collected at 30 June (a), 28 July (b), 30 August (c), and 13 October (d), 2010. Equations and coefficients of determination (R 2) for linear regression were given.
After overwintering, the switchgrass plants were cut at ground level and the residues were spread across the plots. The dry weight of the residue differed significantly among the cultivars (). Weed biomass in the switchgrass plots ranged from 12.9 g m−2 in the Forestberg treatment to 51.2 g m−2 in the Sunburst treatment. There was a significant negative relationship between residue dry weight and weed biomass in the Blackwell (slope = − 0.505, R 2=0.91), Illinois USA (slope = − 2.687, R 2=0.73), and Pathfinder (slope = − 0.445, R 2=0.34) plots. This indicates that residue from these three cultivars had significant suppressive effects on weed growth during the early part of the growing season (i.e., 20 June). There was a significant positive relationship between residue dry weight and weed biomass in the Nebraska 28 plot (slope = 0.496, R 2=0.64). The dry weight of residue had greater effect than the dry weight of growing switchgrass plants on weed biomass on 20 June ().
Figure 2. Linear regression between weed biomass and switchgrass biomass (a) and between weed biomass and switchgrass residue biomass (b). Equations and coefficients of determination (R 2) for linear regression were given.
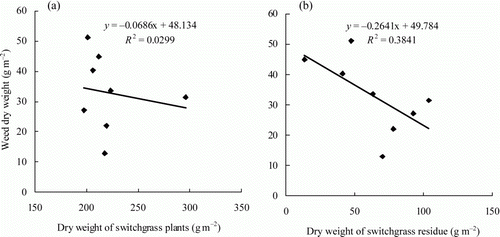
Table IV. Linear regression between weed biomass and switchgrass residue dry weight. Samples were collected at 20 June 2011. Slopes and coefficients of determination (R 2) were given. Means within a column followed by a different lowercase letter are significantly different at the 5% level.
Discussion
The results in this study showed that Cave-in-Rock suppressed weed growth throughout the growing season. This indicated that Cave-in-Rock was more competitive than the other switchgrass cultivars. One explanation is that Cave-in-Rock had greater biomass and a higher photosynthetic rate than the other cultivars (Ma et al., Citation2011). This suggested that Cave-in-Rock was better adapted to the Loess Plateau than the other cultivars were. Previous studies found that the highest yielding and tallest varieties were generally the best weed suppressors (Didon & Hansson, Citation2002; Zhao et al., Citation2006). Compared to the other cultivars, Cave-in-Rock has a longer growing period and a later maturity date (Muir et al., Citation2001). This may explain why weed growth was suppressed even until 13 October. In contrast, Dakota has a short growing season, thus requiring fewer temporal and spatial resources. By the end of the growing season, the Dakota switchgrass plants had died back and the suppressive effects of Dakota switchgrass had disappeared. Dakota is a short cultivar whereas Pathfinder is taller. Both cultivars have relatively few tillers (Einhellig & Rasmussen, Citation1989). The suppressive effect of Dakota and Pathfinder on weed growth was not as strong as the suppressive effect of the other cultivars. Therefore, well-adapted switchgrass cultivars with high tillering rates generally suppressed weed growth most. Some switchgrass cultivars are more competitive than other cultivars. These cultivars are relatively easy to establish, especially in harsh environments with limited resources. Weeds in some switchgrass stands grew well in the early part of the growing season. If these switchgrass cultivars were used, then weeds would need to be removed manually during the early part of the growing season.
Primary productivity is commonly used to evaluate ecosystem productivity. In grasslands, primary productivity is the basic means of assessing the carrying capacity of pastures and the partitioning of grassland types. The most competitive plants out-compete neighboring plants for space and nutrients. Therefore, we analyzed the correlation between switchgrass biomass and weed biomass to evaluate the competiveness of switchgrass at different growth stages. The negative linear relationship between switchgrass biomass and weed biomass indicated the existence of competition. The insignificant correlation between switchgrass biomass and weed biomass in the early growing season suggests that competition was not the only mechanism affecting weed growth at this time. Data in indicated that switchgrass suppressed weed growth in almost every case. The allelopathic effect might be another factor causing the suppression of weed growth in the switchgrass plots. Allelopathic effect in the early growing season would give switchgrass plants an advantage in competing for limited resources (Putnam, Citation1985; George, Citation1987). The significant linear correlation between switchgrass biomass and weed biomass in the middle part of the growing season suggests that the competitive effect of switchgrass on weeds during this period was greater than the allelopathic effect. At the end of the growing season, the switchgrass plants died back and exhibited less competitive effect. Weed growth in most plots increased at that time due to an increase in the availability of nutrient and spatial resources. Allelochemicals leached from the withered switchgrass plants at this time might also affect weed growth.
The effect of crop residue on weed growth has been studied by a number of researchers (Weston, Citation1996; Liebman & Davis, Citation2000; Ohno et al., Citation2000; Kruidhof et al., Citation2008, Citation2009). Compounds produced during the decomposition of plant residue on the soil surface interfere with weed growth. Some of these compounds are allelochemicals. The allelopathic effect of switchgrass varies among ecotypes (Shui et al., Citation2010). Specifically, upland ecotypes displayed greater allelopathic effect than lowland ecotypes. This may explain why the suppressive effects of residue from Blackwell, Illinois USA, and Pathfinder residue were greater than those from the other cultivars. The residue, weed biomass, and switchgrass biomass were collected on 20 June 2011. At that time, the switchgrass plants were growing rapidly and needed resources to support their metabolism. The switchgrass plants could utilize several strategies to compete with weeds for these resources. Our results suggest that allelopathy was one of the most important means by which switchgrass out-competed associated weeds.
The suppression of weed growth by switchgrass residue may have been due to allelochemicals or toxic compounds. The allelochemicals or toxic compounds derived from switchgrass residue on the soil surface can permeate into the soil profile and interfere with weed germination or growth. The optimal residue management strategy for weed suppression depends both on the crop species and the target weed species (Kruidhof et al., Citation2009). Plant species differ in their susceptibility to allelochemicals (Rietveld, Citation1983; Duke et al., Citation1987); therefore, the community structure of such associations may be regulated by a complex three-way interaction between allelopathy, interspecific competition, and intraspecific density effects.
The suppressive effect of switchgrass on associated weed growth is one of the main mechanisms which enables switchgrass to adapt to a new environment. When competition or allelopathic effect occurs in the natural environment, the response of the associated weeds depends on the weed species and the crop growth stage. Competition and allelopathic effect commonly take place during the period of maximum growth. During this period, crops need more resources to support their metabolic needs. Crops often increase their competitiveness with their neighbors in order to improve their productivity. This is the result of natural selection and ecological adaptability for a non-native species in a new environment. It is also important to note that target species differ in their response to competition or allelopathic effect. The response of the associated weeds to switchgrass competition depends on the weed species and the growth stage. However, the allelopathic effect of switchgrass residue on the soil surface depends on the decomposition rate of the residue, the decomposition rate of the allelochemical, and the emergence time of the target (i.e., weed) species. We are currently doing additional laboratory and field studies to learn more about the mechanism for the effect of switchgrass plants and switchgrass residue on target weed species.
Acknowledgements
The study was funded by the State Key Laboratory of Soil Erosion and Dryland Farming on the Loess Plateau (10502) and National Science and Technology Support Program (2011BAD31B05). We thank Dr Jeff Gale, Northwest A & F University, China, for his constructive advice and editorial assistance.
References
- Acciaresi , H. A. , Chidichimo , H. O. and Sarondon , S. J. 2001 . Traits related to competitive ability of wheat (Triticum aestivum) varieties against Italian ryegrass (Lolium multiflorum) . Biological Agriculture & Horticulture , 19 : 275 – 286 . doi: 10.1080/01448765.2001.9754930
- Ben-Hammouda , M. , Kremer , R. J. and Minor , H. C. 1995 . Phytotoxicity of extracts from sorghum plant components on wheat seedlings . Crop Science , 35 : 1652 – 1656 . doi: 10.2135/cropsci1995.0011183X003500060023x
- Bertholdsson , N. O. 2005 . Early vigour and allelopathy – two useful traits for enhanced barley and wheat competitiveness against weeds . Weed Research , 45 : 94 – 102 . doi: 10.1111/j.1365-3180.2004.00442.x
- Beveridge , L. E. & Naylor , R. E. L. 1999 . Options for organic weed control – what farmers do . In G. Marshal Proceedings 1999 Brighton Conference – Weeds , November 15–18 , Brighton , UK . 939 – 944 .
- Brunken , J. N. and Ester , J. R. 1975 . Cytological and morphological variation in Panicum virgatum L . Southwestern Naturalist , 19 : 379 – 385 . doi: 10.2307/3670396
- Callaway , R. M. and Ridenour , W. M. 2004 . Novel weapons: Invasive success and the evolution of increased competitive ability . Frontiers in Ecology and the Environment , 2 : 436 – 444 . doi: 10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2
- Chen , B. M. , Peng , S. L. and Ni , G. Y. 2009 . Effects of the invasive plant Mikania micrantha H.B.K. on soil nitrogen availability through allelopathy in South China . Biological Invasions , 11 : 1291 – 1299 . doi: 10.1007/s10530-008-9336-9
- Cousens , R. D. , Barnett , A. G. and Barry , G. C. 2003 . Dynamics of competition between wheat and oats: I. Effects of changing the time of phenological events . Agronomy Journal , 95 : 1293 – 1304 .
- Creamer , N. G. , Bennett , M. A. , Stinner , B. R. , Cardina , J. and Regnier , E. E. 1996 . Mechanisms of weed suppression in cover crop-based production systems . HortScience , 31 : 410 – 413 .
- Davis , J. R. , Huisman , O. C. , Westermann , D. T. , Hafez , S. L. , Everson , D. O. , Sorensen , L. H. and Schneider , A. T. 1996 . Effects of green manures on Verticillium wilt of potato . Phytopathology , 86 : 444 – 453 . doi: 10.1094/Phyto-86-444
- Del Moral , R. 1975 . Allelopathy: A milestone monograph . Ecology , 56 : 1231 – 1233 . doi: 10.2307/1936167
- Didon , U. M. E. and Hansson , M. L. 2002 . Competition between six spring barley (Hordeum vulgare ssp. vulgare L.) cultivars and two weed flora in relation to interception of photosynthetic active radiation . Biological Agriculture & Horticulture , 20 : 257 – 273 . doi: 10.1080/01448765.2002.9754969
- Doohan , F. M. , Mentebab , A. and Nicholson , P. 2000 . Antifungal activity toward Fusarium culmorum in soluble wheat extracts . Phytopathology , 90 : 666 – 671 . doi: 10.1094/PHYTO.2000.90.6.666
- Duke , S. O. , Vaughn , K. C. , Croom , E. M. Jr and Elsohly , H. 1987 . Artemisinin, a constituent of annual wormwood (Artemisia annua) is a selective phytotoxin . Weed Science , 35 : 499 – 505 .
- Einhellig , F. A. & Rasmussen , J. A. 1989 . Prior cropping with grain sorghum inhibits weeds . Journal of Chemical Ecology 15 , 951 – 960 . doi: 10.1007/BF01015190
- Fisk , J. W. , Hesterman , O. B. , Shrestha , A. , Kells , J. J. , Harwood , R. R. , Squire , J. M. and Sheaffer , C. C. 2001 . Weed suppression by annual legume cover crops in no-tillage corn . Agronomy Journal , 93 : 319 – 325 . doi: 10.2134/agronj2001.932319x
- George , R. W. 1987 . Allelochemicals , Washington , DC : American Chemical Society .
- Huel , D. G. and Hucl , P. 1996 . Genotype variation for competitive ability in spring wheat . Plant Breeding , 115 : 325 – 329 . doi: 10.1111/j.1439-0523.1996.tb00927.x
- Hulquist , S. J. , Vogel , K. P. , Lee , D. J. , Arumuganathan , K. and Kaeppler , S. 1996 . Chloroplast DNA content and nuclear DNA content variations among cultivars of switchgrass, Panicum virgatum L . Crop Science , 36 : 1049 – 1052 . doi: 10.2135/cropsci1996.0011183X003600040039x
- Hulquist , S. J. , Vogel , K. P. , Lee , D. J. , Arumuganathan , K. and Kaeppler , S. 1997 . DNA content and chloroplast DNA polymorphisms among switchgrasses from remnant Midwestern prairies . Crop Science , 37 : 595 – 598 . doi: 10.2135/cropsci1997.0011183X003700020047x
- Kruidhof , H. M. , Bastiaans , L. and Kropff , M. J. 2008 . Ecological weed management by cover cropping: Effects on weed growth in autumn and weed establishment in spring . Weed Research , 48 : 492 – 502 . doi: 10.1111/j.1365-3180.2008.00665.x
- Kruidhof , H. M. , Bastiaans , L. and Kropff , M. J. 2009 . Cover crop residue management for optimizing weed control . Plant and Soil , 318 : 169 – 184 . doi: 10.1007/s11104-008-9827-6
- Lemerle , D. , Verbeek , B. , Cousens , R. D. and Coombes , N. E. 1996 . The potential for selecting wheat varieties strongly competitive against weeds . Weed Research , 36 : 505 – 513 . doi: 10.1111/j.1365-3180.1996.tb01679.x
- Liebman , M. and Davis , A. S. 2000 . Integration of soil, crop and weed management in low-external-input farming systems . Weed Research , 40 : 27 – 47 . doi: 10.1046/j.1365-3180.2000.00164.x
- Ma , Y. Q. , An , Y. , Shui , J. F. and Sun , Z. J. 2011 . Adaptability evaluation of switchgrass (Panicum virgatum L.) cultivars on the Loess Plateau of China . Plant Science , 181 : 638 – 643 . doi: 10.1016/j.plantsci.2011.03.003
- Mack , R. N. , Simberloff , D. , Lonsdale , W. M. , Evans , H. , Clout , M. and Bazzaz , F. A. 2000 . Biotic invasions: Causes, epidemiology, global consequences, and control . Ecological Applications , 10 : 689 – 710 . doi: 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
- Moser , L. E. & Vogel , K. P. 1995 . Switchgrass, big bluestem, and Indian grass . In R. F. Barnes , D.A. Miller and C.J. Nelson Forages: An Introduction to Grassland Agriculture , 1 Ames , IA : Iowa State University Press , 409 – 420 .
- Muir , J. P. , Sanderson , M. A. , Ocumpaugh , W. R. , Jones , R. M. and Reed , R. L. 2001 . Biomass production of ‘Alamo’ switchgrass in response to nitrogen, phosphorus, and row spacing . Agronomy Journal , 93 : 896 – 901 . doi: 10.2134/agronj2001.934896x
- Ohno , T. , Doolan , K. , Zibilske , L. M. , Liebman , M. , Gallandt , E. R. and Berube , C. 2000 . Phytotoxic effects of red clover amended soils on wild mustard seedling growth . Agriculture Ecosystems & Environment , 78 : 187 – 192 . doi: 10.1016/S0167-8809(99)00120-6
- Peter , S. W. and Allison , E. S. 1998 . Where do we go from here? The challenges of risk assessment for invasive plants . Weed Technology , 12 : 744 – 751 .
- Putnam , A. R. 1985 . “ Weed allelopathy ” . In Weed Physiology, Volume I: Reproduction and Ecophysiology , Edited by: Duke , S. O. 131 – 155 . Boca Raton , FL : CRC Press .
- Radosevich , S. R. 1997 . “ Physiological aspects of competition (Chapter 6) ” . In Weed Ecology: Implications for Management , Edited by: Radosevich , S. R. , Holt , J. S. and Ghersa , C. M. 217 – 299 . New York : John Wiley & Sons, Inc. .
- Reddy , K. N. 2003 . Impact of rye cover crop and herbicides on weeds, yield, and net return in narrow-row transgenic and conventional soybean (Glycine max) . Weed Technology , 17 : 28 – 35 . doi: 10.1614/0890-037X(2003)017[0028:IORCCA]2.0.CO;2
- Reichard , S. H. and White , P. 2001 . Horticulture as a pathway of invasive plant introductions in the United States . Bioscience , 51 : 103 – 113 . doi: 10.1641/0006-3568(2001)051[0103:HAAPOI]2.0.CO;2
- Rietveld , W. J. 1983 . Allelopathic effects of juglone on germination and growth of several herbaceous and woody species . Journal of Chemical Ecology , 9 : 295 – 307 . doi: 10.1007/BF00988047
- Sainju , U. M. and Singh , B. P. 1997 . Winter cover crops for sustainable agricultural systems: Influence on soil properties, water quality, and crop yields . HortScience , 32 : 21 – 28 .
- Shui , J. F. , An , Y. , Ma , Y. Q. and Ichizen , N. 2010 . Allelopathic potential of switchgrass (Panicum virgatum L.) on perennial ryegrass (Lolium perenne L.) and alfalfa (Medicago sativa L.) . Environmental Management , 46 : 590 – 598 . doi: 10.1007/s00267-010-9454-x
- Summers , C. G. , Mitchell , J. P. , Prather , T. S. and Stapleton , J. J. 2009 . Sudex cover crops can kill and stunt subsequent tomato, lettuce and broccoli transplants through allelopathy . California Agriculture , 63 : 35 – 40 . doi: 10.3733/ca.v063n01p35
- Teasdale , J. R. 1996 . Contribution of cover crops to weed management in sustainable agricultural systems . Journal of Production Agriculture , 9 : 475 – 479 .
- Teasdale , J. R. and Daughtry , C. S. T. 1993 . Weed suppression by live and desiccated hairy vetch . Weed Science , 41 : 207 – 212 .
- Thomas , F. C. and Peter , A. 1998 . Conservation issues: Lack of public awareness of biological invasions by plants . Natural Areas Journal , 18 : 262 – 266 .
- Weston , L. A. 1996 . Utilization of allelopathy for weed management in agroecosystems . Agronomy Journal , 88 : 860 – 866 . doi: 10.2134/agronj1996.00021962003600060004x
- Weston , L. A. 2005 . History and current trends in the use of allelopathy for weed management . HortTechnology , 13 : 529 – 534 .
- Widmer , T. L. and Abawi , G. S. 2000 . Mechanism of suppression of Meloidogyne hapla and its damage by a green manure of Sudan grass . Plant Disease , 84 : 562 – 568 . doi: 10.1094/PDIS.2000.84.5.562
- Wu , J. R. and Peng , S. L. 2005 . Allelopathy: ‘Novel weapons’ of exotic invasive plants . Acta Ecological Sinica , 25 : 3093 – 3097 .
- Zhao , D. L. , Atlin , G. N. , Bastiaans , L. and Spiertz , J. H. J. 2006 . Cultivar weed-competitiveness in aerobic rice: Heritability, correlated traits, and the potential for indirect selection in weed-free environments . Crop Science , 46 : 372 – 380 . doi: 10.2135/cropsci2005.0192