Abstract
Fusarium spp. in soil are potential threats to crops, so the purpose of this study was to explore the response of their population levels and species composition to long-term fertilizer applications. Two experiments were established in 1993 (consecutive 13 years) and 1997 (consecutive 9 years), where organic fertilizer (OF) and chemical fertilizer (CF) treatments were conducted in the winter wheat–summer maize rotation system in the North China Plain. Unfertilized soil served as controls. Eight Fusarium species were isolated and identified based on their morphological characteristics and nucleic acid analysis. Four dominant species including F. proliferatum, F. solani, F. equiseti, and F. semitectum accounted for 95% of isolates. No differences in total Fusarium population levels and diversity indices with organic and CF were found. Both fertilizer applications increased population levels of F. equiseti in soil, due to its better competition for nutrients. High nutrients also impacted F. solani population. These two species should be monitored when applying OF and CF. The decrease of F. proliferatum population across the growing seasons was attributed to decomposition of maize debris from preceding crop in soil.
Introduction
The majority of Fusarium species are among the most important phytopathogenic fungi, and they produce mycotoxins and other secondary metabolites that are harmful to human and animal health during or after their infection of wheat and maize (Naef & Defago, Citation2006). Severe infection by Fusarium may result in significant losses in yield and grain quality due to mycotoxin contamination (Logrieco et al., Citation2003). F. graminearum and F. culmorum could cause severe stalk rot or crown rot in wheat and maize. F. verticillioides and F. proliferatum are also important in maize (Munkvold, Citation2003; Leslie & Summerell, Citation2006). These species were commonly found temperate regions. F. graminearum and F. culmorum can produce chlamydospores and could survive for a long time in soil. Although F. verticillioides and F. proliferatum cannot produce this kind of spores, their inoculum could survive in the stalk or root debris from host crop. Therefore, both of them are also potential pathogens in soil for next host plants, especially for maize.
Soil is a complex ecosystem composed of multiple minute habitats and harbours almost all Fusarium species. Fertilization can alter soil physico-chemical properties and the composition of the microbial community (Berch et al., Citation2006; Hu et al., Citation2008; Montalba et al., Citation2010; Zhao et al., Citation2011), which in turn may influence Fusarium populations in the soil ecosystem. Yergeau et al. (Citation2006) demonstrated that the application of the recommended phosphorous level (105 kg P2O5 ha−1) in soil increased Fusarium populations and disease incident compared with other phosphorous application rates in field-grown asparagus.
In conventional agriculture, chemical fertilizer (CF) application brought about a series of environmental problems, such as water eutrophication, soil hardening and erosion. In subsistence agriculture, the organic fertilizer (OF) is more preferable (Mäder et al., Citation2002; Bengtsson et al., Citation2005). However, whether OF application means much higher biodiversity and healthier soil environment needs to be further evaluated. Some research has focused on the reduction of Fusarium diseases by using OF in different crop systems (Cotxarrera et al., Citation2002; Postma et al., Citation2003; Montalba et al., Citation2010). Both Zhao et al. (Citation2011) and Qiu et al. (Citation2012) reported that bio-OFs, a combination of OF with antagonistic microbes decreased Fusarium wilt of melon and cucumber, respectively. From OF, certain antagonistic microbial stains were isolated, such as bacterium H9 (Kannangara et al., Citation2004) and Paenibacillus polymyxa SQR-21 (Ling et al., Citation2012), which strengthened the soil suppressive to Fusarium wilt. Until now, these studies on the influences of OFs mainly focused on either one pathological Fusarium species or one strain, but not on the whole Fusarium community in soil. Thus, some pathogenic species affected by fertilizer may be neglected, but they would have a potential to bring about severe plant disease.
Knowledge of long-term fertilization effect on Fusarium populations in soil could help to develop management strategies that will contribute to control of Fusarium diseases before they will make economically damaging. Therefore, the objectives of the study were to identify and quantify the Fusarium populations under different fertilizer treatments in the winter wheat–summer maize rotation system in the North China Plain.
Materials and methods
Study site and experimental design
Experiments were carried out at the Quzhou agricultural experiment station of China Agricultural University in the North China Plain (36°52′N and 115°01′E), located in a continental, temperate monsoon zone, with an average annual temperature of 13.2°C and a mean annual precipitation of 542.7 mm. This region possesses a warm, subhumid climate with a severely dry spring, wet summer and dry–cold winter. The soil type is classified as improved silt fluvo-aquic. A long-term annual rotation of winter wheat (Triticum aestivum L.) and summer maize (Zea mays L.) was implemented by conventional ploughing and soil tillage. The wheat was planted in October and harvested in June, followed by maize which was planted in June and harvested in October. The harvested crop and ground litter were removed from these fields.
Three treatments were applied since 1993 and 1997, respectively: OF, CF, and unfertilized (U) plots that served as control. Treatments were applied in a same manner for consecutive 9 years and consecutive 13 years prior to this study, respectively. The OF containing 100.5 kg N ha 1, consisting of 60% w/w straw (wheat straw in June, maize straw in October), 30% chicken dung, 5% cottonseed-pressed trash, and 5% bran, were applied at 15 t ha−1 to the OF plots and incorporated into the soil by cultivation. The CF was composed of ammonium bicarbonate (750 kg ha−1), urea (300 kg ha−1) and calcium super-phosphate (750 kg ha−1). Each CF plot received a total of 265.5 kg N ha 1 and 39.3 kg P ha−1. Each fertilizer treatment was applied twice annually: in June and October before sowing the winter wheat or the summer maize, respectively. Each experiment (9-year or 13-year) was arranged in a randomized complete block design with nine plots (10.5 m×3 m each). The rates and times of fertilization are typical for this region. The unfertilized wheat and maize plots were cultivated and harvested similarly to the OF and CF plots.
Sampling and identification of Fusarium species
Three replicate samples were collected from each treatment as 3-cm diameter cores, from the 0- to 10-cm soil layers. Each replicate was a composite of five pooled soil cores, which were collected randomly in a zigzag pattern in November 2006 (at the wheat seedling stage) and May 2007 (at the wheat grain filling stage). Soil samples without stem and ground litter debris were thoroughly mixed, air-dried at room temperature and stored at 4°C until the Fusarium strains were isolated. No Fusarium disease was observed in the wheat or maize.
The populations of the Fusarium species were assayed by dilution plating. Each soil sample (1 g) was serially diluted with sterile distilled water, and 1-ml suspension was dispensed uniformly on the Czapek-Dox-Iprodione-Dichloran (CZID)-Agar plates (Abildgren et al., Citation1987), which can inhibit bacterial growth and is specific for Fusarium spp. The plates were incubated at 23°C with a cycle of 12-h white light/12-h darkness for 7 days. The colonies were subcultured on potato dextrose agar (PDA) and single-spore cultures were obtained on water agar. Fusarium isolates were incubated on carnation leaf-piece agar (CLA) and PDA at 23°C with a cycle of 12-h white light/12-h darkness for 14 days for morphological identification (Leslie & Summerell, Citation2006).
The Fusarium population levels from 10−3 to 10−5 diluted suspensions were estimated by counting the number of colony forming units (CFUs) in 14 days. After 14 days, the colonies could be evaluated separately without overgrowing of each other and the CFU colonies considered belonged to Fusariumspp. The Fusarium population diversity under different fertilizer treatment was calculated by the Shannon-Wiener Index (H′) (Krebs, Citation1989; Spellerberg & Fedor, Citation2003).
where S is the taxonomic number of Fusarium species, and P i is the relative abundance of species i.
Nucleic acid-based identification was performed to confirm the identity of the Fusarium isolates determined by morphological characteristics. The isolates randomly selected from each morphological species were tested. The DNA extraction was performed on the Wizard® Magnetic DNA Purification System for Food (Promega, Mannheim, Germany) with standard procedures. The universal Fusarium primers (5′-AACTCCCAAACCCCTGTGAACATA-3′, 5′-TTTAACGGCGTGGCCGC-3′) based on the ITS region were used to confirm the identity of the isolates as Fusarium species (Bluhm et al., Citation2004). The species-specific primers were used to identify F. proliferatum (5′-CTTTCCGCCAAGTTTCTTC-3′, 5′-TGTCAGTAACTCGACGTTGTTG-3′) based on partial calmodulin gene (Mulè et al., Citation2004a); F. equiseti (5′-CATACCTATACGTTGCCTCG-3′, 5′-TTACCAGTAACGAGGTGTATG-3′) based on ITS region (Mishra et al., Citation2003); F. oxysporum (5′-CAGCAAAGCATCAGACCACTATAACTC-3′, 5′-CTTGTCAGTAACTGGACGTTGGTACT-3′) based on calmodulin gene (Mulè et al., Citation2004b); and F. graminearum (5′-CTCCGGATATGTTGCGTCAA-3′, 5′-GGTAGGTATCCG ACATGGCAA-3′) based on RAPD (Nicholson et al., Citation1998; ).
Soil chemistry
Samples from each plot were analyzed for the chemical parameters, including soil organic matter (SOM), total nitrogen (N), hydrolyzable N, available phosphorus (P), available potassium (K), and pH. Soil samples for chemical analysis were air-dried for 14 d at room temperature and sieved through a 2-mm sieve in order to retrieve organic debris. SOM was determined by using the potassium dichromate external heating method (Blakemore et al., Citation1972). Total N was measured by the semi-micro Kjeldahl method (Bremner, Citation1996). Hydrolyzable N was determined by the alkaline-hydrolyzable diffusion method. Available P was measured by the Olsen method (Blakemore et al., Citation1972). Available K was extracted with 1 mol L−1 NH4Ac and determined by flame photometry. Soil pH was measured in 0.01 mol L−1 CaCl2 slurry using a glass electrode.
Data analysis
The overall trends in Fusarium population levels and diversity indices were first evaluated by factorial analysis of variance (ANOVA) with treatment, experiment and sampling time as factors. The Fusarium abundances and indices in two experiments in the three treatments were then compared by one-way ANOVA using two sampling times as replicates. The differences between soil chemical parameters were also tested by one-way ANOVA. Differences between means were evaluated by the least significant difference (LSD) test. Correlations between Fusarium spp. and chemical characteristics were analyzed by determining Pearson correlation coefficients. All statistical tests were conducted at a significance level of p < 0.05 using the SPSS 11.5 software package (SPSS Inc., Chicago, USA).
Results
Soil chemistry
The soil chemical parameters are presented in . Both in the 9-year and in the 13-year experiment, OF treatments significantly increased the contents of SOM, total N, hydrolysable N, available P and available K, and decreased the pH level, compared with no fertilizer treatments (p < 0.05). CF treatments only increased the contents of available P in both experiments.
Table I. Soil chemical characteristics of a winter wheat–summer maize rotation experiment using organic fertilizer (OF) or chemical fertilizer (CF) for 9 years (C9) and 13 years (C13) after establishment. 15 t ha−1 of OF corresponding to a rate of 100.5 kg N ha−1 was applied annually. CF was applied at 265.5 kg N ha−1 and 39.3 kg P ha−1 annually.
Identification of Fusarium species and total colonies
A total of 681 Fusarium isolates were recovered from all plates and all of them were identified mainly based on morphology of macroconidia and other characteristics morphology. Eight Fusarium spp. (F. proliferatum, F. solani, F. equiseti, F. semitectum, F. polyphialidicum, F. merismoides, F. oxysporum, and F. graminearum) were identified (). Macroonidia of F. graminearum are not shown, because only one isolate was obtained and produced no macroconidia.
Figure 1. Macroconidia of Fusarium species from soil in the North China Plain as produced on carnation leaf-piece agar (CLA). A: F. proliferatum; B: F. solani; C: F. equiseti; D: F. semitectum; E: F. polyphialidicum; F: F. oxysporum; and G: F. merismoides (magnification×400; bar = 25 µm).

The identification of Fusarium spp. was further confirmed by nucleic acid-based identification (, ). The use of Fusarium primers confirmed that some isolates randomly selected from each morphological species indeed belonged to Fusarium or specific species. However, neither F. solani nor F. merismoides could be identified by the universal Fusarium primers.
Figure 2. Bands on agarose (1%) gel after electrophoresis with nucleic acid-based determination of Fusarium species. Lane assignments: 1–7, F. proliferatum; 8–10, F. solani; 11, 12, F. semitectum; 13, F. merimoides; 14, 15, F. polyphialidicum; 17, 18 F. equiseti; 16, 19–21 F. oxysporum; 22, 23, F. graminearum (from one isolate). M Lanes represents molecular weight marker.
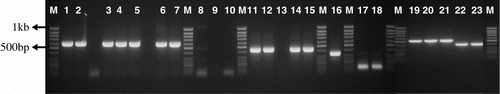
Table II. Identities of eight Fusarium species as determined by morphological and molecular characteristics.
The Fusarium colonies were quantified by counting CFUs on agar plates. No significant differences in total CFUs were observed among the three fertilizer treatments (). However, total CFUs in November 2006 were higher than those in May 2007 (p < 0.001). In addition, total CFUs in the 13-year experiment were higher than in the 9-year experiment (p = 0.005).
Table III. Probability levels of analysis of variance (ANOVA, p values) for population levels and diversity of Fusarium species with treatments (T), sampling times (S), and experiments (E) as factors.
Fusarium spp. populations and diversity
The relative abundance of the Fusarium spp. isolates recovered was quantified. Species including F. proliferatum (48%), F. solani (21%), F. semitectum (15%), and F. equiseti (12%) were dominant, accounting for 95% of all isolates. These dominant species prevailed in all fertilizer plots except F. equiseti under unfertilized plots. Other species occurred sporadically, such as F. polyphialidicum (1%), F. merismoides (2%), F. oxysporum (2%), and F. graminearum (0.1%).
The population levels (CFUs) of F. equiseti and F. solani were significantly affected by treatment (p < 0.05; ). However, when analyzed separately, the significant difference was only observed for F. equiseti in 13-year experiment, where both OF and CF treatments increased the CFUs (). Although this trend was also observed in 9-year experiment, it did not reach the significant level.
Table IV. Mean of Fusarium population levels (103 CFUs/g dry soil) from three fertilizer treatments in two experiments in samples collected in November 2006 and May 2007. For the explanation of C9 and C13, see .
Experiment factor also influenced the CFUs of F. proliferatum, F. solani, F. semitectum, and F. polyphialidicum (p < 0.05; ). The abundances of these four species in the 13-year experiment were higher than those in the 9-year experiment.
Significant differences in F. proliferatem, F. semitectum, F. polyphialidicum, and F. oxysporum were observed between two sampling times (p < 0.05; ). The populations of the first three species in November 2006 were higher than those in May 2007. F. oxysporum was detected only in May 2007.
The diversity of Fusarium populations was evaluated by the Shannon-Wiener Index (H′). Differences were observed under different fertilizer treatments (p = 0.069) and two sampling times (p < 0.001; ). H′ index under OF plots was higher than that under CF and unfertilized plots (p < 0.10; ). Meanwhile, H′ index in November 2006 was higher than that in May 2007.
Relationship of Fusarium populations to soil chemistry
Correlations between Fusarium species and chemical characteristics are presented in . The population levels of F. equiseti were closely positively correlated with the contents of organic matter, total N, hydrolysable N, available P, and available K, and negatively with the pH value. However, the population levels of F. solani were positively correlated only with the contents of organic matter, total N, and hydrolysable N.
Table V. Correlation coefficient for soil chemical parameters to Fusarium population levels (103 CFUs/g soil).
Discussion
Four dominant species, including F. proliferatum, F. solani, F. equiseti, and F. semitectum, were regularly isolated from the wheat field soil in the North China Plain. Differences in predominance of Fusarium species in other geographical locations have been reported in the Slovak Republic, and F. poae was the dominant species in winter wheat crops (Rohacik & Hudec, Citation2005). Crop types and climatic conditions are the main factors that influence dominant Fusarium species. Naef and Defago (Citation2006) reported that F. graminearum, F. avenaceum, and F. proliferatum were the most abundant Fusarium spp. isolated from overwintered maize stalks over two years in a German field, while Görtz et al. (Citation2008) from maize kernels isolated F. verticillioides, F. graminearum, and F. proliferatum as the dominant species in the major maize production areas in Germany.
The increase of Fusarium diversity was mainly attributed to the change to F. equseti. Both organic and CFs increased the F. equiseti population and this trend was clearer in longer period experiment, which suggests the gradual accumulation of its propagules in wheat-maize rotation system. This result was not completely consistent with Toyota and Kimura (Citation1992), which indicated that a soil amended with farmyard manure strongly inhibited the germination of Fusarium oxysporum f. sp. raphani, while the CF was weakly fungistatic. F. equiset is a cosmopolitan soil inhabitant. Although F. equseti is primarily considered as a saprophyte or secondary invader, it is not unusual to recover this species from diseased plants (Leslie & Summerell, Citation2006). F. equseti associated with the higher nutrient status reflects its ability to compete for substrate, suggesting that application of both OF and CF might enhance the risk for occurrence of F. equseti diseases. Thus, when soil environment changed with chemical characteristics, we should pay more attention to this species’ population levels and conduct further biological experiment for assessing the risk, in order to eliminate possible Fusarium disease outbreak.
F. solani also has a cosmopolitan distribution and is recorded as a pathogen on a vast and diverse range of host plants (Montalba et al., 2010). It correlated with soil nutrients, but this correlation was weaker than F. equiseti. Other factors such as block heterogeneity, soil temperature and soil humidity maybe mask the influence of fertilizer. We suggest that the population level of F. solani also should be monitored in high quality nutrient, especially with organic matter and nitrogen fertilizer applications, in order to avoid the F. solani disease occurrence in unfavorable condition.
Fertilization did not influence the population levels of F. proliferatum and F. semitectum. It is very valuable in the control of Fusarium diseases, because some strains of F. proliferatum and F. semitectum cause ear and stalk rot in maize, as well as head blight in small grains such as wheat (Logrieco et al., Citation2003). The total colonies of both species declined from November 2006 to May 2007. This might be attributed to the low temperature during winter and the reduced precipitation in spring, which destroyed most of Fusarium propagules in soil. Bateman and Murray (Citation2001) showed that F. culmorum population levels decreased in late autumn and early winter as the temperature declined in wheat fields in the UK. Edesi et al. (Citation2012) also indicated that the abundance of Fusarium spp. was higher in warm and humid climate. F. proliferatum was isolated in higher abundance in November 2006, mainly because maize, one of main hosts of F. proliferatum, was the preceding crop (Leslie & Summerell, Citation2006). As F. proliferatum cannot form chlamydospore, it survives primarily as mycelial segments in infested plant debris in soil (Cotten & Munkvold, Citation1998). Although stalk and ground litter debris were removed from field, root residues still remained in soil. F. proliferatum could survive in root residues, and it would be more easily influenced by variation in debris across the wheat growing season. Due to the decline of F. proliferatum population with debris decomposition, fewer potential pathogens remain in soil for the subsequent maize crop. Thus, the debris after harvesting maize needs to be efficiently controlled in order to minimize the host's amount of F. proliferatum.
It is remarkable that in this wheat–maize rotation the main pathogens, such as F. graminearum, F. culmorum, and F. verticillioides, were either in less or not found. The isolated species except F. proliferatum are only pathogens of secondary significance, which means that the most important pathogens in soil will not be enhanced by any form of fertilization practice. This also means indirectly that the debris is the most important carriers of the significant pathogens and not the soil itself. From the phytopathological and epidemiological view, organic and CF will not increase the risk of Fusarium disease or mycotoxins, which will give valuable information for growers or breeders.
Acknowledgements
This research was funded by EU Asia-Links project “Organic Farming: ethical, economic, technical and scientific aspects in a global perspective.” Authors thank Andreas Görtz for assisting with the identification of Fusarium species.
References
- Abildgren , M. P. , Lund , F. , Thrane , U. and Elmholt , S. 1987 . Czapek-Dox Agar containing Iprodione and Dicloran as a selective medium for the isolation of Fusarium species . Letter in Applied Microbiology , 5 : 83 – 86 . doi: 10.1111/j.1472-765X.1987.tb01620.x
- Bateman , G. L. and Murray , G. 2001 . Seasonal variations in populations of Fusarium species in wheat-field soil . Applied Soil Ecology , 18 : 117 – 128 . doi: 10.1016/S0929-1393(01)00158-5
- Bengtsson , J. , Ahnström , J. and Weibull , A. C. 2005 . The effects of organic agriculture on biodiversity and abundance: A meta-analysis . Journal of Applied Ecology , 42 : 261 – 269 . doi: 10.1111/j.1365-2664.2005.01005.x
- Blakemore , L. C. , Searle , P. L. & Daly , B. K. 1972 . Methods for chemical analysis of soils . New Zealand Soil Bureau Report 10 A . Wellington : Government Printer .
- Berch , S. M. , Brockley , R. P. and Battigelli , J. P. 2006 . Impacts of repeated fertilization on components of the soil biota under a young lodgepole pine stand in the interior of British Columbia . Canadian Journal of Forest Research , 36 : 1415 – 1426 . doi: 10.1139/x06-037
- Bluhm , B. H. , Cousin , M. A. and Woloshuk , C. P. 2004 . Multiplex real-time PCR detection of fumonisin-producing and trichothecene-producing groups of Fusarium species . Journal of Food Protection , 67 : 536 – 543 .
- Bremner , J. M. 1996 . Nitrogen-total . In D. L. Sparks Methods of Soil Analysis, Part 3 ( Madison , WI : Soil Science Society of America Book Series 5 ), pp. 1085 – 1086 .
- Cotten , T. K. and Munkvold , G. P. 1998 . Survival of Fusarium moniliforme, F. proliferatum, and F. subglutinans in maize stalk residue . Phytopathology , 88 : 550 – 555 . doi: 10.1094/PHYTO.1998.88.6.550
- Cotxarrera , L. , Trillas-Gay , M. I. , Steinberg , C. and Alabouvette , C. 2002 . Use of sewage sludge compost and Trichoderma asperellum isolates to suppress Fusarium wilt of tomato . Soil Biology & Biochemisty , 34 : 467 – 476 . doi: 10.1016/S0038-0717(01)00205-X
- Edesi , L. , Järvan , M. , Noormets , M. , Lauringson , E. , Adamson , A. and Akk , E. 2012 . The importance of solid cattle manure application on soil microorganisms in organic and conventional cultivation . Acta Agriculturae Scandinavica, Section B – Soil & Plant Science , 62 : 583 – 594 . doi: 10.1080/09064710.2012.678380
- Görtz , A. , Oerke , E. C. , Steiner , U. , Waalwijk , C. , de Vries , I. and Dehne , H. W. 2008 . Biodiversity of Fusarium species causing ear rot of maize in Germany . Cereal Research Communication , 36 : 617 – 622 . doi: 10.1556/CRC.36.2008.Suppl.B.51
- Hu , C. , Cao , Z. , Chen , Y. F. and Dawson , R. 2008 . Dynamics of soil microbial biomass carbon, mineral nitrogen and nitrogen mineralization in long-term field experiment, Northern China . Journal of Sustainable Agriculture , 2 : 287 – 302 . doi: 10.1080/10440040802171028
- Kannangara , T. , Utkhede , R. S. and Bactawar , B. 2004 . Compost effect on greenhouse cucumbers and suppression of plant pathogen Fusarium oxysporum . Compost Science & Utilization , 12 : 308 – 313 .
- Krebs , C. 1989 . Ecological Methodology , New York : HarperCollins .
- Leslie , J. F. and Summerell , B. A. 2006 . The Fusarium Laboratory Manual , Ames , IA : Blackwell Professional .
- Ling , N. , Zhang , W. , Tan , S. , Huang , Q. and Shen , Q. 2012 . Effect of the nursery application of bioorganic fertilizer on spatial distribution of Fusarium oxysporum f. sp. niveum and its antagonistic bacterium in the rhizosphere of watermelon . Applied Soil Ecology , 59 : 13 – 19 . doi: 10.1016/j.apsoil.2012.05.001
- Logrieco , A. , Bottalico , A. , Mulé , G. , Moretti , A. and Perrone , G. 2003 . Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops . European Journal of Plant Pathology , 109 : 645 – 667 . doi: 10.1023/A:1026033021542
- Mäder , P. , Flie(bach , A. , Dubois , D. , Gunst , L. , Fried , P. and Niggli , U. 2002 . Soil fertility and biodiversity in organic farming . Science , 296 : 1694 – 1697 . doi: 10.1126/science.1071148
- Mishra , P. K. , Fox , R. T. V. and Culham , A. 2003 . Development of a PCR-based assay for rapid and reliable identification of pathogenic Fusariam . FEMS Microbiology Letters , 218 : 329 – 332 . doi: 10.1111/j.1574-6968.2003.tb11537.x
- Montalba , R. , Arriagada , C. , Alvear , M. and Zúniga , G. E. 2010 . Effects of conventional and organic nitrogen fertilizers on soil microbial activity, mycorrhizal colonization, leaf antioxidant content, and Fusarium wilt in highbush blueberry (Vaccinium corymbosum L.) . Scientia Horticultura , 125 : 776 – 778 .
- Mulè , G. , Susca , A. , Stea , G. and Moretti , A. 2004a . A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans . European Journal of Plant Pathology , 110 : 495 – 502 . doi: 10.1023/B:EJPP.0000032389.84048.71
- Mulè , G. , Susca , A. , Stea , G. and Moretti , A. 2004b . Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences . FEMS Microbiology Letters , 230 : 235 – 240 . doi: 10.1016/S0378-1097(03)00926-1
- Munkvold , G. P. 2003 . Epidemiology of Fusarium diseases and their mycotoxins in maize ears . European Journal of Plant Pathology , 109 : 705 – 713 . doi: 10.1023/A:1026078324268
- Naef , A. and Defago , G. 2006 . Population structure of plant-pathogenic Fusarium species in overwintered stalk residues from Bt-transformed and non-transformed maize crops . European Journal of Plant Pathology , 116 : 129 – 143 . doi: 10.1007/s10658-006-9048-x
- Nicholson , P. , Simpson , D. R. , Weston , G. , Rezanoor , H. N. , Lees , A. K. , Parry , D. W. and Joyce , D. 1998 . Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays . Physiological and Molecular Plant Pathology , 53 : 17 – 37 . doi: 10.1006/pmpp.1998.0170
- Postma , J. , Montanari , M. and van den Boogert , P. H. J. F. 2003 . Microbial enrichment to enhance the disease suppressive activity of compost . European Journal of Soil Biology , 39 : 157 – 163 . doi: 10.1016/S1164-5563(03)00031-1
- Qiu , M. , Zhang , R. , Xue , C. , Zhang , S. , Li , S. , Zhang , N. & Shen , Q. 2012 . Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil . Biology and Fertility of Soils . Accessed February 2012, available at: http://www.springerlink.com/content/j313032uxw45l338/
- Rohacik , T. and Hudec , K. 2005 . Influence of agro-environmental factors on Fusarium infestation and population structure in wheat kernels . Annnals of Agricultural and Enviromental Medicine , 12 : 39 – 45 .
- Spellerberg , I. F. and Fedor , P. J. 2003 . A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species diversity and the “Shannon-Wiener” Index . Global Ecology Biogeography , 12 : 177 – 183 . doi: 10.1046/j.1466-822X.2003.00015.x
- Toyota , K. and Kimura , M. 1992 . Population dynamics of Fusarium oxysporum f. sp. raphani in soils of different fungistatic capacity . FEMS Microbiology Letters , 102 : 15 – 20 . doi: 10.1111/j.1574-6968.1992.tb05790.x
- Yergeau , E. , Sommerville , D. W. , Maheux , E. , Vujanovic , V. , Hamel , C. , Whalen , J. K. and St-Arnaud , M. 2006 . Relationships between Fusarium population structure, soil nutrient status and disease incidence in field-grown asparagus . FEMS Microbiology Ecology , 58 : 394 – 403 . doi: 10.1111/j.1574-6941.2006.00161.x
- Zhao , Q. , Dong , C. , Yang , X. , Mei , X. , Ran , W. , Shen , Q. and Xu , Y. 2011 . Biocontrol of Fusarium wilt disease for Cucumis melo melon using bio-organic fertilizer . Applied Soil Ecology , 47 : 67 – 75 . doi: 10.1016/j.apsoil.2010.09.010