Abstract
Dry rot is a severe fungal disease during potato storage. Fusarium spp. is the pathogen causing severe rot of potato tubers, producing Fusarium toxins and contaminating food. The disease leads to a huge loss to China's potato industry. The present research optimized and screened the culture conditions of the major pathogens causing potato dry rot including Fusarium culmorum, Fusarium avenaceum, Fusarium sambucinum, Fusarium solani var. coeruleum, Fusarium trichothecioides, and Fusarium sporotrioides. The results showed that F. sambucinum and F. culmorum were sensitive to temperature and pH. F. sambucinum, F. trichothecioides, F. Solani var. coeruleum, and F. sporotrioides are favorable to the glucose-based culture while F. culmorum and F. avenaceum were to sucrose-based culture. Illumination stimulated the growth of F. sambucinum and F. sporotrioides but inhibited the growth of F. culmorum and F. avenaceum. It is concluded that the culture conditions are different with species of Fusarium and the understanding of the culture conditions is expected useful in research and the disease control.
Introduction
Potato is susceptible to dry rot during storage. The disease causes many of the potato processing enterprises and farmers suffering huge economic losses and seriously hampers the development of the potato industry (Bojanowski et al. Citation2013). The toxins produced from the pathogen, Fusarium spp., during the destruction of the potato tissue lower the quality of the potato and contaminate human food. According to statistics and reports, there are dozens of Fusarium spp. that can cause dry rot worldwide. The types of Fusarium spp. and their virulence are different in variant countries (Hide et al. Citation1992; Mecteau et al. Citation2002; Peters et al. Citation2008; Sharifi et al. Citation2009). The isolation and identification of the pathogens for dry rot started in the 1990s in China and indicated that dozens of Fusarium spp. could cause dry rot in China too. It was reported that the pathogens causing potato dry rot included Fusarium coeruleum, Fusaruim solani, Fusarium oxysporum, F. moniliforme, Fusarium flocciferum, Fusarium semitectum, Fusarium tricintum, Fusarium solani var. coeruleum, Fusarium roseum, Fusarium sulphureum, Fusarium sambucinum, Fusarium sporotrichioides, Fusarium trichothecioides, and Fusarium redolens (Li et al. Citation2009; Du et al. Citation2012; Hu et al. Citation2014). Our research group isolated and identified the major pathogens causing dry rot in Heilongjiang province and the results indicated the main pathogens causing potato dry rot were Fusarium avenaceum (Corda ex Fries) Sacc., Fusarium culmorum (W.G. Smith) Sacc., F. solani var. coeruleum (Sacc.) Booth, F. sambucinum Fuckel, Fusarium sporotrioides Fuckel, and F. trichothecioides Wollenweber, as listed in , where the pathogenicity to other diseases in addition to potato dry rot was also mentioned with references attached.
Table 1. List of the Fusarium spp. used in this study that cause dry rot of potato.
There are large differences in the pathogenicity and virulence of potato dry rot pathogens in different regions and they are closely related to the phenological characteristics of different regions and environmental conditions for pathogens growth. Many factors affect the growth of fungi, especially environmental factors including temperatures, humidity, air conditions, pH, illumination, and wind speed. These conditions have large influences on the incidences of specific pathogens and their virulence (Chen et al. Citation2013). Temperature, pH of the medium, different sources of carbon, and nitrogen have great influence on the growth of pathogens and illumination is the major factor for colony growth and sporulation according to studies on the biological characteristics of papaya Fusarium, Fusarium solani, and Ginko endophytic Fusarium (Brennan et al. Citation2003; Doohan et al. Citation2003). The present experiment explored the optimal culture conditions for F. culmorum, F. sambucinum, F. trichothecioides, F. avenaceum, F. Solani var. coeruleum, and F. sporotrioides (), which will provide theoretical basis for early prediction and rational prevention of potato dry rot and will provide technical support for the storage of potato seed and the quality assurance of commodity potato seed.
Materials and methods
Experimental materials
The fungal strains used in the experiment were all from our own laboratory and incubated on potato dextrose agar (PDA) medium. The fungal strains included F. culmorum, F. sambucinum, F. trichothecioides, F. avenaceum, F. solani var. coeruleum, and F. sporotrioides. The media used in the experiment were PDA and potato sucrose agar (PSA).
Experimental methods
The PDA medium was melted and poured onto the marked plates. The tip portions of the newly grown Fusarium hyphae were transferred into a new medium using a sterile needle.
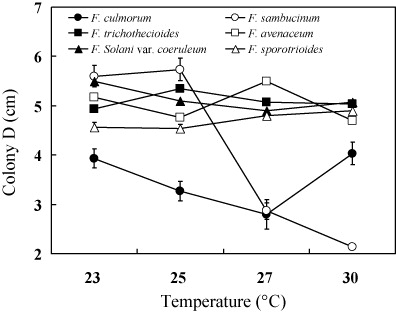
The effects of temperature on the growth of Fusarium spp. The plates with Fusarium spp. were cultured at four different temperature levels of 23°C, 25°C, 27°C, and 30°C. Each treatment was repeated three times and the colony diameters were measured and recorded using criss-cross method for 4–6 days.
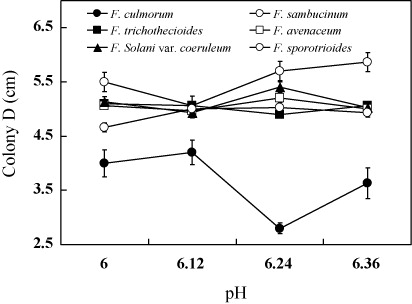
The effects of pH on the growth of Fusarium spp. The pH of the PDA medium was adjusted using 1 mol L−1 HCl and 1 mol L−1 NaOH. The plates were placed at 25°C with four pH gradients: 6.00, 6.12, 6.24, and 6.36. Each treatment was repeated three times and the colony diameters were measured and recorded by cross method for 4−6 days.
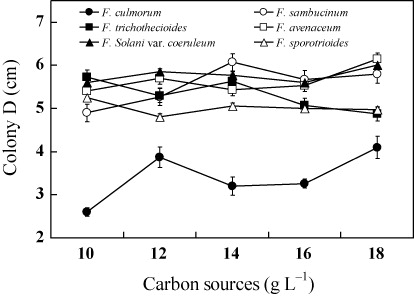
The effects of carbon concentration and types on the growth of Fusarium spp. The PDA media were made from different carbon sources, sucrose and glucose, respectively. The glucose concentrations were 10 g L−1, 12 g L−1, 14 g L−1, 16 g L−1, and 18 g L−1, while the sucrose added was 14 g L−1 and all other culture conditions were maintained the same. The colony diameters were measured and recorded for 4–6 days.
The effects of illumination on the growth of Fusarium spp. The experimental plates were placed in incubators either with or without illumination and the default temperature was 25°C. Incubation methods and measurement methods were the same as described above.
Results
The effects of temperature on the growth of Fusarium spp.
The investigated strains grew slowly at 5–15°C while the growth of mycelium was totally inhabited at 37°C. The culture temperature in the experiment was 23°C, 25°C, 27°C, and 30°C because the colony diameters were smaller at temperatures below 15°C and above 30°C. The results and analysis of variance are shown in and . The results indicated that the optimal temperature for the six pathogens causing potato dry rot was 23–27°C. The growth of F. Solani var. coeruleum, F. trichothecioides, F. avenaceum, and F. sporotrioides were less affected by culture temperature, while the growths of F. sambucinum and F. culmorum were greatly influenced by temperature. The optimal temperature for F. sambucinum was 25°C and its growth was inhabited with temperature increasing. There was almost no growth of the mycelium with temperature at 30°C and the analysis of variance showed that the growth was significantly affected by temperature. The results indicated that lower temperature was suitable to F. sambucinum and this was probably the main reason why the pathogen was more pathogenic in the potato storage in Heilongjiang Province, China.
Table 2. t-Test for effects of temperature on growth of potato dry rot pathogens.
The effects of pH on the growth of Fusarium spp.
The results from the pre-experiment indicated that all the six Fusarium spp. could survive within pH 2 and 9. The colony diameters were smaller below pH 5 and above pH 6.5. Four pH levels were chosen to incubate Fusarium spp. in the experiment and the results and analysis of variance were shown in and . As can be seen from the results, all the six strains were suitable to grow in the weak alkaline environment. Changing pH had minor effect on the growth of F. solani var. coeruleum, F. trichothecioides, F. avenaceum, and F. sporotrioides, while F. culmorum and F. sambucinum were more sensitive to the pH change. Especially, the growth of F. culmorum was significantly affected by pH, indicating that the infection of this strain would be interfered by the environmental factors.
Table 3. t-Test for effects of pH on growth of potato dry rot pathogens.
Effects of carbon sources on growth of Fusarium spp.
Glucose and sucrose are the most commonly used carbon sources and the optimal nutrient requirements are different to different Fusarium (Zhang & Wolf Citation2010). Five gradients of glucose (10 g L−1, 12 g L−1, 14 g L−1, 16 g L−1 and 18 g L−1) were used to incubate Fusarium spp. and the results are shown in . As shown from the figure, with exception of F. culmorum, the growth of Fusarium spp. was not significantly affected by the added carbon and the optimal adding amount was between 12–16 g L−1.The growth of F. culmorum was unstable on glucose-based medium and the growth of mycelia was totally inhabited under low glucose concentrations.
While comparing different types of carbon sources, media with14g L−1 glucose and sucrose were used to incubate the six strains of Fusarium. As can be seen from , F. sambucinum, F. trichothecioides, F. solani var. coeruleum, and F. sporotrioides were suitable to the media with glucose as carbon sources while the growth of F. trichothecioides and F. sporotrioides were significantly different with different media. F. culmorum and F. avenaceum were suitable to the media with sucrose as carbon source but the variances were not significant.
Table 4. Effects of carbon sources on growth of potato dry rot pathogens.
The effects of illumination on the growth of Fusarium spp.
Fusarium spp. was treated with or without illumination and the results are shown in . The growth of Fusarium responded differently to illumination. F. culmorum and F. avenaceum grew better without illumination while F. sambucinum and F. sporotrioides grew better with illumination and showed significant variances. F. trichothecioides and F. solani var. coeruleum were insensitive to illumination and there were no significant variances within the two treatments.
Table 5. Effects of light treatment on growth of potato dry rot pathogens.
Discussion
Dehydration shrinkage and rot are common phenomena during potato storage that will cause the quality decline of consumption, processing and breeding. This has become a bottleneck for further development of the potato industry. The loss caused by the disease during storage accounted for 20–30% of total production. The main diseases affecting the potato storage are dry rot, late blight, ring rot, and blackleg, bacterial rot, bud rot, blackheart and hollow disease (Fiers et al. Citation2012). The loss caused by dry rot accounted for 80% and led to enormous economic losses to potato processing enterprises and farmers which severely hampered the development of potato industry (Sharifi et al. Citation2009). The present experiment investigated the factors affecting growth of the pathogens causing dry rot and the results indicated that Fusarium spp. could grow within 20–40°C with the optimal temperature as 25–30°C. The effect of temperature on the growth of Fusarium mycelium was very similar to each other among species and there were wide ranges of temperature for optimal growth. Dry rot will cause large amount of rotten if temperature control is improper during potato seed and commodity potato storage (Doohan et al. Citation2003).
High or low pH will change the charge on protein or nucleic acid and hence affecting their biology activity leading to their inactivation, or even cause the charge changes on the cell membrane thus affecting the nutrient absorption of the cells, which will further change the nutrient supply of the environment and the toxicity of hazardous substances (Yamanaka Citation2003; Mo et al. Citation2005). Each pathogen needs specific pH to survive (Alexander Citation1990) and the results of the experiment found the main pathogen causing potato dry rot in Heilongjiang province appeared suitable to grow in a medium with weak acid environment. Basic environment can be considered to inhibit the growth of the pathogen and to achieve the purpose of prevention.
Carbon sources are the most important nutrient to fungi and sugar is the most widely used carbon source, for example, glucose and sucrose. Fu et al. investigated the effects of different carbon sources at the same concentration on the growth of fungi and found different fungi had preferences to different carbon sources (Mo et al. Citation2005). The present experiment used sucrose and glucose as carbon sources to incubate the pathogens causing dry rot and the results indicated that the preferences to the carbon source were different among different species of Fusarium. F. culmorum preferred sucrose-based medium while F. sambucinum preferred glucose-based medium. In the experiments using glucose as carbon source, the optimal range of glucose concentration was 12–16 g L−1 to the Fusarium spp. and mycelium grew the best when glucose concentration was 14 g L−1. These data can provide basis for the isolation and identification of Fusarium spp. in different crops.
Illumination can either stimulate or inhibit the growth of fungi. The mechanism of illumination is complicated and is affected by other environmental or nutrient factors. Fungi can “memorize” the illumination and will affect the subsequent development in the dark environment even just under very limited illumination (Mukherjee et al. Citation2003). The experiment showed different responses of different fungi causing dry rot in Heilongjiang province, some of them preferred illumination, for example, F. sambucinum and F. sporotrioides, some preferred dark, for example, F. culmorum and F. avenaceum, and some were insensitive to illumination, for example, F. trichothecioides and F. Solani var. coeruleum. These results explained that the occurrence of dry rot could be caused by different pathogens either with or without illumination during potato storage.
Fusarium pathogens are not only able to damage potato and affect the commercial value but also they generate toxins which are chemically stable and can not be degraded or destroyed by heating and processing (Bojanowski et al. Citation2013). The toxins can accumulate in human and animal bodies and lead to teratogenicity, neurotoxicity, embryo toxicity and immunosuppression. Long-term intake of food contaminated by Fusarium toxins will harm heart, kidney, and liver and even cause death due to the damage to the hematopoietic system. Numerous studies showed that Fusarium toxins and human disease incidences were correlated. Therefore, to further determine the relevance between toxigenic and pathogenic through the exploration of optimal growth conditions for Fusarium spp. become one of the research directions in microbiology.
Funding
This research was supported by Natural Science Foundation for Distinguished Young Scholars of China [grant number 31201470].
Additional information
Funding
References
- Abbatecola A, Pollastro S, Pichierri A, Faretra F. 2006. Survey on the presence of Phaeomoniella chlamydospora in grapevine rootstocks. J Plant Pathol. 88(Suppl. 3):31–63.
- Alexander SK. 1990. Laboratory exercises in organism and molecular microbiology. New York: McGraw-Hill; p. 1, 39–241.
- Baayen RP, van den Boogert PHJF, Bonants PJM, Poll JTK, Blok WJ, Waalwijk C. 2000. Fusarium Redolens f.sp asparagi, causal agent of asparagus root rot, crown rot and spear rot. Eur J Plant Pathol. 106:907–912.
- Bojanowski A, Avis TJ, Pelletier S, Tweddell RJ. 2013. Management of potato dry rot. Postharvest Biol Technol. 84:99–109.10.1016/j.postharvbio.2013.04.008
- Brennan JM, Fagan B, Maanen A, Cooke BM, Doohan FM. 2003. Studies on in vitro growth and pathogenicity of European Fusarium fungi. Eur J Plant Pathol. 109:577–587.
- Chehri KM, Salleh B, Yli-Mattila T, Soleimani MJ, Yousef AR. 2010. Occurrence, pathogenicity and distribution of Fusarium spp. in stored wheat seeds in Kermanshah Province, Iran. Pak J Biol Sci. 13:1178–1186.10.3923/pjbs.2010.1178.1186
- Chen LH, Huang XQ, Yang XM, Shen QR. 2013. Modeling the effects of environmental factors on the population of Fusarium oxysporum in cucumber continuously cropped soil. Commun Soil Sci Plant Anal. 44:2219–2232.10.1080/00103624.2012.760577
- Cook RJ. 1980. Fusarium foot rot of wheat and its control in the Pacific Northwest. Plant Dis. 64:1061–1066.10.1094/PD-64-1061
- Cumagun CJR, Aguirre JA, Relevante CA, Balatero CH. 2010. Pathogenicity and aggressiveness of Fusarium oxysporum Schl. in bottle gourd and bitter gourd. Plant Prot Sci. 46:51–58.
- Doohan FM, Brennan J, Cooke BM. 2003. Influence of climatic factors on Fusarium species pathogenic to cereals. Eur J Plant Pathol. 109:755–768.
- Du MR, Ren XY, Sun QH, Wang Y, Zhang RF. 2012. Characterization of Fusarium spp. causing potato dry rot in China and susceptibility evaluation of Chinese potato germplasm to the pathogen. Potato Res. 55:175–184.10.1007/s11540-012-9217-6
- Fiers M, Edel-Hermann V, Chatot C, Le HY, Alabouvette C, Steinberg C. 2012. Potato soil-borne diseases. A review. Agron Sustain Dev. 32:93–132.10.1007/s13593-011-0035-z
- Gulsoy HE. 1979. Behavior of the chlamydospores of Fusarium solani var. coeruleum Sacc. Booth in soil. J Turk Phytopathol. 8:81–96.
- Hide GA, Read PJ, Hall SM. 1992. Resistance to thiabendazole in Fusarium species isolated from potato tubers affected by dry rot. Plant Pathol. 41:745–748.10.1111/j.1365-3059.1992.tb02558.x
- Hu LG, Li YC, Bi Y, Li JP, Bao GH, Liu JJ, Yu XY. 2014. Effects of nitric oxide on growth of Fusarium sulphureum and its virulence to potato tubers. Eur Food Res Technol. 238:1007–1014.10.1007/s00217-014-2180-5
- Jelén HH, Mirocha CJ, Wasowicz E, Kamiński E. 1995. Production of volatile sesquiterpenes by Fusarium sambucinum strains with different abilities to synthesize trichothecenes. Appl Environ Microbiol. 61:3815–3820.
- Li WJ, Feng J, Chang KF, Conner RL, Hwang SF, Strelkov SE, Gossen BD, McLaren, DL. 2012. Microsatellite DNA markers indicate quantitative trait loci controlling resistance to pea root rot caused by Fusarium avenaceum (Corda ex Fries) Sacc. Plant Pathol J. 11:114–119.10.3923/ppj.2012.114.119
- Li YC, Bi Y, Ge YH, Sun XJ, Wang Y. 2009. Antifungal activity of sodium silicate on Fusarium sulphureum and its effect on dry rot of potato tubers. J Food Sci. 74:213–218.10.1111/j.1750-3841.2009.01154.x
- Mecteau MR, Arul J, Tweddell RJ. 2002. Effect of organic and inorganic salts on the growth and development of Fusarium sambucinum, a causal agent of potato dry rot. Mycol Res. 106:688–696.10.1017/S0953756202005944
- Michail SH, Ibrahim IA, Abd-El-Rehim MA, Padel FM. 1971. Damping-off of cucurbitaceous plants in U.A.R. III. Fusarium semitectum Berk, et Rav., a damping-off causal organism of watermelon. Phytopathol Mediterr. 10:46–49.
- Mo MH, Xu CK, Zhang KQ. 2005. Effects of carbon and nitrogen sources, carbon-to-nitrogen ratio, and initial pH on the growth of nematophagous fungus Pochonia chlamydosporia in liquid culture. Mycopathologia. 159:381–387.
- Moussa TAA, Rizk M. 2002. Biocontrol of sugarbeet pathogen Fusarium solani (Mart.) Sacc. by Streptomyces aureofaciens. Pak J Biol Sci. 14:59–559.
- Mukherjee T, Duke M, Copperman A, Grunfeld L, Sandler B, Barritt J. 2003. Value of sequential ultraviolet illumination in reducing ambient fungi, bacteria, and non viable fungal structures (NVFS) in an IVF laboratory. Fertil Steril. 80:289–290.10.1016/S0015-0282(03)01742-4
- Peters JC, Lees AK, Cullen DW, Sulivan L, Stroud GP, Cunnington AC. 2008. Characterization of Fusarium spp. responsible for causing dry rot of potato in Great Britain. Plant Pathol. 2:262–271.10.1111/j.1365-3059.2007.01777.x
- Pettitt TR, Parry DW, Polley RW. 1996. Effect of temperature on the incidence of nodal foot rot symptoms in winter wheat crops in England and Wales caused by Fusarium culmorum and Microdochium nivale. Agric For Meteorol. 79:233–242.10.1016/0168-1923(95)02281-3
- Popiel D, Kwasna H, Chelkowski J, Stępień L, Laskowska M. 2008. Impact of selected antagonistic fungi on Fusarium species – toxigenic cereal pathogens. Acta Mycol. 43:29–40.10.5586/am.2008.004
- Popovski S, Celar FA. 2013. The impact of environmental factors on the infection of cereals with Fusarium species and mycotoxin production – a review. Acta Agric Slov. 101:105–116.
- Scherm B, Balmas V, Spanu F, Pani G, Delogu G, Pasquali M, Migheli Q. 2013. Fusarium culmorum: causal agent of foot and root rot and head blight on wheat. Mol Plant Pathol. 14:323–341.10.1111/mpp.12011
- Semaškienė R, Mankevičienė A, Dabkevičius Z, Supronienė S. 2006. Effect of fungicides on Fusarium infection and production of deoxynivalenol in spring cereals. Agric Res. 4:363–366.
- Sharifi K, Rasoul Z, Zamanizadeh HR, Arjmandian A. 2009. Fusarium species causing dry rot of potatoes in Ardabil, Tehran and Hamedan Provinces. Appl Entomol Phytopathol. 2:93–114.
- Wharton PS, Tumbalam P, Kirk WW. 2006. First report of potato sprout rot caused by Fusarium sambucinum in Michigan. J Plant Dis. 90:1460.10.1094/PD-90-1460B
- Yamanaka T. 2003. The effect of pH on the growth of saprotrophic and ectomycorrhizal ammonia fungi in vitro. Mycologia. 95:584–589.10.2307/3761934
- Zhang HM, Wolf HC. 2010. The effect of different carbon sources on phenotypic expression by Fusarium graminearum strains. Eur J Plant Pathol. 127:137–148.10.1007/s10658-010-9578-0