Abstract
In the last years, ‘Cuore di Bue’ tomato has been one of the most important types requested from the market because of its special flesh texture and flavour, which is very pleasing to the consumer. The present study aims to verify if rootstock affects some specific qualitative traits of this tomato fruits during ripening process. The main quality traits during ripening of tomato fruits of cultivar ‘Profitto’ ungrafted and grafted on ‘Beaufort’ and ‘Big Power’ rootstocks were determined. Results showed that different ripening stages and grafting combinations affected, sometimes strongly, tomato fruits' quality. Among phenolics, gallic, cinnamic and p-coumaric acids declined during ripening, whereas chlorogenic, caffeic and ferulic acids increased in the last ripening stages. The quality of tomato fruits was also influenced by grafting. As far as quality parameters (colour, °Brix, electrical conductivity, pH, titratable acidity) and sugar content are concerned, there were significant differences. Strong differences are attributable also to the contents of phenolic acids. In particular, ungrafted Profitto plants showed more p-coumaric and caffeic acid content than the other two grafted treatments. Profitto and Profitto×Beaufort showed more chlorogenic and cinnamic acid than Profitto×Big Power variety. The last one variety showed a completely different chlorogenic acid accumulation pattern and contained more gallic and ferulic acid than the other two. The nutritional value of grape tomatoes ‘Cuore di Bue’ considerably increased with ripening stage. Grafting has a significant effect on various biochemical and nutritional properties in tomato fruit. Many chemical compounds related to the antioxidants group have been involved in these changes showing a significant decrease in grafted fruits. Concerning other parameters such as pH, EC and Brix, the graft leads to very small changes.
Introduction
In the last years, ‘Cuore di Bue’ tomato, a salad type, gained more and more importance in Italy for its taste and flesh firmness. Its cultivation usually takes place in greenhouse in a very intensive way all year-round. The cultivation of the same crop through years often determines soil-born diseases that, since 2005, cannot be controlled by methyl bromide. Mainly for this reason, the number of tomato grafted plants increased a lot in the last decade, with more than 47 million in 2008 (Morra and Bilotto, Citation2009) if we consider the vegetable grafted plants on the whole. Although in the beginning, tomato grafting was adopted in order to limit only the effects of Fusarium wilt (Lee, Citation1994), the reasons for grafting have increased dramatically over the years. For example, grafts have been used to induce resistance against low (Bulder et al., Citation1990) and high (Rivero et al., Citation2003) temperatures; to enhance nutrient uptake (Ruiz et al., Citation1997); to improve yield when plants are cultivated in infected soils (Bersi, Citation2002; Kacjan-Marsic and Osvald, Citation2004); to affect the levels of endogenous phytohormones due to the characteristics of the rootstock and the rootstock–scion combination (Proebsting et al., Citation1992); to improve water use (Cohen and Naor, Citation2002); to increase flower and seed production (Lardizabal and Thompson, Citation1990) and to enhance vegetable tolerance to drought, salinity and flooding (AVRDC, Citation2000; Estan et al., Citation2005). Moreover, many researchers reported that an interaction between rootstocks and scions results in high vigour of the root system and greater water and mineral uptake, which leads to increased yield and fruit enhancement (Lee, Citation1994; Oda, Citation1995; Bersi, Citation2002; Ioannou et al., Citation2002; Kacjan-Marsic and Osvald, Citation2004).
There are some contradictory results about the fruit quality traits and how grafting affects them (Rouphael et al., Citation2010). For example, Traka-Mavrona et al. (Citation2000) reports that the solutes associated with fruit quality are translocated in the scion through the xylem, whereas Lee (Citation1994) states that quality traits such as fruit shape, skin colour, skin or rind smoothness, flesh texture and colour, soluble solids concentration, etc. are influenced by the rootstock. However, other researchers showed that grafting did not affect fruit quality (Romano and Paratore, Citation2001). From these information appear that the majority of research papers studied only how grafting affect the main qualitative traits like soluble solids, pH and electrical conductivity (EC), but the determination of others parameters like antioxidants and phenolics with regard to grafting technique is lacking especially for ‘Cuore di Bue’ tomato.
Tomato contains different classes of antioxidants such as carotenoids, ascorbic acid, phenolic compounds and tocopherols, and, due to its high consumption rates, it can provide a significant part of the total intake of these components (Beecher, Citation1998). The antioxidant content of fresh tomatoes can be affected by many pre- and postharvest factors: the influence of cultivar (Abushita et al., Citation2000), cultural practices (Audisio et al., Citation1993) and ripening stage at harvest (Buta and Spaulding, Citation1997). Phenolic acids have received considerable attention as potentially protective factors against cancer and heart diseases, in part because of their potent antioxidant properties and their ubiquity in a wide range of commonly consumed foods of plant origin (Shahidi and Naczk, Citation1995; Breinholt, Citation1999). Phenolic acids are hydroxylated derivatives of benzoic and cinnamic acids. The most common hydroxycinnamic acid derivatives are p-coumaric, caffeic and ferulic acids, which frequently occur in foods as simple esters with quinic acid or glucose. Likely, the most familiar is chlorogenic acid.
The present study aims to verify how grafting affect some specific biochemical and nutritional qualitative traits in tomato fruits. The influence of ripening process was considered too, since ‘Cuore di Bue’ tomato, a salad tomato type with increasing interest in Italy, is usually eaten at different ripening stages depending on consumer preferences.
Materials and methods
Experimental treatment and plant samples
The experiment was conducted during spring-summer in high tunnel (7 m wide × 30 m long) in north-east of Italy using tomato ‘Cuore di bue’ cultivar ‘Profitto’, ungrafted (P) and grafted on rootstock ‘Beaufort’ (B) or ‘Big Power’ (BP) using a randomized blocks with three replications experimental design. Further information on crop management is reported in and . In the middle of harvest season (30 July), all fruits of each replication (20 plants) coming from the third truss were harvested when the first fruit reached the full ripening stage. All fruits of each grafting combination were sorted in six groups following the sample of California Tomato Ripening Scale corresponding to the following ripening stages: green (G, fully green skin), green-yellow (G-Y, 30% yellow skin), green-orange (G-O, 50% orange skin), orange-red (O-R, >90% orange or red skin), light red (L-R, fully orange or red skin) and red (R, fully red skin).
Table I. Crop management practices during the experiment.
Table II. Nutrient solution composition.
Carpometric characteristics
All tomato fruits (whole fruit) were first evaluated individually for physical traits like colour and fresh weight. Then fruits were gently washed, homogenized by means of a blender, transferred into pre-labelled individual vinyl bags and then immediately frozen at 80°C. The pH, EC, refraction index (°Brix) and titratable acidity (ISO 750 method) were measured on fruit juice obtained after filtering unfrozen sample fractions. Chromatic coordinates (L*, a*, b*) were measured on homogenized sample in order to avoid the high colour heterogeneity along the fruit typical for ‘Cuore di Bue’ tomato. A tristimulus Minolta Chroma meter (model CR-300, Minolta Corp.) was used to obtain an objective colour value. Colour was described by the ratio a*/b*, lightness (L*) and chroma .
Chemical reagents
Acetic acid (glacial) and sodium carbonate anhydrous were purchased from Riedel-de Haën (Hanover, Germany). Gallic acid monohydrate was obtained from Fluka (Sigma-Aldrich, Italy); methanol from VWR Prolabo (France) and Folin–Ciocalteu's (FC) reagent from Labochimica (Padova, Italy). Chlorogenic acid, methanol ferulic acid, D-(+)-glucose and D-(−)-fructose were purchased from Aldrich Chemical Company (Sigma-Aldrich, Italy); p-coumaric acid, formic acid and caffeic acid from Sigma (Sigma-Aldrich, Italy) and methanol from Carlo Erba (Milano, Italy). Deionized water (18 ΩA) was prepared using ultrapure water by Arium® pro purification system (Sartorius, Italy). All reagents and standards were of analytical and high-performance liquid chromatography (HPLC) grades.
Extraction of phenols for analysis
Fruit tissues (5 g) were homogenized in methanol (20 mL) with an Ultra Turrax T25 until uniform consistency at 13500 rpm. Samples were filtered (filter paper, 589 Schleicher) and appropriate aliquots of extracts were assayed by an FC assay for total phenols (TP) content. Concerning HPLC analysis, extracts were further filtered through cellulose acetate syringe filters (0.45 µm). For each sample, triplicate extractions and analyses were carried out.
Dry matter was obtained in a PID system ventilated oven (model M80-VF; Instruments s.r.l.; Bernareggio, MI) set at 65°C for 72 hours.
Determination of total phenols by the Folin–Ciocalteu assay
The content of TP was determined using the FC assay using gallic acid as calibration standard, by a Shimadzu UV-1800 spectrophotometer (Columbia, MD, USA). The FC assay was carried out by pipetting 200 µL of tomato extract into a 10 mL polypropylene tube. This was followed by addition of 1 mL of FC's reagent. The mixture was vortexed for 20–30 seconds, and 800 µL of filtered 20% sodium carbonate solution was added after 1 minute and before 8 minutes of addition of the FC reagent. This was recorded as time zero; the mixture was then vortexed for 20–30 seconds after addition of sodium carbonate. After 2 hours at room temperature, the absorbance of the coloured reaction product was measured at 765 nm. The content of TP in the extracts was calculated from a standard calibration curve, built with different concentrations of gallic acid, ranging from 0 to 600 µg mL−1 (Correlation coefficient: R 2=0.9994). Results were expressed on the basis of mg of Gallic Acid Equivalent per 100 g (mg GAE kg−1) of dried tomato powder (Singleton et al., Citation1974).
Determination of total antioxidant activity by ferric reducing antioxidant power
The assay was based on the methodology of Benzie and Strain (Citation1996). The ferric reducing antioxidant power (FRAP) reagent was prepared fresh so that it contained 1mM 2,4,6-tripyridyl-2-triazine and 2 mM ferric chloride in 0.25 M sodium acetate at pH 3.6. A 100 µL aliquot of the methanol extract prepared as above was added to 1900 µL of FRAP reagent and accurately mixed. After leaving the mixture at 20°C for 4 minutes, the absorbance at 593 nm was determined. Calibration was against a standard curve (0–1200 µg mL−1 ferrous ion) produced by the addition of freshly prepared ammonium ferrous sulfate. FRAP values were calculated as µg mL−1 ferrous ion (ferric reducing power) from three determinations and are presented as mg kg−1 of Fe2 + E (ferrous ion equivalent).
Separation and analysis of free phenolic acids by HPLC
The p-coumaric, chlorogenic, caffeic and ferulic acids were separated and quantified using a HPLC-DAD constituted of a Jasco X-LC system, consisting of a model PU-2080 pump, a multiwavelength detector (mod. MD-2015), an autosampler (mod. AS-2055) and a column oven (mod. CO-2060). ChromNAV Chromatography Data System software was used for result analyses. The separation of phenolic acids was achieved on a Tracer Extrasil OSD2 column (5µm, 250×4.6 mm), operating at 35°C, at a flow rate of 1 mL/min. The mobile phase consisted of two solvents: 0.1% acid formic (A) and methanol (B). Gradient elution was as follows: 0–100% B over 50 minutes and held at 100% B for an additional 10 minutes to clean up the column. Two wavelengths (310 and 325 nm) were used to detect eluent composition. HPLC analysis at 325 nm was used for quantification of chlorogenic, caffeic and ferulic acids. Quantification of p-coumaric acid was performed at 310 nm. Phenolic acids were quantified following a calibration method. Four standards ranging from 0.3 to 30 mg/L of chlorogenic acid hemihydrate, p-coumaric acid, caffeic acid and ferulic acid were used.
Quantitative determination of sugars by HPLC
The liquid chromatography apparatus utilized in these analysis was a Jasco X.LC system consisting of a model PU-2080 pump, a model RI-2031 refractive index detector, a model AS-2055 autosampler and a model CO-2060 column. ChromNAV Chromatography Data System was used as software. The separation of sugars was achieved on a HyperRez XP Carbohydrate Pb+ + analytical column (7.7mm×300mm, ThermoScientific), operating at 80°C. Isocratic elution was effected using water at a flow rate of 0.6 mL min−1. D-(+)-glucose and D-(−)-fructose were quantified following a calibration method. All standards utilized in the experiments were accurately weighed, dissolved in water and the calibration curves were generated with concentrations ranging from 100 mg L−1 to 1000 mg L−1 of standards.
Nutritional qualitative index (I quan )
In this work, a tomato nutritional quality index based on the antioxidant content was also calculated according to Frusciante et al. (Citation2007) method in order to identify specific properties linked to antioxidant using a comprehensive tool that could be used to evaluate the nutritional quality of tomato grafting combination.
Statistical analysis
Statistical analysis was performed according to a factorial design [3 genotypes (P, B and BP) and 6 ripening stages to obtain 18 treatments]. Data were analysed by means of ANOVA. In the case of a significant F-value, the means were compared with Tukey's Honestly Significant Difference test at the significance level of P≤0.01.
Results
Ripening stages
In our study, we have considered the a*/b* ratio determined on the blended sample for each ripening stage instead of a visual classification in order to overcome the typical differences of pigmentation in this kind of tomato fruits. Tomato fruits picked at six different stages showed a*/b* values significantly increasing from −0.63 to 1.27, whereas both colour lightness (L*) and chroma (C*) values varied only slightly (). Lightness decreased during ripening, showing a reverse relationship with respect to a*/b* values. Soluble solids (°Brix) slightly increased (only 4.3%) during ripening, whereas dry matter content did not vary significantly (5.51–5.84%, respectively) at G and R stage (). About salts concentration (EC) and pH, higher values were observed in green and red tomatoes following a mirror-like curve with a minimum value at intermediate ripening stages. Titratable acidity was maximal in O-R stage at 0.35% citric acid eq. and was minimal in G-O fruit.
Table III. Effect of ripening stages (green, green-yellow, green-orange, orange-red, light red, red) on chromaticity values.
Table IV. Effect of ripening stages (green, green-yellow, green-orange, orange-red, light red, red) and grafting combinations (Profitto, Profitto×Beaufort, Profitto×Big Power) on dry matter, soluble solids, EC, pH and titratable acidity.
Reducing sugars () concomitantly increased during fruit ripening by 20.4 and 11.8%, respectively for fructose and glucose compared to immature green fruit.
Table V. Effect of ripening (green, green-yellow, green-orange, orange-red, light red, red) stages and grafting combination (Profitto, Profitto×Beaufort, Profitto×Big Power) on sugars, carotenoids content and nutritional qualitative index (I quan ).
During fruit ripening, lycopene and β-carotene increased because chloroplasts are transformed into chromoplasts. Immature green fruit contained a very low quantity of lycopene (16 µg 100 g−1 fw); it did, however, contain 657 µg of β-carotene 100 g−1 fw, which represents around 80% of its final concentration. Red fruit contained the greatest amount of both lycopene (847 µg 100 g−1 fw) and β-carotene (789 µg 100 g−1 fw).
Concerning antioxidant properties during ripening (A), tomato fruits showed increasing values for both antioxidant activity (AOA) and TP content till G-O and O-R stages, respectively. Then values decreased 42.4 and 34.5% respectively for AOA and TP reaching 17423 mg Fe2 + E kg−1 dw and 2000 mg GAE kg−1 dw.
Figure 1. Changes in tomato fruit biochemical composition during ripening stages (G, G-Y, G-O, O-R, L-R, R) expressed in relation to a*/b* ratio: (A) antioxidant activity (AOA) and total phenols (TP); (B) gallic acid and chlorogenic acid; (C) cinnamic acid and p-coumaric acid; (D) caffeic acid and ferulic acid. Data are means (± pooled standard deviation, SD) of 12 batches of 5 fruits.
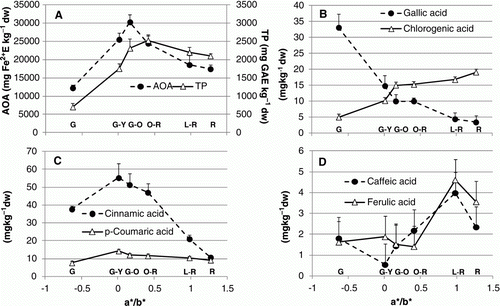
With regard to phenolic acids, ripening process played an interesting role in determining significant changes. In particular, gallic acid (B) strongly decreased ( 89.7%) from G to R, whereas chlorogenic acid slightly increased showing a quite linear trend till R stage. The pattern of cinnamic acid (C) was quite different from the others already described; in fact, its concentration increased intensely from G to G-Y (+31.8%), then decreased during ripening and was minimal in red fruit (stage R). A similar pattern, but with lower values, was observed for p-coumaric acid. Caffeic acid and ferulic (D) acid showed a significant increase of values from O-R to L-R ripening stages to decrease again in red ripe tomatoes; during the first stages, instead, their concentration was lower than 2.5 mg kg−1 dw.
The definition of I QUAN (), a tomato nutritional index proposed by Frusciante et al. (Citation2007), showed that ripening process affected tomato fruits from a nutritional point of view. In fact, the significant higher values were found in the last ripening stages and the maximum was found in R (I QUAN 28.45), whereas the minimum value was recorded in G-O stage (−20%).
Grafting effect
From the data presented in , dry matter, soluble solids and EC content higher values were observed in P with 5.3, 3.2 and 5.6% more than P×BP. pH, instead increased in grafted plants with P×B and P×BP showing values higher than 4.35. About reducing sugar concentration in tomato fruits (), only fructose was significantly affected by grafting and the higher content (1.20 g 100 g−1 fw) was expressed by P. Considering now carotenoids, the lower amount of lycopene and β-carotene was registered respectively in P×B (285 µg 100 g−1 fw) and P×BP (669 µg 100 g−1 fw); the last one grafting combination differed by 6.7% from P×B.
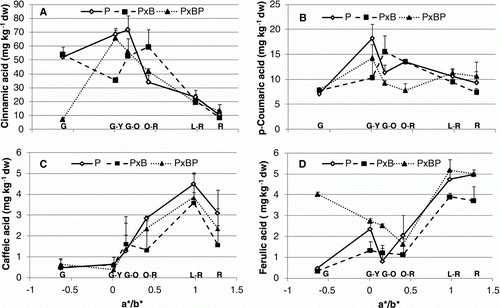
Antioxidant properties were affected sometimes strongly by grafting combination. About the antioxidant capacity, grafting combinations showed the same trend from G to O-R, then P×B value stayed higher (15% on average) till fully red ripe stage (A). Also, for TP, main differences among treatments were observed in the final stages where P showed a significant increase (37.4%) followed by P×BP (14.9%), whereas P×B decreased till R stage reaching 1998 mg GAE kg−1 fw (B). Concerning phenolic acids, it is quite interesting to observe that grafting combinations showed significant differences for some acids. In particular, if we consider chlorogenic acid (A), P and P×B expressed a completely different trend if compared with P×BP. For the last one, in fact, chlorogenic acid content slightly decreased from G to G-O, then increased in an exponential way (>95% from G-O to R stage). A significant increase in the first stages for P and P×B till O-R was registered, then chlorogenic acid concentration dropped away. Gallic acid content (B) decreased for all grafting combination during ripening, but P seemed to fall out quicker than P×B and P×BP till G-O stage reaching 3.21 mg kg−1 dw. Among phenolics, the main compound was cinnamic acid (A) for all grafting combinations, but trends were slightly different especially in the first stages of ripening process. P×BP cinnamic acid content was very low (7.05 mg kg−1 dw) in green fruits, but increased almost by 90% from G to G-Y; P and P×B showed the same cinnamic acid concentration in green fruits, but, differently from P; its concentration fell out in the second ripening stage and was maximum in O-R (59.2 mg kg−1 dw). p-Coumaric acid (B) content at G stage was the same (7.89 mg kg−1 dw) for all treatments; then P and P×BP showed a significant increase of 56.5 and 44.5% in G-Y to decrease later, whereas P×B was maximum in G-O. At the end of ripening process (R stage), except for P×BP, both P and P×B p-coumaric acid content was lower than 10 mg kg−1 dw. About caffeic acid (C), all grafting combinations expressed a similar trend during ripening process with the highest amount in L-R stage around 4 mg kg−1 dw. For ferulic acid (D), instead, P×BP moved differently from G to O-R decreasing almost of 60%, then strongly rose up like others treatments reaching a stable concentration in red ripe fruits.
Figure 2. Effect of grafting combination on changes in tomato fruit biochemical composition during ripening stages (G, G-Y, G-O, O-R, L-R, R) expressed in relation to a*/b* ratio: (A) antioxidant activity; (B) total phenols. Data are means (± standard deviation, SD) of four batches of five fruits.
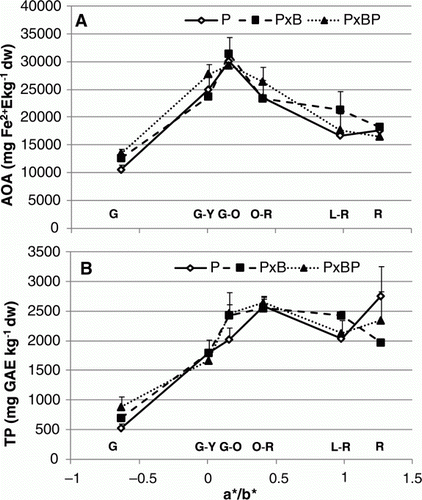
Figure 3. Effect of grafting combination on changes in tomato fruit biochemical composition during ripening stages (G, G-Y, G-O, O-R, L-R, R) expressed in relation to a*/b* ratio: (A), chlorogenic acid; (B) gallic acid. Data are means (± standard deviation, SD) of four batches of five fruits.
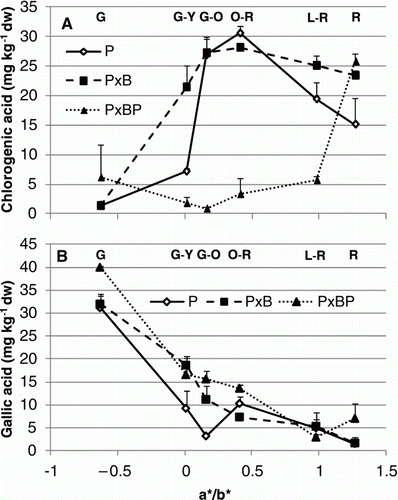
Figure 4. Effect of grafting combination on changes in tomato fruit biochemical composition during ripening stages (G, G-Y, G-O, O-R, L-R, R) expressed in relation to a*/b* ratio: (A) cinnamic acid; (B) p-coumaric acid; (C) caffeic acid; (D) ferulic acid. Data are means (± standard deviation, SD) of four batches of five fruits.
Grafting combinations affected also I QUAN index (), in fact P and P×B showed the highest value, whereas P×BP significantly differed showing I QUAN 5% lower.
Discussion
Tomato fruit content in ‘primary metabolites’ was subject to considerable changes during ripening and considering grafting combinations. Dry matter content was quite stable during ripening and similar to other tomato salad varieties (Leonardi et al., Citation2000). Significant changes in grafting combinations were observed, especially in P×BP where dry matter content was lower. This result could be due to the different rate of absorption of water and nutrient from the soil by roots of the rootstock Big Power as observed for other rootstocks (Khah et al., Citation2006). About sugar content, the pattern of accumulation was quite similar to cherry tomato (Raffo et al., Citation2002) and reducing sugars (glucose and fructose) increased due to starch degradation (Grierson and Kader, Citation1986). Secondary metabolites also showed strong variations. Lycopene and β-carotene reached maximum levels in the deep red tomatoes; the first one, that is the most important carotenoid in tomato, increased during ripening approximately 50-fold enhancing the fruit nutritional profile. Lycopene and β-carotene values were lower than other fresh consumption varieties as reported by Gautier et al. (Citation2008); in a general review on the carotenoid content of vegetables, Mangels et al. (Citation1993) reported the range from 880 to 4200 µg/100 g fw. The carotenoid concentration was affected also by grafting, and the combination P×B and P×BP showed the lower value respectively for lycopene and β-carotene.
Concerning phenolic acids, the results showed that their total content (TP) was different among ripening stages studied and grafting combinations. Different patterns of change in phenolic content were observed between grafted plants and P especially in the last ripening stages. If we consider the most important phenolics compounds, chlorogenic acid showed a different pattern of accumulation if compared with the one reported by Raffo et al. (Citation2002) and Buta and Spaulding (Citation1997) for tomato. In this case, a gradual decline of concentration during ripening was not detected, but a significant increase in P and P×B from G to O-R ripening to decrease till R stage was observed. In P×BP instead, always increased till R stage, and this contradictory result could be attributed to a differential synthesis of phenolics due to Big Power grafting. This behaviour is quite interesting since chlorogenic acid has a potential protective effect on human health, being characterized by a medium AOA (Rice-Evans et al., Citation1996). It can also contribute significantly to the AOA of the tomato because of its relatively high concentration. Free gallic acid, instead showed a noticeable decline during ripening in all grafting combinations. Cinnamic, p-coumaric, caffeic and ferulic acid concentrations, as reported in literature (Rice-Evans et al., Citation1997) is connected by a biosynthetic pathway (cinnamic acid > p-coumaric acid > caffeic acid > ferulic acid). In fact, both cinnamic acid and p-coumaric acid decreased during ripening in a noticeable way and with no rapid decline, respectively. Caffeic acid and especially ferulic acid, instead, increased in the last stages of ripening process; this could be due also to chlorogenic acid that could be further catabolized to produce other phenolics such as caffeic acid derivatives that accumulate during ripening (Gautier et al., Citation2008).
With regard to the I quan , even if we did not find an optimal value in literature, results highlighted that this index can be affected mainly by ripening process. In fact, this index significantly increased in the last ripening stages, and this result showed that ‘Cuore di Bue’ tomato expressed the best quality from the antioxidant content point of view from O-R to R stages. About grafting combinations, instead, only P×BP showed a slightly lower value, and this result was probably due to the different accumulation patter of chlorogenic acid that was very low in the first stages of ripening process. Differences are quite small, and this could be useful for producers because they could use these grafting solutions without losing important qualitative traits in the production.
References
- Abushita , A. A. , Daood , H. G. and Biacs , P. A. 2000 . Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors . Journal of Agriculture and Food Chemistry , 48 : 2075 – 2081 . doi: 10.1021/jf990715p
- Audisio , M. , Dante , D. , De Cicco , A. and Suraci , C. 1993 . Il contenuto di vitamina C nei pomodori in relazione ai metodi di coltivazione . Rivista di Scienza dell'Alimentazione , 22 : 513 – 518 . (In Italian)
- AVRDC 2000 . Grafting takes root in Taiwan. Center Point, the quarterly newsletter of Asian Vegetable Research and Development Centre , September 1–3
- Beecher , G. R. 1998 . Nutrient content of tomatoes and tomato products . Proceedings of the Society for Experimental Biology and Medicine , 218 : 98 – 100 .
- Benzie , I. F. F. and Strain , J. J. 1996 . The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP essay . Analytical Biochemistry , 239 : 70 – 76 . doi: 10.1006/abio.1996.0292
- Bersi , M. 2002 . Tomato Grafting as an Alternative to Methyl Bromide in Marocco , Marocco : Institut Agronomieque et Veterinaire Hasan II .
- Breinholt , V. 1999 . “ Desirable versus harmful levels of intake of flavonoids and phenolic acids ” . In Natural Antioxidants and Anticarcinogens in Nutrition, Health and Disease , Edited by: Kumpulainen , J. and Salonen , J. Cambridge : The Royal Society of Chemistry .
- Bulder , H. A. M. , van Hasselt , P. R. , Kuiper , P. J. C. , Speek , E. J. and Den Nijs , A. P. M. 1990 . The effect of low temperature in growth and lipid composition of low temperature tolerant rootstock genotypes for cucumber . Journal of Plant Physiology , 138 : 661 – 666 . doi: 10.1016/S0176-1617(11)81312-X
- Buta , J. G. and Spaulding , D. W. 1997 . Endogenous levels of phenolics in tomato fruits during growth and maturation . Journal of Plant Growth Regulation , 16 : 43 – 46 . doi: 10.1007/PL00006973
- Cohen , S. and Naor , A. 2002 . The effect of three rootstocks on water use, canopy conductance and hydraulic parameters of apple trees and predicting canopy from hydraulic conductance . Plant Cell and Environment , 25 : 17 – 28 . doi: 10.1046/j.1365-3040.2002.00795.x
- Estan , M. T. , Martinez-Rodriguez , M. M. , Perez-Alfoce , F. , Flowers , T. J. and Bolarin , M. C. 2005 . Grafting raises the salt tolerance of tomato through limiting the transport of sodium and chloride to the shoot . Journal of Experimental Botany , 56 : 703 – 712 . doi: 10.1093/jxb/eri027
- Frusciante , L. , Carli , P. , Ercolano , M. R. , Pernice , R. , Di Matteo , A. , Fogliano , V. and Pellegrini , N. 2007 . Antioxidant nutritional quality of tomato . Molecular Nutrition & Food Research , 51 : 609 – 617 . doi: 10.1002/mnfr.200600158
- Gautier , H. , Diakou-Verdin , V. , Bénard , C. , Reich , M. , Buret , M. , Bourgaud , F. , Poëssel , J. L. , Caris-Veyrat , C. and Génard , M. 2008 . How does tomato quality (sugar, acid and nutritional quality) vary with ripening stage, temperature and irradiance? . Journal of Agriculture and Food Chemistry , 56 : 1241 – 1250 . doi: 10.1021/jf072196t
- Grierson , D. and Kader , A. A. 1986 . “ Fruit ripening and quality: Physiology and biochemistry of ripening ” . In The Tomato Crop: A Scientific Basis for Improvement , Edited by: Atherton , J. G. and Rudich , J. 241 – 280 . London : Chapman and Hall .
- Ioannou , N. , Ioannou , M. and Hadijparaskevas , K. 2002 . Evaluation of watermelon rootstocks for off-season production in heated greenhouses . Acta Horticulturae , 579 : 501 – 506 .
- Kacjan-Marsic , N. and Osvald , J. 2004 . The influence of grafting on yield of two tomato cultivars (Lycopersicon esculentum Mill.) grown in a plastic house . Acta Agriculturae Slovenica , 83 : 243 – 249 .
- Khah , E. M. , Kakava , E. , Mavromatis , A. , Chachalis , D. and Goulas , C. 2006 . Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field . Journal of Applied Horticulture , 8 : 3 – 7 .
- Lardizabal , R. D. and Thompson , P. G. 1990 . Growth regulators combined with grafting increase flower number and seed production in sweet potato . HortScience , 25 : 79 – 81 .
- Lee , J. M. 1994 . Cultivation of grafted vegetables I: Current status, grafting methods and benefits . HortScience , 29 : 235 – 239 .
- Leonardi , C. , Ambrosino , P. , Esposito , F. and Fogliano , V. 2000 . Antioxidative activity and carotenoid and tomatine contents in different typologies of fresh consumption tomatoes . Journal of Agriculture and Food Chemistry , 48 : 4723 – 4727 . doi: 10.1021/jf000225t
- Mangels , A. R. , Holden , J. M. , Beecher , G. R. , Forman , M. R. and Lanza , E. 1993 . Carotenoid content of fruits and vegetables: An evaluation of analytical data . Journal of the American Dietetic Association , 93 : 284 – 296 . doi: 10.1016/0002-8223(93)91553-3
- Morra , L. and Bilotto , M. 2009 . Mercato in fortissima ascesa per i portinnesti orticoli. L . Informatore Agrario , 1 : 51 – 54 . (In Italian)
- Oda , M. 1995 . New grafting method for fruit-bearing vegetables in Japan . Japan Agricultural Research Quarterly , 29 : 187 – 194 .
- Proebsting , W. M. P. , Hedden , M. J. , Lewis , S. J. and Coker-Proebsting , L. N. 1992 . Gibberellin concentration and transport in genetic lines of pea . Plant Physiology , 100 : 1354 – 1360 . doi: 10.1104/pp.100.3.1354
- Raffo , A. , Leonardi , C. , Fogliano , V. , Ambrosino , P. , Salucci , M. , Gennaro , L. , Bugianesi , R. , Giuffrida , F. and Quaglia , G. 2002 . Nutritional value of cherry tomatoes (Lycopersicon esculentum cv Naomi F1) harvested at different ripening stages . Journal of Agriculture and Food Chemistry , 50 : 6550 – 6556 . doi: 10.1021/jf020315t
- Rice-Evans , C. A. , Miller , N. J. and Paganga , G. 1996 . Structure-antioxidant activity relationships of flavonoids and phenolic acids . Free Radical Biology & Medicine , 20 : 933 – 956 . doi: 10.1016/0891-5849(95)02227-9
- Rice-Evans , C. A. , Miller , N. J. and Paganga , G. 1997 . Antioxidant properties of phenolic compounds . Trends in Plant Science , 2 : 152 – 159 . doi: 10.1016/S1360-1385(97)01018-2
- Rivero , R. M. , Ruiz , J. M. and Romero , L. 2003 . Role of grafting in horticultural plants under stress conditions . Food, Agriculture and Environment , 1 : 70 – 74 .
- Romano , D. and Paratore , A. 2001 . Effects of grafting on tomato and eggplant . Acta Horticulturae , 559 : 149 – 153 .
- Rouphael , Y. , Schwarz , D. , Krumbein , A. and Colla , G. 2010 . Impact of grafting on product quality of fruit vegetables . Scientia Horticolturae , 127 : 172 – 179 . doi: 10.1016/j.scienta.2010.09.001
- Ruiz , J. M. , Belakbir , L. , Ragala , J. M. and Romero , L. 1997 . Response of plant yield and leaf pigments to saline conditions: Effectiveness of different rootstocks in melon plants (Cucumis melo L.) . Soil Science Plant Nutrition , 43 : 855 – 862 . doi: 10.1080/00380768.1997.10414652
- Shahidi , F. and Naczk , M. 1995 . Food Phenolics , Lancaster , PA/Basel : Technomic Publishing Co, Inc. .
- Singleton , V. L. , Orthofer , R. and Lamuela-Raventos , R. M. 1974 . Analysis and total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent . Methods of Enzymology , 229 : 152 – 178 .
- Traka-Mavrona , E. , Koutsika-Sotiriou , M. and Pritsa , T. 2000 . Response of squash (Cucurbita spp.) as rootstock for melon (Cucumis melo L.) . Scientia Horticulturae , 83 : 353 – 362 . doi: 10.1016/S0304-4238(99)00088-6