Abstract
Quantity and quality of soil organic matter (SOM) affect physical, chemical, and biological soil properties, and are pivotal to productive and healthy grasslands. Thus, we analyzed the distribution of soil aggregates and assessed quality, quantity, and distribution of SOM in two unimproved and improved (two organic and two conventional) grasslands in subarctic Iceland, in Haplic and Histic Andosols. We also evaluated principal physicochemical and biological soil properties, which influence soil aggregation and SOM dynamics. Macroaggregates (>250 µm) in topsoils were most prominent in unimproved (62–77%) and organically (58–69%) managed sites, whereas 20–250 µm aggregates were the most prominent in conventionally managed sites (51–53%). Macroaggregate stability in topsoils, measured as mean weight diameter, was approximately twice as high in organically managed (12–20 mm) compared with the conventionally managed (5–8 mm) sites, possibly due to higher organic inputs (e.g., manure, compost, and cattle urine). In unimproved grasslands and one organic site, macroaggregates contributed between 40% and 70% of soil organic carbon (SOC) and nitrogen to bulk soil, whereas in high SOM concentration sites free particulate organic matter contributed up to 70% of the SOC and nitrogen to bulk soil. Aggregate hierarchy in Haplic Andosols was confirmed by different stabilizing mechanisms of micro- and macroaggregates, however, somewhat diminished by oxides (pyrophosphate-, oxalate-, and dithionite-extractable Fe, Al, and Mn) acting as binding agents for macroaggregates. In Histic Andosols, no aggregate hierarchy was observed. The higher macroaggregate stability in organic farming practice compared with conventional farming is of interest due to the importance of macroaggregates in protecting SOM and soils from erosion, which is a prerequisite for soil functions in grasslands that are envisaged for food production in the future.
Introduction
Physical, chemical, and biological properties are strongly influenced by quantity and quality of soil organic matter (SOM). Being the key attributes of soil quality and productivity (Manlay et al. Citation2007; Feller et al. Citation2012), SOM-induced changes in soil quality strongly affect productivity and health of grasslands. Land misuse and soil mismanagement may deplete SOM, while sustainably managed farms can maintain and enhance SOM levels and soil structure by e.g., application of organic amendments (Siegrist et al. Citation1998). Stable aggregates physically protect the encapsulated SOM against decomposition, especially in soil systems with less physical disturbances such as no-tillage or conservation agriculture (Six et al. Citation2000). Formation and stabilization of aggregates are influenced by several factors such as input of biomass-C, soil fauna (most importantly earthworms) (Siegrist et al. Citation1998), soil microorganisms such as fungi and bacteria and their activity (Eash et al. Citation1994), roots and mycorrhiza (Rilling & Mummey Citation2006; Graf & Frei Citation2013), inorganic binding agents such as carbonates and oxy(hydr)oxides, nutrient availability (Six et al. Citation2004) and environmental variables such as freeze/thaw and drying/wetting cycles (Six et al. Citation2000; Citation2004). Tisdall and Oades (Citation1982) proposed the hierarchical model of soil aggregates, and hypothesized that macroaggregates (>250 µm) are comprised of microaggregates (<250 µm), mineral particles, and particulate organic matter (POM). These components are bound together and stabilized by transient or temporary binding agents such as microbial- and plant-derived polysaccharides, roots, and fungal hyphae. Microaggregates are associations of free primary particles bound together by organic molecules, oxy(hydr)oxides, polyvalent cations, Ca and Mg carbonates, and CaSO4 (Tisdall & Oades Citation1982; Amézketa Citation1999). The hierarchical order indicates that macroaggregates are less stable and more influenced by farming practices (e.g., tillage) than microaggregates (Six et al. Citation2004).
SOM originates primarily from plant litter and microbial biomass and consists of numerous compounds with varying structure, concentration, and recalcitrance (Kögel-Knabner Citation2002). Tillage is one of the most important factors that may reduce soil organic carbon (SOC) stocks and also decrease aggregate stability in Icelandic grasslands. Tillage may disrupt macroaggregates and thus aggravate decomposition of SOM (Elliott Citation1986). Farming practices that apply organic amendments (e.g., animal manure, green manure, compost, and/or crop residues) may improve physical, chemical, and biological soil properties, and result in enhanced overall soil fertility (e.g., increased plant nutrients, SOM, and soil structure) (Watson et al. Citation2002; Sodhi et al. Citation2009; Diacono & Montemurro Citation2011). Regardless of few comparative land management studies on soil aggregate stability, previous research by Siegrist et al. (Citation1998) and Shepherd et al. (Citation2002) suggests that organic farming practices increase macroaggregate stability.
Separation of SOM fractions, based on density fractionation in combination with ultrasonic dispersion, enables separation of free particulate organic matter (fPOM, consisting of undecomposed plant residue, hyphae, and their partial decomposition products), occluded particulate organic matter (oPOM, consisting of POM occluded in aggregates), and organo-mineral associations with more processed SOM in the heavy fraction (sediment of the density fractionation procedure) (Christensen Citation1992; Golchin et al. Citation1994; Kölbl & Kögel-Knabner Citation2004). In general, POM fractions respond more sensitively to management changes than total SOC (Golchin et al. Citation1994; Chan et al. Citation2002; Steffens et al. Citation2009). Marriott and Wander (Citation2006) observed increased oPOM in organic farming systems over conventionally managed farming systems, and oPOM was more decomposed in manure + legume than in legume-based organic systems. Most of the studies on agricultural soils focus on temperate cropland soils (e.g., Gattinger et al. Citation2012), whereas grassland soils from sub-arctic and of volcanic origin have received less attention, as well as on-farm studies are lacking in the research literature (Siegrist et al. Citation1998; Stavi et al. Citation2011). Approximately, 90% of farmland in Iceland is under grassland management for hay production (Helgadóttir et al. Citation2013). Normally, the soils are ploughed when the fields are renewed from several to tens of year's interval, and the fields are fertilized annually.
Therefore, the objective of this research was to assess: (1) distribution of soil aggregates in Icelandic Andosols, (2) quality, quantity, and distribution of SOM in unimproved and improved grasslands in subarctic Iceland, and (3) principal physicochemical and biological soil properties influencing soil aggregation and SOM dynamics. We hypothesized that (1) unimproved grasslands without ploughing would have higher SOM levels and thus higher distribution of macroaggregates compared with improved grasslands, (2) organically managed grassland have a closer resemblance to unimproved grassland than to conventionally managed grassland, and that (3) organic matter (OM) and fungi are the major aggregating agents.
Materials and methods
Site description
The sites were selected to represent the major grassland soil types in Iceland (Haplic Andosol and Histic Andosol), and a range of grassland management practices (). Samples obtained from the first four sites were from Haplic Andosols [according to WRB, according to Icelandic classification Brown Andosols (Arnalds Citation2004), hereafter referred to as HaA]. Samples obtained from the two last sites were from Histic Andosols (according to WRB, hereafter referred to as HiA). Altogether, six grassland sites located in South and Southwest Iceland comprised of ():
Table 1. Background information of the studied sites.
Grass1: unimproved grassland that has not been ploughed or fertilized. The field is lightly used as a pasture for young cattle and sheep for a short time in the autumn. The site is located in between HaAorg and HaAcon.
HaAorg: improved grassland where organic fertilizers (manure, compost, and cattle urine from the farm) and biodynamic preparations such as humus and silica preparations are used (). Biodynamic preparations are, according to the farmer, used to enhance sprouting and early spring growth, to enhance ripeness of the crop and to protect from fungal diseases, as well as to enhance plant availability of phosphates (). Ploughing of HaAorg was done during the first three consecutive years of the crop rotation, most recently in 2001, 2002, and 2003. The field has not been ploughed since. Since 1996, the field has been managed according to Icelandic guidelines for organic and sustainable production and resource utilization, which are based on the guidelines from Soil Association (TÚN Citation2013). Before 1996, the field had never been ploughed or fertilized.
HaAcon: improved grassland where organic (manure) and supplemental inorganic fertilizers are used. The last ploughing was done in 1995 when the field was renewed; the cultivation of the field started in the 1960s.
Grass2: unimproved grassland that has neither been ploughed nor fertilized. The site is located in between HiAorg and HiAcon, and is not used for pasture.
HiAorg: improved grassland that receives organic fertilizers (manure and compost from the farm) and is managed according to the Icelandic guidelines for organic and sustainable production and resource utilization (TÚN Citation2013) since 1994. During the first three consecutive years of the crop rotation (1994–1996), the field was ploughed but has not been ploughed since 1996. Before 1994, the studied field had never been ploughed or fertilized.
HiAcon: improved grassland that receives organic (manure) and supplementary inorganic fertilizers. The soil is ploughed when the field is renewed, which was in 1998. Cultivation of the field started in the 1960s.
For all improved grassland sites (HaAorg, HaAcon, HiAorg, and HiAcon), the soils were ploughed with a moldboard plow to a depth of 15–20 cm. The improved grasslands were generally cut twice each year for forage production, and on a good year a third time, while Grass1 and Grass2 sites were not harvested. Thus, principal differences between the selected grasslands are the start of the cultivation of the grasslands, usage of mineral fertilizers on the conventional sites, and input of organic material as well as crop rotation at the organically managed sites. The organically managed sites have higher diversity of grasses and related fodder plants including legumes such as clover (Trifolium repens and Trifolium pratense). None of the sites received pesticides.
Soil sampling
Soil samples were obtained in June 2011. Samples at each randomly selected site were obtained in triplicate from 0 to 10 cm and 10 to 20 cm soil depth using a core sampler (8 cm diameter). The replicates were approximately 30–40 m apart. Each replicate comprised of 10–15 subsamples, which were sampled within a 1–2 m radius and were composited. Thus, there were a total of 36 homogeneous samples. Soils for biological analyses were sampled only from 0 to 10 cm depth in a similar fashion to that of other samples, in triplicates. Soil samples were gently sieved through a 5 mm sieve in the field, and kept at 4°C for the biological analyses, and air-dried in the laboratory pending other analyses. Air-drying of the samples may have caused irreversible changes to the soil structure, which is known to happen for Andosols (e.g., Kubota Citation1972; Bartoli & Burtin Citation2007), but this was done due to long transports and future comparison to other soil types from the same project.
Physicochemical and biological characterization of soils at the grassland sites
Soil pH was measured electrochemically (Microprocessor pH Meter pH196 WTW, Weilheim, Germany) in H2O at a soil:solution ratio of 1:2.5 (Soil Survey Staff Citation2004). Particle-size distribution was determined by a combination of sieve and pipette method after removal of OM with 10% hydrogen peroxide and dispersion by reciprocal shaking with sodium metaphosphate solution for 12 h (Soil Survey Staff Citation2004). However, because of the presence of active amorphous materials, complete dispersion may not have been achieved. Ammonium-oxalate-extractable Fe, Mn, Al, and Si (Feo, Mno, Alo, and Sio) were determined according to Schwertmann (Citation1964). Dithionite-citrate-bicarbonate-extractable Fe, Mn, and Al (Fed, Mnd, and Ald) were quantified according to Mehra and Jackson (Citation1960). Allophane concentration was estimated by multiplying Sio by 6, based on an average Al/Si ratio of 1.5 (Parfitt Citation1990), and ferrihydrite concentration was estimated by multiplying Feo by 1.7 (Parfitt & Childs Citation1988). Total carbon (Ct) and total nitrogen (Nt) were quantified by the dry combustion method (Tabatabai & Bremner Citation1991) using an elemental analyzer (Carlo Erba Nitrogen Analyser 1500, Milano, Italy). The Icelandic volcanic soils contain no carbonate minerals (e.g., Gislason et al. Citation2008), and therefore, Ct values obtained were assumed to be SOC. Plant available phosphorous (P) and potassium (K) were determined by the calcium-acetate-lactate (CAL)-extraction (ÖNORM, L1087). Cation exchange capacity (CEC) and exchangeable cations were determined using an unbuffered 0.1 M BaCl2 extraction (Soil Survey Staff Citation2004). Extracted exchangeable cations (K, Na, Ca, Fe, Mg, Mn, and Al) were measured by flame atomic absorption spectrophotometry (Perkin-Elmer 2100). Sampling cores of a known volume determined bulk density. Samples were dried, and sieved through a 2 mm sieve. The volume of coarse fragments (>2 mm) was determined by the water displacement method. The bulk density of the <2 mm fraction was calculated after subtracting the weight and volume of the coarse fraction from the weight and volume of the total sample.
Dissolved organic carbon (DOC) was determined by UV adsorption at 254 nm (Brandstetter et al. Citation1996) and microbial biomass C by chloroform fumigation-extraction method (Vance et al. Citation1987). Hot water (16 h at 80°C) extractable C (HWC) was determined according to Ghani et al. (Citation2003). Mineralizable nitrogen was measured as the NH4 production during 1 week of anaerobic incubation in slurry at 40°C (Canali & Benedetti Citation2006). For determination of hyphal length and bacterial numbers, microscopic slides were prepared as described by Bloem and Vos (Citation2004) after a pre-incubation period of 2 weeks at 20°C. The equation of a cylinder with spherical ends (V = (π/4) W2 (L − (W/3)), where V = volume (µm³), L = length (µm), and W= width (µm), a mean hyphal diameter of 2.5 µm and a specific C concentration of 130 fg C µm−3, were used to estimate fungal biomass. Bacterial biomass was calculated using a specific C concentration of 320 fg C µm−3 and bacterial cell numbers and volume were determined by confocal laser scanning microscopy combined with an image analysis system (Bloem et al. Citation1995). Bacterial activity was estimated by measuring incorporation rates of [³H]thymidine and [14C]leucine (Bloem & Bolhuis Citation2006).
Density and aggregate fractionation
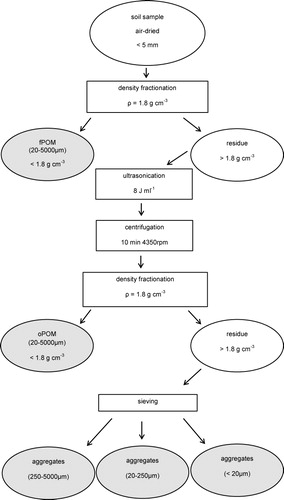
A three-step density and aggregate fractionation procedure, modified from Mueller et al. (Citation2009) and Steffens et al. (2009), was used in triplicate for all sites (). Briefly, 20 g of air-dried soil (<5 mm) was capillary-saturated with Na-polytungstate solution (density of 1.8 g cm−3) and allowed to settle overnight. The floating POM (referred to as fPOM, 20–5000 µm) was extracted by aspiration via a water jet pump. To obtain POM occluded in aggregates (referred to as oPOM, 20–5000 µm), the subsequent heavy fraction (>1.8 g cm−3) was treated by ultrasound. The application of low energy ultrasound of 8 J ml−1 was used to maintain stable macroaggregates as well as to minimize the production of artifacts (Lehtinen et al. Citation2014). Calibration of the output power of the sonicator was done calorimetrically according to North (Citation1976). With a subsequent density fractionation step (Na-polytungstate solution, 1.8 g cm−3), the oPOM floating on the suspension was obtained after centrifugation (10 minutes at 4350 rpm). All POM fractions were washed with deionized water until the electric conductivity dropped below 5 µS cm−1 (Mueller et al. Citation2009; Steffens et al. Citation2009) and then freeze-dried for further analyses. The residues of the density fractionation procedure (mineral particles and organo-mineral associations) were sieved at 250 µm and 20 µm to obtain macroaggregates (250–5000 µm) and microaggregates (20–250 µm and < 20 µm). All aggregate fractions were washed with deionized water until the electronic conductivity dropped below 5 µS cm−1, oven-dried at 100°C, weighed, and ground for further analyses. The weights of aggregates were corrected for the sand concentration of the same size (for aggregates 20–250 µm, and >250 µm), in order to exclude sand particles to be weight as aggregates (Six et al. Citation2000; Lehtinen et al. Citation2014). Mean weight diameter (MWD, mm) of the sand-corrected aggregates was calculated according to Kemper and Rosenau (Citation1986) as follows:
where is the geometric mean of aggregate size on sieve i, and wi is the fraction of aggregates on sieve i.
Solid-state 13C NMR spectroscopy
The chemical quality of selected POM fractions and bulk soils from the improved grasslands (HaAorg, HaAcon, HiAorg, and HiAcon) was analyzed by solid-state 13C NMR (Nuclear Magnetic Resonance) spectroscopy (DSX 200 NMR spectrometer, Bruker, Karsruhe, Germany). Composite bulk soil and POM samples were prepared by mixing equal amounts of the replicates. To improve the signal-to-noise ratio, bulk soil samples were treated with 10% HF (Schmidt et al. Citation1997). The cross-polarization magic angle spinning (CPMAS) technique with a 13C-resonance frequency of 50.32 MHz and a spinning speed of 6.8 kHz was applied. A ramped 1H-pulse starting at 100% to 50% of the initial power was used during a contact time of 1 ms in order to circumvent spin modulation during the Hartmann–Hahn contact. Pulse delays between 0.8 and 1 s were used for all spectra. Depending on the C concentrations of the samples, between 2000 and 320,000 scans were accumulated and a line broadening of 50 Hz was applied. The 13C chemical shifts were calibrated relative to tetramethylsilane (0 ppm). The relative contributions of the various C groups were determined by integration of the signal intensity in their following respective chemical shift regions (Knicker et al. Citation2005) assignable to alkyl-C (−10 to 45 ppm), N-alkyl-C (45–60 ppm), O-alkyl-C (60–110 ppm), olefinic and aromatic C (110–160 ppm), and carbonyl (aldehyde and ketone) and carboxyl/amide C (160–220 ppm).
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics 20 software package for Mac. Normality was tested with Shapiro–Wilkinson's test and all data were log-transformed before analyses to obtain homogeneity of variances. One-way analysis of variance followed by Tukey's significant difference (p < 0.05) as a post-hoc test (Tukey Citation1957) was used to compare means of the different soil properties between the different grassland sites. Correlations between variables were calculated with the Pearson correlation coefficient.
Results
Physicochemical and biological characterization of soils at the grassland sites
The physicochemical and biological characteristics of the bulk soils are summarized in and in Table S1, Supplemental Material. Clay concentration was higher and soil pH lower in HiAs compared with HaAs, indicating the differences in parent material between the soil types. Concentrations of SOC and Nt were significantly higher in HiAs compared with HaAs and CAL-extractable K concentration was higher in the HaAs compared with HiAs. Significantly higher concentrations of SOC and CAL-extractable P in HiAcon compared with HiAorg were observed. CEC followed SOC and clay concentrations, being the highest in HiAorg and HiAcon with the highest SOC and clay concentrations. Fungal biomass was significantly higher in HiAorg compared with HiAcon, and higher fungal biomass was detected in HaAs compared with HiAs although the difference was not significant. Bacterial biomass did not differ among sites, while leucine incorporation (bacterial activity), mineralizable N, and HWC all differed significantly among the soil types but not among the grassland management practices ().
Table 2. Key physicochemical and biological properties of the studied soils.
Distribution of soil fractions in the grassland sites
Total soil recovery after fractionation into fPOM, oPOM, and various aggregate sizes: <20 µm, 20–250 µm, and >250 µm was between 92% and 97% for all sites, indicating negligible soil losses during the fractionation procedure. The fPOM concentration in 0–10 cm depth ranged from the minimum of 41 g kg−1 in HaAorg to the maximum of 556 g kg−1 in HiAcon (). The amount of oPOM in 0–10 cm depth ranged from 4 g kg−1 to 92 g kg−1 in Grass2 and Grass1, respectively, and higher values were observed in HiAs than in HaAs. The MWD was highest in Grass2 and among the lowest in HiAcon at both soil depths. The aggregate distribution showed that macroaggregates (>250 μm) were most prominent in Grass1, Grass 2, HaAorg, and HiAorg, followed by HaAcon and HiAcon (). The amounts of microaggregates (<20 μm) in 0–10 cm depth were the highest in HaAcon (121 g kg−1) and the lowest in Grass2 (37 g kg−1) (). In Haplic Andosols, stable macroaggregates (>250 µm) were most strongly positively correlated with HWC (r = 0.863, p < 0.01), SOC (r = 0.623, p < 0.01), and Nt (r = 0.602, p < 0.01). As expected, we also found a positive correlation with fungal biomass (r = 0.666, p < 0.05). MWD was found to correlate strongly with HWC, SOC, Nt, and DOC (r = 0.872, p < 0.01; r = 0.676, p < 0.001, r = 0.669, p < 0.001, r = 0.630, p < 0.001; respectively). Strong positive correlations were also found with the pyrophosphate-extractable Fe, Mn, and Al, as well as dithionite and oxalate-extractable Mn (). On the contrary, fPOM was most strongly positively correlated with P (r = 0.466, p < 0.05) (). In Histic Andosols, the observed correlations were quite the opposite. Stable macroaggregates were negatively correlated with SOC and Nt (r = −0.840, p < 0.001, r = −0.775, p < 0.01; respectively), and the same was also observed for MWD (r = −0.827, p < 0.001, r = −0.755, p < 0.01; respectively). Both fPOM and oPOM were positively correlated with SOC and Nt (r = 0.755, p < 0.01, r = 0.765, p < 0.01; and r = 0.684, p < 0.05, r = 0.659, p < 0.05; respectively), as well as pyrophosphate-extractable Fe (r = 0.643, p < 0.05, r = 0.593, p < 0.05; for fPOM and oPOM, respectively) ().
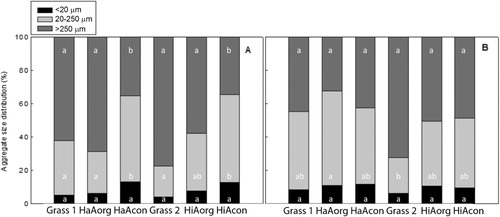
Table 3. Means (standard deviations) of fPOM, oPOM, and MWD in the studied sites.
Table 4. Pearson correlation coefficients between the particulate organic matter and soil aggregate fractions, MWD, and the key physicochemical and biological soil properties.
Distribution of SOC and Nt in the grassland sites
Total loss of SOC and Nt during fractionation was negligible (recoveries between 92% and 99% for all sites). We found that the distribution of SOC and N differed between the soil types (). In 0–10 cm depth, in Grass1, HaAorg and Grass2 macroaggregates >250 µm contributed the greatest quantities of SOC and N to bulk soil (44%, 65%, and 70% for SOC; 54%, 65%, and 71% for N, respectively). In comparison, in HiAorg and HiAcon, fPOM contributed the largest quantities of SOC and N to bulk soil (61% and 69%, respectively). The differences between the soil types remained in 10–20 cm depth, but for Grass1, HaAorg and HaAcon microaggregates 20–250 µm were the largest contributor of SOC and N to bulk soil (44%, 50%, and 41% for OC; 43%, 48%, and 40% for N, respectively). The C/N ratio decreased in the following order in both soil layers: fPOM > oPOM >macroaggregates 250 µm > macroaggregates 20–250 µm > microaggregates <20 µm.
![Figure 3. The C and N distribution within particle-size fractions and C/N ratios of different soil fractions of soils of different grassland sites. (Note: The C concentration of each fraction was calculated by taking total soil C as the sum of the C associated with all separate particle-size fractions, including particulate organic matter [POM] fractions). A, C, E = 0–10 cm and B, D, F = 10–20 cm.](/cms/asset/80b29422-d1d8-439a-935a-6c6fbae2d690/sagb_a_1001778_f0003_b.jpg)
13C CPMAS-NMR spectra revealed a large contribution of O-alkyl-C in all analyzed fractions (). In HaAorg and HaAcon, alkyl-C increased in the order fPOM < oPOM < bulk soil and O-alkyl-C decreased in the same order. Thus, alkyl-C to O-alkyl-C ratios increased in the order fPOM < oPOM < bulk soil. Aryl-C decreased in the order: fPOM > oPOM > bulk soil. In HiAorg and HiAcon, similar differences were found in alkyl-C to O-alkyl-C ratio, whereas chemical shift regions were fairly similar between fPOM, oPOM, and bulk soil. The chemical quality differed between the soil types, with an especially larger contribution of alkyl-C in HiAorg and HiAcon compared with HaAorg and HaAcon.
Table 5. Integrated chemical shift regions (% of total signal intensity) obtained by 13C CPMAS NMR spectroscopy for the extracted fPOM, oPOM, and bulk soil.
Discussion
Soil structure in the grassland sites
The hierarchical model of aggregates was confirmed in the studied soils by evidence of different stabilizing mechanisms for microaggregates (<20 µm and 20–250 µm) and macroaggregates (>250 µm). Persistent binding agents including oxalate-extractable Fe and Si as well as allophanes and ferrihydrites were positively connected to the microaggregates, whereas macroaggregates were bound together by temporary binding agents including HWC, SOC, DOC, and fungal biomass. However, this was only observed in Haplic Andosols but not in Histic Andosols. In those soils, no significant positive correlations were found with >250 µm aggregates or with MWD. Macroaggregates in Haplic Andosols were also positively correlated with pyrophosphate-extractable Fe, Mn, and Al, as well as with oxalate-extractable Fe and dithionite-extractable Fe, Al, and Mn. This is not in accord with identification of aggregate hierarchy based on Elliott (Citation1986) and Oades and Waters (Citation1991), but is very typical for Andosols in general where organo-mineral complexes and their positive effect on aggregation are well known (e.g., Bartoli & Burtin Citation2007). Our results also agree with Lehtinen et al. (Citation2014), who indicated the role of Mn in macroaggregation. The role of Mn in aggregation may be explained by it being associated with SOM (Navrátil et al. Citation2007), which in turn functions as an aggregating agent. In contrast to Haplic Andosols, in Histic Andosols, the aggregation properties were negatively correlated with SOC and pyrophosphate- and dithionite-extractable Fe and Al. Some explanations for negative correlations could be that there is a level above which extra SOC does not increase aggregation anymore, that it is the composition of SOM rather than the amount of SOC that influences soil aggregation (Dutarte et al. Citation1993), or that soils with high SOC might be so hydrophobic (Bartoli et al. Citation2007) that aggregation is inhibited. Stability of microaggregates is in agreement with Tisdall and Oades (Citation1982) and Dexter (Citation1988), who observed that stability of microaggregates depends on persistent forms of stabilizing agents such as organic carbon materials and sesquioxides and hence tends to be more resistant to management practices (Six et al. Citation2004). In Andosols, it is well known that amorphous inorganic materials such as allophane and ferrihydrite (as estimated based on oxalate-extractable Fe and Si) affect the aggregation (Hoyos & Comerford Citation2005), for which we found evidence, especially for Haplic Andosols, in this study. The role of organo-mineral complexes was observed as strong correlations of macroaggregates and MWD with pyrophosphate-extractable Fe, Mn, and Al (). These organo-mineral complexes are known to be very strong in volcanic soils and very hard to disperse (Bartoli & Burtin Citation2007).
Thus, we suggest that in Icelandic Haplic Andosol grassland soils the aggregate hierarchy is somewhat diminished and in Histic Andosols it does not exist. In Haplic Andosols, this is because oxides diminish the expression of the aggregate hierarchy, as was also suggested by Oades and Waters (Citation1991) in Oxisols. The main aggregating agents in macroaggregates in Haplic Andosols are SOM (as HWC, SOC, DOC, fungal biomass) together with pyrophosphate-extractable Fe, Mn, Al, oxalate-extractable Mn, and dithionite-extractable Fe, Mn, and Al.
Soil structure, measured, as MWD and amount of macroaggregates was highest in the unimproved grasslands Grass 1 and Grass 2 that had never been ploughed, followed by the improved grasslands (, ). It is well known that tillage breaks down aggregates and subsequently SOM is mineralized when not physically protected in the aggregates (e.g., Madejón et al. Citation2007; Laudicina et al. Citation2011). Our data may indicate that the application of OM in organic farming practices may have had positive effects on soil structure (, ). HaAorg received the highest OM inputs (manure, compost, and cattle urine; ), which may have contributed to the closest resemblance of macroaggregates to the Grass 1 and Grass 2 sites (which have never been disturbed by tillage and with insignificant grazing intensity) compared with the other sites (). Connection between OM inputs and increased aggregate stability is supported by several previous studies (Siegrist et al. Citation1998; Shepherd et al. Citation2002; Karami et al. Citation2012). In our study, we link higher amount of macroaggregates and higher MWD with higher fungal biomass (cf. Tisdall & Oades Citation1982). Organic inputs entering the soil provide substrate for the soil fungi, which further physically stabilize soil particles into larger aggregates when fungal growth increases and hyphae enmesh soil particles (Eash et al. Citation1994). We found higher macroaggregate stability in the topsoil, as explained by Tisdall (Citation1991). The concentrations of fine roots, OM, and fungi are the highest in the topsoil and provide therefore a favorable environment for macroaggregation. Fungal hyphae and extracellular polysaccharides produced by fungi enhance formation and stabilization of aggregates. In our study, conventional farms also used manure, but the amount may not have been sufficient to increase macroaggregation or other parts of the management practice did not support higher aggregation. The compost input, used at HaAorg and HiAorg, may have attributed to the higher microbial activity and the production of microbial decomposition products that bind the soil particles into microaggregates, and microaggregates further into macroaggregates (Sodhi et al. Citation2009).
SOM in the grassland sites
Significantly higher bulk SOC concentrations were observed in HiAs compared with HaAs (), as expected for these soil types (Arnalds Citation2004). The higher SOC concentration in HiAcon compared with HiAorg contradicts previous studies that have found evidence for significantly more SOC in organically managed topsoils compared with conventional farming practice (Leifeld & Fuhrer Citation2010; Gattinger et al. Citation2012). A major contributing factor for lower SOC concentrations in HiAorg compared with HiAcon could be ploughing history, which increases decomposition of SOM, damages fungal hyphae, and reduces root biomass (cf. Bolinder et al. Citation2002). However, duration since the last ploughing does not support this hypothesis (HiAcon was last ploughed in 1998 and HiAorg in 1994, 1995, and 1996), while the intensity might. Addition of compost and manure can increase SOC concentrations compared with conventional farming practice (Leifeld & Fuhrer Citation2010). However, the SOC concentration at the organic farm (HaAorg) did not differ significantly from the SOC concentration at the neighboring conventional farm (HaAcon) that both applied manure on their fields. The organic farms had slightly higher yields compared with the conventional farms (), which might have increased the carbon input by plant roots into the soil by increased root and shoot remains as well as increased root exudates and other root-borne organic substances (Kuzyakov & Domanski Citation2000). However, as previously described, the SOC was lower in the organic sites, and not the opposite. Historical SOC values for the sites could give more insight into the differences between the farming practices but those were unfortunately not available. In addition, landscape features such as changes in slope position, even if small, may have caused differences in SOM (Wang et al. Citation2008). The lower SOC and Nt concentrations in the unimproved grassland sites (Grass 1 and Grass 2) may be caused by a number of factors, including lower OM input to the soil from the vegetation (Kuzyakov & Domanski Citation2000) and/or lack of inorganic fertilization (Guo & Gifford Citation2002). Sites Grass 1 and Grass 2 are both on HaAs, and therefore have significantly lower SOC concentrations than the sites on HiAs that are organic-rich soils () and low concentration of plant available P in HaAs may limit plant growth.
Density fractionation revealed that fPOM reflected higher SOC concentrations, being the highest in HiAs and the lowest in HaAs as well as higher (but not significantly) in conventional sites compared with organic sites (). The higher concentration of fPOM in these soils may be a result from the timing and frequency of tillage; HaAcon was last ploughed earlier and less frequent (1995) than HaAorg (2001, 2002, 2003) and the HiAcon had a lower ploughing frequency compared with HiAorg (1998 for HiAcon; 1994, 1995, and 1996 for HiAorg). The negative effect of tillage on fPOM concentration in the soil is in accord with some previous studies (Chan et al. Citation2002; Linsler et al. Citation2013). However, we observed no significant differences between unimproved grasslands and improved grasslands. Presence of allophane, which is the case for low-density allophane-rich soils, has been observed as an explanation for a large percentage of POM of the bulk soil (Golchin et al. Citation1997), but contradicts this study because HiAcon and HiAorg had significantly lower concentrations of allophane compared with HaAcon and HaAorg, yet these soils had the highest fPOM concentrations. The generally high contents of fPOM in all of the studied soils may reflect the organo-mineral associations described above, that might hinder the microbial decomposition of SOM in these soils (Zagal et al. Citation2013).
SOM distribution and chemical quality in the grassland sites
The results of the SOM distribution showed that the SOC and Nt in oPOM and in <20 µm aggregates were different between farming practices and soil types (). Macroaggregate-associated OM fraction was the highest and less susceptible to mineralization in the Grass1, HaAorg, HaAcon, and Grass2 sites (). In HiAorg and HiAcon, however, the fPOM-associated SOC and Nt fraction had by far the greatest storage capacity. The soil fractions that were observed in the highest proportion to the bulk soil contained the most SOC and Nt, which is in accordance to Poll et al. (Citation2003). In general, the distribution and dynamics of Nt concentration paralleled those of the SOC concentration. Organic farming practices favored macroaggregate-associated OM fraction and had a closer resemblance to Grass 1 and Grass 2 sites compared with conventional farming practice. The increase in fPOM-associated SOC and Nt concentration measured in the cultivated sites compared with the unimproved grassland sites (Grass 1 and Grass 2) is consistent with other studies that have shown that animal manure can increase the particulate OM fraction (Whalen & Chang Citation2002; Courtier-Murias et al. Citation2013). However, the fact that the C/N ratio of the soil fractions decreased from POM fractions to the largest aggregates and further to the smallest aggregates suggests that the nitrogen-rich organic materials were associated with mineral particles, and indicates the plant-like character of fPOM and oPOM. This is in accordance with previous studies (Baldock et al. Citation1997; Golchin et al. Citation1997). Because the SOC concentrations and C/N ratios were not significantly different between the 20–250 µm and >250 µm sized aggregates, the SOM distribution in our soils is not in accord to the aggregate hierarchy model (Six et al. Citation2004).
The solid-state 13C NMR spectroscopy of all analyzed fractions showed an increasing degree of decomposition in the order fPOM < oPOM < bulk soil, shown as increased Alkyl-C to O-Alkyl-C ratio (Baldock et al. Citation1997). Despite the differences in farming practices, the chemical characteristics of the OM in soil fractions were similar in organic and conventional farming practices. A similar trend was reported by Golchin et al. (Citation1994), where five different soils with different environmental conditions and vegetation were compared. The organic inputs did not appear to affect the amount (except at 10–20 cm depth in HiAs) or the composition of the OM in the studied soils. Our results indicate that the fPOM and oPOM consisted mainly of plant material at different stages of decomposition, and were less decomposed compared with the SOM in the bulk soil (). Courtier-Murias et al. (Citation2013) showed that organic amendments could affect the amount but not necessarily the composition of the OM stabilized in agricultural soils.
To summarize, this study has demonstrated that macroaggregates were the most prominent soil aggregates in the topsoils of unimproved and organically managed sites, whereas 20–250 µm aggregates were the most prominent in conventionally managed topsoils. Aggregate stability decreased under cultivation of grasslands, but less so under organic farming practices compared with conventional farming practice. This may be due to later start of cultivation at the organic grassland sites and the higher usage of organic fertilizers (e.g., manure, compost, and urine) compared with the conventional grassland sites. Macroaggregates in the topsoils were the biggest contributor of SOC and N to bulk soil at Grass1, HaAorg, and Grass2 macroaggregates, whereas the fPOM did so for the HiAorg and HiAcon. In Haplic Andosols, stability of microaggregates (<250 µm) was related to oxalate-extractable Fe and Si, whereas macroaggregates (>250 µm) correlated with higher fungal biomass and higher concentration of SOM. Because also oxides were one of the binding agents in macroaggregates, the aggregate hierarchy was somewhat diminished in Haplic Andosols. In Histic Andosols, aggregate hierarchy could not be confirmed due to any positive significant correlations found with macroaggregates. The temporal variation of aggregation and SOM should be investigated in future studies, in order to monitor the long-term effects of different management practices.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/09064710.2014.1001778.
Supplementary_Material.pdf
Download PDF (13.7 KB)Acknowledgments
Dr. Axel Mentler (BOKU) and Dr. Carsten Müller (Technical University of Munich) are thanked for their advice on method development; E. Brauner, E. Kopecky, A. Hobel, K. Hackl, A. Hromatka, G. Heranney, and F. Brocza for technical assistance and laboratory work; Dr. Anu Mikkonen (University of Helsinki, Finland) for comments on the manuscript; Dr. Hans Göransson and Dr. Ika Djukic (BOKU) for advice on statistics; farmers are gratefully acknowledged for their cooperation and permission to take samples from their properties; Louise Hamilton, Jo Reilly, and James Salter are acknowledged for English proofreading.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amézketa E. 1999. Soil aggregate stability: a review. J Sustain Agric. 14:83–151.
- Arnalds O. 2004. Volcanic soils of Iceland. Catena. 56:3–20.
- Baldock JA, Oades JM, Nelson PN, Skene TM, Golchin A, Clarke P. 1997. Assessing the extent of decomposition of natural organic materials using solid-state 13C NMR spectroscopy. Aust. J. Soil Res. 35:1061–1083.
- Bartoli F, Burtin G. 2007. Organo-mineral clay and physical properties in COST 622 European volcanic soils. In: Arnalds Ó, Bartoli F, Buurman P, Óskarsson H, Stoops G, García-Rodeja E, editors. Soils of volcanic regions in Europe. Berlin: Springer Verlag; p. 469–491.
- Bartoli F, Regalado CM, Basile A, Buurman P, Coppola A. 2007. Physical properties in European volcanic soils: a synthesis and recent developments. In: Arnalds Ó, Bartoli F, Buurman P, Óskarsson H, Stoops G, García-Rodeja E, editors. Soils of volcanic regions in Europe. Berlin: Springer Verlag; p. 515–537.
- Bloem J, Bolhuis PR. 2006. Thymidine and leucine incorporation to assess bacterial growth rate. In: Bloem J, Hopkins DW, Benedetti A, editors. Microbiological methods for assessing soil quality. Wallingford: CABI.
- Bloem J, Vos A. 2004. Fluorescent staining of microbes for total direct counts. In: Kowalchuk GA, de Bruijn FJ, Head IM, Akkermans ADL, van Elsas JD, editors. Molecular microbial ecology manual. 2nd ed. Dordrecht: Kluwer Academic Publishers.
- Bloem J, Veninga M, Shepherd J. 1995. Fully-automatic determination of soil bacterium numbers, cell volumes, and frequencies of dividing cells by confocal laser-scanning microscopy and image-analysis. Appl Environ Microbiol. 61:926–936.
- Bolinder MA, Angers DA, Bélanger G, Michaud R, Laverdière MR. 2002. Root biomass and shoot to root ratios of perennial forage crops in eastern Canada. Can J Plant Sci. 82:731–737.
- Brandstetter A, Sletten RS, Mentler A, Wenzel WW. 1996. Estimating dissolved organic carbon in natural waters by UV absorbance (254 nm). Zeitschrift für Pflanzenernährung und Bodenkunde. J Plant Nutr Soil Sci. 159:605–607.
- Canali S, Benedetti A. 2006. Soil nitrogen mineralization. In: Bloem J, Hopkins DW, Benedetti A, editors. Microbiological methods for assessing soil quality. Wallingford: CABI Publishing.
- Chan KY, Heenan DP, Oates A. 2002. Soil carbon fractions and relationship to soil quality under different tillage and stubble management. Soil Tillage Res. 63:133–139.
- Christensen BT. 1992. Physical fractionation of soil and organic matter in primary particle size and density separates. Adv Soil Sci. 20:1–90.
- Courtier-Murias D, Simpson AJ, Marzadori C, Baldoni G, Ciavatta C, Fernández JM, López-de-Sá EG, Plaza C. 2013. Unraveling the long-term stabilization mechanisms of organic materials in soils by physical fractionation and NMR spectroscopy. Agric Ecosyst Environ. 171:9–18.
- Diacono M, Montemurro F. 2011. Long-term effects of organic amendments on soil fertility. In: Lichtfouse E, Hamelin M, Navarrete M, Debaeke P, editors. Sustainable agriculture, vol. 2. Dordrecht: Springer; p. 761–787.
- Dexter AR. 1988. Advances in characterization of soil structure. Soil Tillage Res. 11:199–238.
- Dutarte P, Bartoli F, Andreux F, Portal JM, Ange A. 1993. Influence of content and nature of organic matter on the structure of some sandy soils from West Africa. Geoderma. 54:478–495.
- Eash NS, Karlen DL, Parkin TB. 1994. Fungal contributions to soil aggregation and soil quality. Soil Sci Soc Am J. special publication. 35:221–228.
- Elliott ET. 1986. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci Soc Am J. 50:627–633.
- Feller C, Blanchart E, Bernoux M, Lal R, Manlay R. 2012. Soil fertility concepts over the past two centuries: the importance attributed to soil organic matter in developed and developing countries. Arch Agron Soil Sci. 58(Suppl 1):S3–S21. 10.1080/03650340.2012.693598
- Gattinger A, Muller A, Haeni M, Skinner C, Fliessbach A, Buchmann N, Mäder P, Stolze M, Smith P, El-Hage Scialabba N, Niggli U. 2012. Enhanced top soil carbon stocks under organic farming. Proc Natl Acad Sci. 109:18226–18231.
- Ghani A, Dexter M, Perrott KW. 2003. Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol Biochem. 35:1231–1243.
- Gislason SR, Oelkers EH, Eiriksdottir ES, Kardjilov MI, Gisladottir G, Sigfusson B, Snorrason A, Elefsen S, Hardadottir J, Torssander P, Oskarsson N. 2008. The feedback between climate and weathering. Mineral Mag. 72:317–320.
- Golchin A, Baldock JA, Clarke P, Higashi T, Oades JM. 1997. The effects of vegetation and burning on the chemical composition of soil organic matter of a volcanic ash soil as shown by 13C NMR spectroscopy. II. Density fractions. Geoderma. 76:175–192.
- Golchin A, Oades JM, Skjemstad JO, Clarke P. 1994. Study of free and occluded particulate organic matter in soils by solid state 13C CP/MAS NMR spectroscopy and scanning electron microscopy. Aust J Soil Res. 32:285–309.
- Graf F, Frei M. 2013. Soil aggregate stability related to soil density, root length, and mycorrhiza using site-specific Alnus incana and Melanogaster. Ecol Eng. 57:314–323.
- Guo LB, Gifford RM. 2002. Soil carbon stocks and land use changes: a meta analysis. Global Change Biol. 8:345–360.
- Helgadóttir A, Eythórsdóttir E, Jóhannesson T. 2013. Agriculture in Iceland – a grassland based production. In: Helgadóttir A, Hopkins A, editors. The role of grasslands in a green future – threats and perspectives in less favoured areas. Proceedings of the 17th Symposium of the European Grassland Federation, 2013 June 23–26, Akureyri. Iceland.
- Hoyos N, Comerford NB. 2005. Land use and landscape effects on aggregate stability and total carbon of Andisols from the Columbian Andes. Geoderma. 129:268–278.
- Karami A, Homaee M, Afzalinia S, Ruhipour H, Basitar S. 2012. Organic resource management: impacts on soil aggregate stability and other soil physic-chemical properties. Agric Ecosyst Environ. 148:22–28.
- Kemper WD, Rosenau RC. 1986. Aggregate stability and size distribution. In: Klute A, editor. Methods of soil analysis. Part I. Agronomy Monography 9. 2nd ed. ASA, SSSA, Madison, WI; p. 425–442.
- Knicker H, Gonzalez-Vila FJ, Polvillo O, Gonzalez JA, Almendros G. 2005. Fire-induced transformation of C- and N-forms in different organic soil fractions from a Dystric Cambisol under a Mediterranean pine forest (Pinus pinaster). Soil Biol Biochem. 37:701–718.
- Kubota T. 1972. Aggregate-formation of allophanic soils: effect of drying on the dispersion of the soils. Soil Sci Plant Nutr. 18:79–97.
- Kuzyakov Y, Domanski G. 2000. Carbon input by plants into the soil. J Plant Nutr Soil Sci. 163:421–431.
- Kögel-Knabner I. 2002. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem. 34:139–162.
- Kölbl A, Kögel-Knabler I. 2004. Content and composition of free and occluded particulate organic matter in a differently textured arable Cambisol as revealed by solid-state 13C NMR spectroscopy. J Plant Nutr Soil Sci. 167:45–53.
- Laudicina VA, Badalucco L, Palazzolo E. 2011. Effects of compost input and tillage intensity on soil microbial biomass and activity under Mediterranean conditions. Biol Fertil Soils. 47:63–70.
- Lehtinen T, Lair GJ, Mentler A, Gisladóttir G, Ragnarsdóttir KV, Blum WEH. 2014. Soil aggregate stability in different soil orders quantified by low dispersive ultrasonic energy levels. Soil Sci Soc Am J. 78:713–723.
- Leifeld J, Fuhrer J. 2010. Organic farming and soil carbon sequestration: what do we really know about the benefits? Ambio. 39:585–599.
- Linsler D, Geisseler D, Loges R, Taube F, Ludwig B. 2013. Temporal dynamics of soil organic matter composition and aggregates distribution in permanent grassland after a single tillage event in a temperate climate. Soil Tillage Res. 126:90–99.
- Madejón E, Moreno F, Murillo JM, Pelegrín F. 2007. Soil biochemical response to long-term conservation tillage under semi-arid Mediterranean conditions. Soil Tillage Res. 94:346–352.
- Manlay RJ, Feller C, Swift MJ. 2007. Historical evolution of soil organic matter concepts and their relationships with the fertility and sustainability of cropping systems. Agric Ecosyst Environ. 119:217–233.
- Marriott EE, Wander M. 2006. Qualitative and quantitative differences in particulate organic matter fractions in organic and conventional farming systems. Soil Biol Biochem. 38:1527–1536.
- Mehra OP, Jackson ML. 1960. Iron oxide removal from soils and clays by dithionite-citrate systems buffered with sodium bicarbonate. Clays Clay Miner. 7:317–327.
- Mueller CW, Brüggemann N, Pritsch K, Stoelken G, Gayler S, Winkler JB, Kögel-Knabler I. 2009. Initial differentiation of vertical soil organic matter distribution and composition under juvenile beech (Fagus szlvatica L.) trees. Plant Soil. 323:111–123.
- Navrátil T, Shanley JB, Skrivan P, Krám P, Mihaljevic M, Drahota P. 2007. Manganese biochemistry in a Central Czech Republic catchment. Water Air Soil Pollut. 186:149–165.
- North PF. 1976. Towards an absolute measurement of soil structural stability using ultrasound. J Soil Sci. 27:451–459. 10.1111/j.1365-2389.1976.tb02014.x
- Oades JM, Waters AG. 1991. Aggregate hierarchy in soils. Aust J Soil Res. 29:815–828. 10.1071/SR9910815
- Parfitt RL. 1990. Allophane in New Zealand - a review. Aust J Soil Res. 28:343–360. 10.1071/SR9900343
- Parfitt RL, Childs CW. 1988. Estimation of forms of Fe and Al: a review, and analysis of contrasting soils by dissolution and Moessbauer methods. Aust J Soil Res. 26:121–144. 10.1071/SR9880121
- Poll C, Thiede A, Wermbter N, Sessitsch A, Kandeler E. 2003. Micro-scale distribution of microorganisms and microbial enzyme activities in a soil with long-term organic amendments. Eur J Soil Sci. 54:715–724. 10.1046/j.1351-0754.2003.0569.x
- Rilling MC, Mummey DL. 2006. Mycorrhizas and soil structure. New Phytol. 171:41–53.
- Schmidt MWI, Knicker H, Hatcher PG, Kogel-Knabner I. 1997. Improvement of 13C and 15N CPMAS NMR spectra of bulk soils, particle size fractions and organic material by treatment with 10% hydrofluoric acid. Eur J Soil Sci. 48:319–328. 10.1111/j.1365-2389.1997.tb00552.x
- Schwertmann U. 1964. Differentiation of iron oxides of soil by extraction with ammonium oxalate solution. J Plant Nutr Soil Sci. 105:194–202. doi:10.1002/jpln.3591050303.
- Shepherd MA, Harrison R, Webb J. 2002. Managing soil organic matter – implications for soil structure on organic farms. Soil Use Manage. 18:284–292.
- Siegrist S, Schaub D, Pfiffner L, Mäder P. 1998. Does organic agriculture reduce soil erodability? The results of a long-term field study on loess in Switzerland. Agric Ecosyst Environ. 69:253–264.
- Six J, Bossuyt H, Degryze S, Denef K. 2004. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 79:7–31.
- Six J, Elliott ET, Paustian K. 2000. Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem. 32:2099–2103.
- Sodhi GPS, Beri V, Benbi DK. 2009. Soil aggregation and distribution of carbon and nitrogen in different fractions under long-term application of compost in rice-wheat system. Soil Tillage Res. 103:412–418.
- Soil Survey Staff. 2004. Soil Survey Laboratory Methods Manual. Soil Survey Investigations Rep. 42. Washington, DC: USDA – NRCS.
- Stavi I, Lal R, Owens LB. 2011. On-farm effects of no-till versus occasional tillage on soil quality and crop yields in eastern Ohio. Agron Sustain Dev. 31:475–482.
- Steffens M, Kölbl A, Kögel-Knabler I. 2009. Alteration of soil organic matter pools and aggregation in semi-arid steppe topsoils as driven by organic matter input. Eur J Soil Sci. 60:198–212. 10.1111/j.1365-2389.2008.01104.x
- Tabatabai MA, Bremner JM. 1991. Automated instruments for determination of total carbon, nitrogen, and sulfur in soils by combustion techniques. In: Smith KA, editor. Soil analysis. New York: Marcel Dekker.
- Tisdall JM. 1991. Fungal hyphae and structural stability of soil. Aust J Soil Res. 29:729–743. 10.1071/SR9910729
- Tisdall JM, Oades JM. 1982. Organic matter and water-stable aggregates in soils. Eur J Soil Sci. 33:141–163.
- Tukey JW. 1957. On the comparative anatomy of transformations. Ann Math Stat. 28:602–632.
- TÚN. 2013. Rules for organic and sustainable production and resource utilization. 2nd ed. Reykjavik: Vottunastofan Tún ehf.
- Vance ED, Brookes PC, Jenkinsson DS. 1987. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem. 19:703–707.
- Wang ZM, Zhang B, Song KS, Liu DW, Guo ZX, Zhang SM. 2008. Soil organic carbon under different landscape attributes in croplands of Northeast China. Plant Soil Environ. 54:420–427.
- Watson CA, Atkinson D, Gosling P, Jackson LR, Rayns FW. 2002. Managing soil fertility in organic farming systems. Soil Use Manage. 18:239–247.
- Whalen JK, Chang C. 2002. Macroaggregate characteristics in cultivated soils after 25 annual manure applications. Soil Sci Soc Am J. 66:1637–1647.
- Zagal E, Córdova C, Sohi SP, Powlson DS. 2013. Free and intra-aggregate organic matter as indicators of soil quality change in volcanic soils under contrasting crop rotations. Soil Use Manage. 29:531–539.
