Abstract
We investigated the effects of inoculation of Rhodobacter sphaeroides, Lactobacillus plantarum, and Saccharomyces cerevisiae on cucumber plant growth promotion and on the contents of plant hormones, amino acids, and mineral nutrients. We showed that treatment with all three bio-inoculants significantly increased the shoot length, root length, shoot fresh weight, shoot dry weight, and chlorophyll content, via secretion of indole acetic acid and/or organic acids. Inoculation with R. sphaeroides had more favorable effect on plant growth than did inoculation with L. plantarum or S. cerevisiae, by significantly enhancing the gibberellin and reducing the abscisic acid contents. The results of amino acid analysis revealed that inoculation with R. sphaeroides, L. plantarum, and S. cerevisiae generally increased the contents of 17 amino acids, namely, aspartic acid, threonine, serine, glutamic acid, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tyrosine, phenylalanine, lysine, histidine, arginine, and proline. With the exception of cysteine, all these amino acids were present in higher concentrations in plants inoculated with R. sphaeroides than in control plants or in plants inoculated with L. plantarum and S. cerevisiae. Furthermore, inoculation with R. sphaeroides significantly increased the calcium, potassium, magnesium, and phosphate contents. Our results suggest that the use of R. sphaeroides, L. plantarum, and S. cerevisiae in agricultural fields can improve plant growth. Moreover, inoculation of cucumber plants with R. sphaeroides regulates plant functional metabolites, thereby promoting plant growth.
Introduction
Organic farming is an emerging technique designed to ensure food safety, and it is leading to increased demand for biofertilizers as alternatives to agro-chemicals (Araujo et al. Citation2008; Raja Citation2013). The exploitation of chemical fertilizers, fungicides, and pesticides in agricultural fields causes pollution of the air, soil, and ground water, thereby negatively affecting human health (Radhakrishnan, Khan, & Lee Citation2013; Youssef & Eissa Citation2014). Soil-borne microbes, including bacteria and fungi, have been identified as biofertilizers. The application of plant growth-promoting microorganisms to crop plants enhance plant growth and yield via nitrogen fixation, phosphate and potassium solubilization, secretion of plant growth-regulating substances, production of antibiotics, and degradation of organic matter in the soil (Sinha et al. Citation2014). Bacteria belonging to the genera Acetobacter, Azospirillum, Azotobacter, Bacillus, Burkholderia, Klebsiella, Pseudomonas, and Serratia have been reported to be plant growth-promoting bacteria (PGPB) (Glick Citation1995; Jones et al. Citation2007). Other bacterial species can also be used for crop improvement provided these microorganisms are classified as “generally recognized as safe” and have low harmful effects on human health (Limanska et al. Citation2013).
In the present study, we investigated the effects of inoculation of Rhodobacter sphaeroides, Lactobacillus plantarum, and Saccharomyces cerevisiae on cucumber plant growth promotion. R. sphaeroides is a purple non-sulfur photosynthetic bacterium. Photosynthetic bacteria (PSB) can utilize various types of organic matter as carbon and energy substrates. Several studies have reported the use of PSB in the treatment of sewage and wastewater, the bioremediation of sediment mud polluted with organic matter, and as fertilizers for crop improvement (Sasaki et al. Citation1998; Takeno et al. Citation1999; Nagadomi et al. Citation2000; Yin et al. Citation2012). L. plantarum is commonly found in fermented food products and anaerobic plant matter and is used in human probiotics and also to stimulate plant growth and control plant diseases (Limanska et al. Citation2013). Yeasts exhibit plant growth-promoting effects by inhibiting pathogens, producing phytohormones, and solubilizing phosphate (Nassar et al. Citation2005; El-Tarabily & Sivasithamparam Citation2006; Mirabal-Alonso et al. Citation2008). For instance, S. cerevisiae promotes plant growth even under unfavorable environmental stress conditions (Gao et al. Citation2014).
Cucumber is a good source of vitamin A, vitamin C, mineral nutrients, and dietary fibers and is cultivated worldwide; however, it is highly sensitive to environmental conditions (Radhakrishnan & Lee Citation2013; Radhakrishnan, Shin, et al. Citation2014). Farmers use various chemical and organic fertilizers to obtain higher yields, but this practice leads to pollution of soil and ground water (Kang, Khan, Waqas, et al. Citation2014). The inoculation of cucumber plants with beneficial microbes may represent a valuable tool for increasing plant growth (Khan et al. Citation2012; Kang, Khan, Waqas, et al. Citation2014). However, data regarding the plant growth-promoting properties of bacteria and fungi are lacking. Investigation of the changes in plant growth hormones, amino acids, and mineral nutrients induced by microbial associations are essential and may help to clarify the mechanisms of plant microbe interactions. Few studies regarding the plant growth-promoting properties of R. sphaeroides, L. plantarum, and S. cerevisiae have previously been conducted. In the present study, we investigated the effects of inoculation of R. sphaeroides, L. plantarum, and S. cerevisiae on cucumber plant growth via modulation of the contents of gibberellin (GA4), abscisic acid (ABA), a diverse range of amino acids, and the macronutrients calcium, potassium, magnesium, and phosphate.
Materials and methods
Microbial cultures and conditions
The bacteria R. sphaeroides and L. plantarum and the yeast S. cerevisiae were obtained from PGPR Corp. (Chilgok, Gyeongbuk, Korea). R. sphaeroides, L. plantarum, and S. cerevisiae were cultured in Luria-Bertania broth, modified Rimler–Shotts broth, and yeast mannitol broth, respectively, for three days at 30°C in a shaking incubator at 200 rpm.
Plant growth and microbial inoculation
Cucumber (Cucumis sativus L. cv. Black Pearls) seeds were purchased from Seminis Korea Co., Korea. The seeds were surface sterilized with NaOCl (5%) for 10 min and then thoroughly rinsed with autoclaved distilled water. Fifty seeds were sown in autoclaved plastic pots under controlled greenhouse conditions at 30 ± 2°C. All experiments were conducted in triplicate. Two weeks after sowing, cucumber seedlings were treated with 5-mL suspensions of R. sphaeroides (106 CFU/mL), L. plantarum (108 CFU/mL), and S. cerevisiae (107 CFU/mL). Two weeks after treatment, the shoot length, root length, shoot fresh and dry weights, root fresh and dry weights, and chlorophyll content were recorded.
Determination of Indole acetic acid (IAA) and organic acids in microbial culture filtrates
For IAA (Indole acetic acid), R. sphaeroides, L. plantarum, and S. cerevisiae were cultured in 50 mL of medium with or without 0.5 g/L of D-tryptophan and incubated for seven days at 28 ± 2°C in a shaking incubator at 200 rpm. The cultures were then centrifuged at 10,000 × g for 20 min at 4°C to separate the microbes from the culture, and the cell-free cultures were filtered through 0.45-µm cellulose acetate filters. The filtrate pH was adjusted to 2.8–3.0, and ethyl acetate was then added. The obtained organic layer was evaporated under vacuum using a rotary evaporator at 45°C. The extracts were re-suspended in 5 mL of 0.1 M acetic acid and transferred to a reverse-phase C18 column. The extract was eluted using stepwise elution with 30% MeOH, 50% MeOH, and 100% MeOH. All the eluted samples were combined and dried. Methyl esters of the samples were prepared by dissolving the residues in 1 mL of methanol and adding 1.5 mL of ethereal diazomethane; the methyl esters were then re-dissolved in ethyl acetate and analyzed using gas chromatography–mass spectrometry (GC-MS) with selected ion monitoring (SIM) (6890N Network GC System and 5973 Network Mass Selective Detector; Agilent Technologies, Palo Alto, CA, USA). The quantity of IAA was calculated using a known standard peak area.
To evaluate organic acid production in the culture medium, the bacteria and fungus were separated from the culture medium and filtered through a 0.22-μm Millipore filter. Next, 10 μL of each filtrate was injected into a high-performance liquid chromatography (HPLC; Waters 600E) equipped with a refractive index detector (Waters 410) and a PL Hi-Plex H column (internal diameter, 7.7 mm; length, 300 mm) at 65°C. The mobile phase was 0.005 M H2SO4 in H2O at 0.6 mL/min. Organic acid production in culture medium was determined using the peak values of acetic acid, malic acid, citric acid, lactic acid, and succinic acid (Sigma-Aldrich).
Analysis of plant hormones
To determine variation in the GA4 concentration among the bio-inoculant–treated cucumber plants, we used the lyophilized samples to extract and quantify endogenous GA4 according to the method of Lee et al. (Citation1998). Briefly, crude GA4 extracts were prepared from plant samples (0.5 g), and 20 ng of GA standard (obtained from Professor Lewis N. Mander, Australian National University, Canberra, Australia) was added to each sample. The crude extracts were fractionated using HPLC, and the fractions were identified and quantified using GC-MS with SIM (6890N Network GC System and 5973 Network Mass Selective Detector; Agilent Technologies). The endogenous GA4 was calculated from the peak area ratios of 284/286.
We quantified the ABA content of cucumber plant samples according to the method described by Qi et al. (Citation1998). Crude ABA extracts of plant samples were prepared using isopropanol and glacial acetic acid. In each extract, 10 ng of ABA was added as an internal standard. The dried extracts were methylated by adding diazomethane, prior to analysis using GC-MS with SIM (6890N Network GC System and 5973 Network Mass Selective Detector; Agilent Technologies). For quantification, we used the Lab-Base (ThermoQuest, Manchester, UK) data system software to monitor responses to ions of m/e 162 and m/e 190 for Me-ABA and m/e 166 and m/e 194 for Me-[2H6]-ABA.
Determination of amino acid
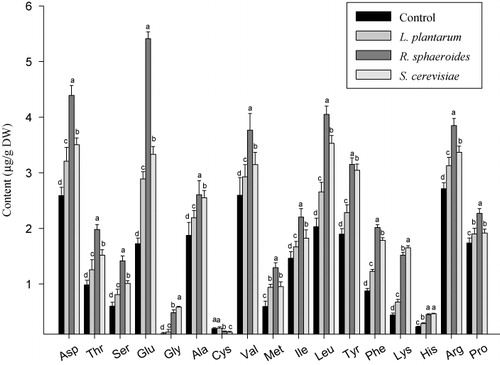
We determined the amino acid composition of cucumber plant samples according to the method of Kang et al. (Citation2012), using an amino acid analyzer (L-8900; Hitachi, Tokyo, Japan) after hydrolysis of a 50-mg sample with 0.02 N HCl at 110°C for 24 h. We quantified the aspartic acid (Asp), threonine (Thr), serine (Ser), glutamic acid (Glu), glycine (Gly), alanine (Ala), cysteine (Cys), valine (Val), methionine (Met), isoleucine (Ile), leucine (Leu), tyrosine (Tyr), phenylalanine (Phe), lysine (Lys), histidine (His), arginine (Arg), and proline (Pro) contents using standard peak values.
Minerals nutrient analysis
Calcium, potassium, magnesium, and phosphate were extracted from dried cucumber plant samples and analyzed using inductively coupled plasma mass spectroscopy (VG Elemental, PlasmaQuad 3; Perkin Elmer, Massachusetts, MA, USA). The mineral nutrients were quantified using known standard values.
Statistical analysis
The results of morphological and biochemical analysis were expressed as mean ± standard error. Significant differences between the microbial treatments and the control were determined using Duncan's multiple-range tests with SPSS software version 11.5. The significance level was P < 0.05.
Results and discussion
Effect of microbial inoculants on cucumber plant growth
Inoculation with beneficial microorganisms has a potentially important function in the development of sustainable crop production and may play a valuable role in promoting cucumber plant growth (Sturz et al. Citation2000). PGPB belonging to the genera Pseudomonas and Bacillus have been intensively studied in many agricultural crops. Some other strains have also been identified as PGPB (Sturz & Nowak Citation2000). R. sphaeroides, L. plantarum, and S. cerevisiae are commonly used in industrial applications; however, few studies regarding the plant growth-promoting properties of these microbes have previously been conducted. In the present study, inoculation of cucumber plants with R. sphaeroides, L. plantarum, and S. cerevisiae significantly enhanced plant growth ( and ). Inoculation with R. sphaeroides significantly increased the shoot length (25%), root length (35%), fresh weight (50%), and dry weight (twofold). Inoculation with L. plantarum and S. cerevisiae enhanced the shoot length, root length, fresh weight, and dry weight by 7–11%, 19–33%, 63–85%, and 40–75%, respectively. Yin et al. (Citation2012) reported that spraying with the purple non-sulfur bacterium, Rhodopseudomonas palustris, significantly increased the fresh weight and leaf area of Chinese dwarf cherry plants. Gao et al. (Citation2014) showed that exogenous application of S. cerevisiae enhanced plant growth and yield and alleviates the adverse effects of drought on rice. In addition, tomato seeds inoculated with L. plantarum NRRL B-4524 significantly increased the shoot length and root length (Hamed et al. Citation2011). In the present study, the results of chlorophyll content analysis revealed that inoculation of cucumber plants with R. sphaeroides, L. plantarum, and S. cerevisiae significantly enhanced the chlorophyll content. The concentration of chlorophyll was highest in plants inoculated with R. sphaeroides, followed by plants inoculated with S. cerevisiae and L. plantarum (). R. sphaeroides is a photosynthetic bacterium that interacts with plant roots, thereby stimulating photosystems and improving the photosynthetic efficiency of cucumber plants. Our results are in accordance with those of Yin et al. (Citation2012), who suggested that treatment of Chinese dwarf cherry seedlings with R. palustris significantly promoted plant growth by enhancing photosynthesis. In contrast, Gao et al. (Citation2014) reported that S. cerevisiae promoted plant growth, but reduced the photosynthetic rate and transpiration rate.
Table 1. Effects of inoculation of L. plantarum, R. sphaeroides, and S. cerevisiae on the shoot length, root length, fresh weight, dry weight, and chlorophyll content of cucumber plants.

Recently, Limanska et al. (Citation2013) demonstrated that pre-treatment of tomato seeds with L. plantarum stimulated seed germination, root length, and shoot length, possibly via the activity of released microbial metabolites. In the present study, we examined the contents of the plant growth stimulation hormone IAA and organic acids in culture filtrates of R. sphaeroides, S. cerevisiae, and L. plantarum (). We analyzed the IAA in the culture filtrates using GC-MS with SIM. We identified IAA in the R. sphaeroides culture filtrate, but not in the L. plantarum and S. cerevisiae culture filtrates. Plant growth regulators produced by fungi and bacteria are known to play important roles in the growth and development of crop plants (Radhakrishnan, Khan, Kang, et al. Citation2013; Kang, Khan, You, et al. Citation2014). Several species of IAA-producing microorganisms have been isolated from rhizosphere soil, and their positive interactions with plants are well documented in published literature (Radhakrishnan, Shim, Lee, et al. Citation2013). The result of our present study suggests that the secretion of a substantial amount of IAA by R. sphaeroides in the culture medium may have growth-promoting effect on cucumber plants. In addition, the production of organic acids by L. plantarum and S. cerevisiae in the culture medium may have enhanced plant growth (). L. plantarum produced succinic acid and lactic acid, whereas S. cerevisiae produced acetic acid; on the other hand, we were not able to detect organic acids in the R. sphaeroides culture filtrate. Thus, the organic acids produced by L. plantarum and S. cerevisiae may be indirectly involved in cucumber plant growth.
Table 2. Production of IAA and organic acids by L. plantarum, R. sphaeroides, and S. cerevisiae in the culture medium.
Effects of microbial inoculants on endogenous plant hormone contents of cucumber plants
Plant growth hormones a play major role in plant growth and development. For instance, GAs are responsible for a range of developmental processes including seed germination, dormancy, stem elongation, flowering, sex expression, and senescence. The biosynthesis of several GAs is controlled by non–C-13-hydroxylated and C-13-hydroxylated GAs pathways. GA4 is reported to be a major bioactive GA in Arabidopsis and in some Cucurbitaceae members (Yamaguchi Citation2008). In the present study, the contents of GA4 and ABA in cucumber plants were significantly influenced by inoculation with R. sphaeroides and S. cerevisiae (). In comparison with control plants, plants inoculated with R. sphaeroides or S. cerevisiae, but not with L. plantarum had significantly higher contents of the shoot elongation plant hormone, GA. The increases in GA contents were partially correlated with plant growth. We previously showed that the inoculation of cucumber plants with Acinetobacter calcoaceticus markedly increased the level of GA4, implying that the major GA biosynthesis pathway in cucumber is the non–C-13-hydroxylatation pathways (Kang et al. Citation2012). The results of our present study concur with our previous findings. We furthermore showed that inoculation of cucumber plants with the photosynthetic bacterium R. sphaeroides had a more favorable effect on plant growth than did inoculation with L. plantarum or S. cerevisiae, by significantly enhancing the GA.
Note: Means with the same letter do not differ significantly (P < 0.05) according to Duncan's multiple-range test.
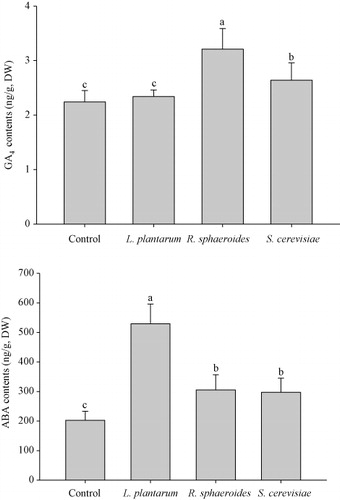
Next, we examined the effects of inoculation of R. sphaeroides, L. plantarum, and S. cerevisiae on the ABA content in cucumber plants (). We determined an increase in the ABA content after treatment with all three bio-inoculants. Moreover, inoculation with L. plantarum stimulated higher ABA production than did inoculation with R. sphaeroides and S. cerevisiae. Thus, the results of our study demonstrate a reciprocal relationship between GA and ABA in cucumber plants. ABA is involved in stomatal closure and is considered to be a plant stress hormone; in general, the ABA content is increased in plants subjected to abiotic and biotic stresses (Radhakrishnan, Khan, & Lee Citation2013). The relatively low ABA contents in cucumber plants inoculated with R. sphaeroides and S. cerevisiae may therefore be partially responsible for the improvement in plant growth.
Effects of microbial inoculants on amino acids contents of cucumber plants
Soil microorganisms release nitrogen in the form of amino acids, via nitrogen fixation or amino acid secretion, and these amino acids are absorbed by plants (Ferrara et al. Citation2012; Whiteside et al. Citation2012). In the present study, we examined the effects of inoculation of R. sphaeroides, L. plantarum, and S. cerevisiae on the quantities of a diverse range of amino acids. We identified and quantified 17 amino acids, namely, Asp, Thr, Ser, Glu, Gly, Ala, Cys, Val, Met, Ile, Leu, Tyr, Phe, Lys, His, Arg, and Pro (). In general, treatment with the three bio-inoculants increased the quantities of the investigated amino acids. Asp, Glu, Val, Leu, and Arg occurred in higher quantities, whereas Gly, Cys, and His occurred in lower quantities than did the remaining amino acids. With the exception of Cys, the quantities of all the investigated amino acids were increased by inoculation with R. sphaeroides; furthermore, the quantities of Thr, Ser, Glu, Gly, Met, Leu, Phe, Lys, and His were increased by more than twofold. Inoculation with the yeast S. cerevisiae resulted in a similar, but less marked pattern of amino acid enhancement. Data regarding microbial-induced changes of amino acids in crop plants are lacking. Previous studies have suggested that bacterial and fungal inoculation of plants can elevate the quantities of plant amino acids (Tchameni et al. Citation2011; Kang et al. Citation2012; Radhakrishnan, Khag, Baek, et al. Citation2014). Plant growth-promoting rhizobacteria are involved in fixing atmospheric nitrogen in the soil; furthermore, amino acid transporters to transport the resulting amino acids from the soil into the roots have been identified in plants (Jones et al. Citation2005; Kang, Radhakrishnan, You, et al. Citation2014). Recently, we showed that inoculation with A. calcoaceticus and Bacillus megaterium increased the quantities of amino acids in cucumber and mustard plants (Kang et al. Citation2012; Kang, Radhakrishnan, You, et al. Citation2014). Thus, in the present study, the increase in amino acids content of cucumber plants after inoculation with R. sphaeroides, L. plantarum, and S. cerevisiae may have been caused by increased rates of photosynthesis and nitrogen fixation.
Effects of bacterial and yeast inoculants on mineral nutrient contents of cucumber plants
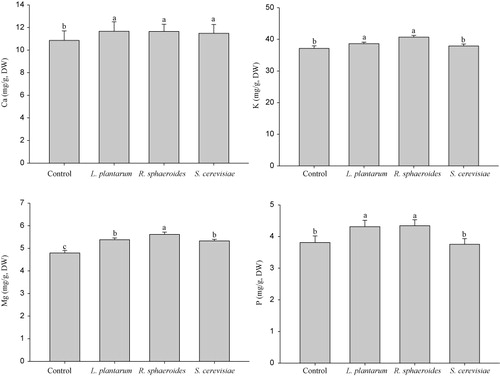
Plants generally absorb mineral nutrients from the soil; however, environmental factors can affect the efficiency of nutrient acquisition (Morgan & Connolly Citation2013). Soil microbes facilitate the uptake of mineral nutrients by plant roots. Most soil-living microorganisms degrade complex organic substances and convert these into simple forms. For instance, phosphate-solubilizing microorganisms solubilize organic phosphate and promote the uptake of plant nutrients (Cakmakci et al. Citation2007). In the present study, we quantified the quantities of the macronutrients potassium, magnesium, phosphate, and calcium in cucumber plants inoculated with R. sphaeroides, L. plantarum, and S. cerevisiae (). We found that treatment with all three bio-inoculants significantly increased the calcium and magnesium contents. The increase in magnesium content was higher in plants inoculated with R. sphaeroides. Inoculation with R. sphaeroides, and L. plantarum increased the potassium and phosphate contents. Phosphate, potassium, magnesium, and calcium are important components of proteins and nucleic acids, which are involved in many cellular functions. Mineral nutrient uptake by plant roots is known to be increased by IAA and by IAA-producing microorganisms (San-Francisco et al. Citation2005; El-Tarabily et al. Citation2008; Nimnoi et al. Citation2014). Thus, in the present study, the significant increase in calcium, phosphate, potassium, and magnesium contents of cucumber plants after inoculation with R. sphaeroides may indicate that this microbe is an IAA-producing photosynthetic bacterium. On the other hand, organic acid–producing bacteria have the potential to solubilize phosphate and increase the phosphorus nutrition and also the availability of other nutrients (Goldstein Citation1986; Gyaneshwar et al. Citation2002; Wang et al. Citation2014). Therefore, the observed secretion of organic acids by L. plantarum and S. cerevisiae may facilitate nutrient uptake by cucumber plants.
Note: Means with the same letter do not differ significantly (P < 0.05) according to Duncan's multiple-range test.
In conclusion, we have shown that inoculation of cucumber plants with R. sphaeroides, L. plantarum, and S. cerevisiae has the potential to enhance plant growth via an increase in the production of IAA and/or organic acids. Treatment with all three bio-inoculants positively altered the plant metabolic pathways, by increasing the contents of chlorophyll, GA4, and ABA and range of amino acids and mineral nutrients. The utilization of R. sphaeroides in cucumber cultivation fields may represent a valuable tool for enhancing plant growth and promoting sustainable agriculture.
Funding
This work was financially supported by the National Research Foundation of Korea, Ministry of Science, Information and Communication Technology (ICT) and Future Planning through Basic Science Research Program [grant number 2014R1A1A1004918].
Additional information
Funding
References
- Araujo ASF, Santos VB, Monteiro RTR. 2008. Responses of soil microbial biomass and activity for practices of organic and conventional farming systems in Piauistate, Brazil. Eur J Soil Biol. 44:225–230. 10.1016/j.ejsobi.2007.06.001
- Cakmakci R, Donmez MF, Erdogan U. 2007. The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk J Agric For. 31:189–199.
- El-Tarabily KA, Nassar HA, Sivasithamparam K. 2008. Promotion of growth of bean (Phaseolus vulgaris L.) in a calcareous soil by a phosphate-solubilizing, rhizosphere-competent isolate of Micromonospora endolithica. Appl Soil Ecol. 39:161–171. 10.1016/j.apsoil.2007.12.005
- El-Tarabily KA, Sivasithamparam K. 2006. Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience. 47:25–35. 10.1007/S10267-005-0268-2
- Ferrara FIS, Oliveira ZM, Gonzales HHS, Floh EIS, Barbosa HR. 2012. Endophytic and rhizospheric enterobacteria isolated from sugar cane have different potentials for producing plant growth-promoting substances. Plant Soil. 353:409–417. 10.1007/s11104-011-1042-1
- Gao J, Wang N, Li Y, Wang Y, Wang GX. 2014. Influence of Saccharomyces cerevisiae on gas exchange and yield attributes in rice under drought conditions. Biol Agric Hortic. 30:52–61. 10.1080/01448765.2013.845608
- Glick BR. 1995. The enhancement of plant growth by free-living bacteria. Can J Microbiol. 41:109–117. 10.1139/m95-015
- Goldstein AH. 1986. Bacterial phosphate solubilization: Historical perspective and future prospects. Am J Alt Agric. 1:57–65.
- Gyaneshwar P, Kumar N, Parekh LJ, Poole PS. 2002. Role of soil microorganisms in improving P nutrition of plants. Plant Soil. 245:83–93. 10.1023/A:1020663916259
- Hamed HA, Moustafa YA, Abdel-Aziz SM. 2011. In vivo efficacy of lactic acid bacteria in biological control against Fusarium oxysporum for protection of tomato plant. Life Sci J. 8:462–468.
- Jones DL, Healey JR, Willett VB, Farrar JF, Hodge A. 2005. Dissolved organic nitrogen uptake by plants an important N uptake pathway? Soil Biol Biochem. 37:413–423. 10.1016/j.soilbio.2004.08.008
- Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. 2007. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat Rev Microbiol. 5:619–633. 10.1038/nrmicro1705
- Kang S-M, Khan AL, Hamayun M, Shinwari ZK, Kim Y-H, Joo G-J, Lee I-J. 2012. Acinetobacter calcoaceticus ameliorated plant growth and Influenced gibberellins and functional biochemical. Pak J Bot. 44:365–372.
- Kang S-M, Khan AL, Waqas M, You Y-H, Kim J-H, Kim J-G, Hamayun M, Lee I-J. 2014. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact. 9:673–682. 10.1080/17429145.2014.894587
- Kang S-M, Khan AL, You Y-H, Kim J-G, Kamran M, Lee I-J. 2014. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J Microbiol Biotechnol. 24:106–112. 10.4014/jmb.1304.04015
- Kang S-M, Radhakrishnan R, You Y-H, Joo G-J, Lee I-J, Lee K-E, Kim J-H. 2014. Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J Microbiol. doi:10.1007/s12088-014-0476-6
- Khan AL, Hamayun M, Radhakrishnan R, Waqas M, Kang S-M, Kim Y-H, Shin J-H, Choo Y-S, Kim J-G, Lee I-J. 2012. Mutualistic association of Paecilomyces formosus LHL10 offers thermotolerance to Cucumis sativus. Anton Leeuw Int J G. 101:267–279. 10.1007/s10482-011-9630-x
- Lee I-J, Foster KR, Morgan PW. 1998. Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol. 116:1003–1011. 10.1104/pp.116.3.1003
- Limanska N, Ivanytsia T, Basiul O, Krylova K, Biscola V, Chobert JM, Ivanytsia V, Haertle T. 2013. Effect of Lactobacillus plantarum on germination and growth of tomato seedlings. Acta Physiol Plant. 35:1587–1595. 10.1007/s11738-012-1200-y
- Mirabal-Alonso L, Kleiner D, Ortega E. 2008. Spores of the mycorrhizal fungus Glomus mosseae host yeasts that solubilize phosphate and accumulate polyphosphates. Mycorrhiza. 18:197–204. 10.1007/s00572-008-0172-7
- Morgan JB, Connolly EL. 2013. Plant-soil interactions: nutrient uptake. Nat Educ Knowl. 4:2.
- Nagadomi H, Kitamura T, Watanabe M, Sasaki K. 2000. Simultaneous removal of chemical oxygen demand (COD), phosphate, nitrate and H2S in the synthetic sewage wastewater using porous ceramic immobilized photosynthetic bacteria. Biotechnol Lett. 22:1369–1374. 10.1023/A:1005688229783
- Nassar A, El-Tarabily K, Sivasithamparam K. 2005. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol Fert Soils. 42:97–108. 10.1007/s00374-005-0008-y
- Nimnoi P, Pongsilp N, Lumyong S. 2014. Co-inoculation of soybean (Glycine max) with actinomycetes and Bradyrhizobium japonicum enhances plant growth, nitrogenase activity and plant nutrition. J Plant Nut. 37:432–446. 10.1080/01904167.2013.864308
- Qi QG, Rose PA, Abrams GD, Taylor DC, Abrams SR, Cutler AJ. 1998. (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme a synthase gene expression, and very long chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol. 117:979–987. 10.1104/pp.117.3.979
- Radhakrishnan R, Khag S-M, Baek J-Y, Lee I-J. 2014. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J Plant Interact. 9:754–762. 10.1080/17429145.2014.930524
- Radhakrishnan R, Khan AL, Kang SM, Lee I-J. 2013. A comparative study of phosphate solubilization and the host plant growth promotion ability of Fusarium verticillioides RK01 and Humicola sp. KNU01 under salt stress. Ann Microbiol. doi:10.1007/s13213-014-0894-z
- Radhakrishnan R, Khan AL, Lee I-J. 2013. Endophytic fungal pre-treatments of seeds alleviates salinity stress effects in soybean plants. J Microbiol. 51:850–857. 10.1007/s12275-013-3168-8
- Radhakrishnan R, Lee I-J. 2013. Regulation of salicylic acid, jasmonic acid and fatty acids in cucumber (Cucumis sativus L.) by spermidine promotes plant growth against salt stress. Acta Physiol Plant. 35:3315–3322. 10.1007/s11738-013-1364-0
- Radhakrishnan R, Shim K-B, Lee B-W, Hwang C-D, Pae S-B, Park C-H, Kim S-U, Lee C-K, Baek I-Y. 2013. IAA-producing Penicillium sp. NICS01 triggers plant growth and suppresses Fusarium sp.-induced oxidative stress in sesame (Sesamum indicum L.). J Microbiol Biotechnol. 23:856–863. 10.4014/jmb.1209.09045
- Radhakrishnan R, Shin J-H, Choo Y-S, Kim J-G, Lee I-J. 2014. Studies on toxic effects of nitrogenous compound, putrescine and spermine on cucumber plant growth. J Environ Biol. 35:247–251.
- Raja N. 2013. Biopesticides and biofertilizers: ecofriendly sources for sustainable agriculture. J Biofertil Biopestici. 4:e112. 10.4172/2155-6202.1000e112
- San-Francisco S, Houdusse F, Zamarreno AM, Garnica M, Casanova E, Garcia-Mina JM. 2005. Effects of IAA and IAA precursors on the development, mineral nutrition, IAA content and free polyamine content of pepper plants cultivated in hydroponic conditions. Sci Hortic. 106:38–52. 10.1016/j.scienta.2005.03.006
- Sasaki K, Tanaka T, Nagai S. 1998. Use of photosynthetic bacteria for the production of SCP and chemicals from agroindustrial wastes. In: Martin AM, editor. Bioconversion of waste materials to industrial products. London: Blackie Academic and Professional; p. 247–291.
- Sinha RK, Valani D, Chauhan K, Agarwal S. 2014. Embarking on a second green revolution for sustainable agriculture by vermiculture biotechnology using earthworms: reviving the dreams of Sir Charles Darwin. Int J Agric Health Saf. 1:50–64.
- Sturz AV, Christie BR, Novak J. 2000. Bacterial endophytes: potential role in developing sustainable system of crop production. Crit Rev Plant Sci. 19:1–30. 10.1016/S0735-2689(01)80001-0
- Sturz AV, Nowak J. 2000. Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Appl Soil Ecol. 15:183–190. 10.1016/S0929-1393(00)00094-9
- Takeno K, Sasaki K, Watanabe M, Kaneyasu T, Nishio N. 1999. Removal of phosphorus from oyster farm mud sediment using a photosynthetic bacterium, Rhodobacter shaeroides IL106. J Biosci Bioeng. 88:410–415. 10.1016/S1389-1723(99)80218-7
- Tchameni SN, Ngonkeu MEL, Begoude BAD, Nana LW, Fokom R, Owona AD, Mbarga JB, Tchana T, Tondje PR, Etoa FX, Kuate J. 2011. Effect of Trichoderma asperellum and arbuscular mycorrhizal fungi on cacao growth and resistance against black pod disease. Crop Prot. 30:1321–1327. 10.1016/j.cropro.2011.05.003
- Wang T, Liu M-Q, Li H-X. 2014. Inoculation of phosphate-solubilizing bacteria Bacillus thuringiensis B1 increases available phosphorus and growth of peanut in acidic soil. Acta Agr Scand B-S P. 64:252–259.
- Whiteside MD, Digman MA, Gratton E, Treseder KK. 2012. Organic nitrogen uptake by Arbuscular mycorrhizal fungi in a boreal forest. Soil Biol Biochem. 55:7–13. 10.1016/j.soilbio.2012.06.001
- Yamaguchi S. 2008. Gibberellin metabolism and its regulation. Ann Rev Plant Biol. 59:225–251. 10.1146/annurev.arplant.59.032607.092804
- Yin ZP, Shang ZW, Wei C, Ren J, Song XS. 2012. Foliar sprays of photosynthetic bacteria improve the growth and anti-oxidative capability on Chinese dwarf cherry seedlings. J Plant Nutr. 35:840–853. 10.1080/01904167.2012.663439
- Youssef MMA, Eissa MFM. 2014. Biofertilizers and their role in management of plant parasitic nematodes – a review. J Biotechnol Pharm Res. 5:1–6.