Abstract
Onion cultivar “Sunpower” has growing popularity in Korea due to high storability. The present study considers changes in flavonols, sugars, and two amino acids in onion bulbs during a long-term storage in the ambient dark storage room and in glasshouse equipped with a climate control system. Flavonol and sugar contents were found to fluctuate noticeably during the storage period. Amino acid content remained relatively unchanged till the onset of inner sprouting, increasing afterward. Visible sprouts appeared at week 22 in the dark storage room and for four weeks later in the glasshouse. The bulbs lost 20–30% of their weight depending on storage conditions at the end of the storage trials. At the same time, the content of nutraceutics in study expressed on the dry weight basis remained of the same order of magnitude. The nature of observed variations in chemical composition of onions as well as relation of this phenomenon to physiological development of stored onion bulbs is discussed. A conclusion is made that the “Sunpower” onion cannot sustain overwinter storage.
Introduction
Onion (Allium cepa L.) is an important crop cultivated worldwide and is used mostly for various culinary purposes (Bosch Serra & Currah Citation2002; Brewster Citation2008). In regions with warm climate, distinct seasonal cultivars of onion are successfully grown to provide supplies throughout the year (Bosch Serra & Currah Citation2002). In countries like Korea, where winter is relatively cold and long, there is just one summer production season and onion bulbs must be stored for a year-round supply. Large growers, retailers, and food companies follow different practices of pre- and post-harvest treatment to protect onion quality during storage. Those include storage at low temperatures, the application of mitosis inhibitors, ethylene atmosphere, etc. (Gubb & MacTavish Citation2002; Brewster Citation2008). On the contrary, small food and agricultural enterprises as well as householders usually store onion under ambient conditions. In this case, the storability of onion is an important attribute influencing the choice of a cultivar for overwinter storage. Among the onions grown in Korea, cv. Sunpower seems to have the highest storability (Nam et al. Citation2011).
Irregular fluctuations in chemical composition of onion (A. cepa L.) bulbs during storage are not an uncommon phenomenon. Hurst et al. (Citation1985) and Benkeblia et al. (Citation2004) reported strong variations in carbohydrate levels in stored onion bulbs. Patil et al. (Citation1995a) observed fluctuations in total quercetin content in a five months storage trial unless the bulbs were kept under controlled atmosphere (CA). Downes et al. (Citation2009) described abrupt changes in total phenolics in onions stored for eight months. Some authors tried to explain this phenomenon by complex cycles of metabolic activity in bulbs (Benkeblia et al. Citation2004), in particular, by those associated with the break of dormancy (Benkeblia & Selselet-Attou Citation1999). Other researchers noticed the effect of fluctuating storage conditions on attributes of onions (Thamizharasi & Narasimham Citation1991). Frequently, however, the cases of the fluctuative behavior remained unexplained.
Intrigued by the phenomenon and having not found a satisfactory explanation in the literatures, we undertook a systematic investigation of the mentioned fluctuations in a long-term storage trial in 2011/2012. The present paper aimed to describe variations in a long-term storage trial. The onion cultivar “Sunpower” was chosen for study because of its high storability under ambient conditions. Three groups of organic compounds were monitored during the trial: quercetin and its glucosidase, sugars and aromatic amino acids phenylalanine (Phe) and tryptophan (Trp). Flavonols and sugars are typical phytochemicals considered in storage studies thank to their nutritional importance. Amino acids were chosen as one more groups of reference compounds because Phe is a key compound in the phenylpropanoid pathway (Dixon & Paiva Citation1995). Besides, a little is known about amino acid composition of onions (Kuon & Bernard Citation1963; Griffiths et al. Citation2002).
Materials and methods
Chemicals and standard solutions
All solvents used in this study were of high performance liquid chromatography (HPLC) grade. Water was obtained from J. T. Baker (Phillipsburg, NJ, USA), methanol from Duksan (Seoul, Korea), acetonitrile from Daejung (Gyonggi-do, Korea), and chloroform from Burdick and Jackson (Ulsan, Korea). Trifluoroacetic acid (extra pure grade) was supplied by Alfa Aesar (Ward Hill, MA, USA). Trp, Phe, and quercetin used as standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). Quercetin-3, 4′-O-diglucoside (QDG) and quercetin-4′-O-monoglucoside (QMG) were supplied by Polyphenols Laboratories AS (Sandnes, Norway). The purity of the flavonol standards was controlled by HPLC and was found to be > 99%. The saccharide standards sucrose (> 99.5%), D-glucose (>99.5%), and D-fructose (guaranteed reagent grade) were from Fluka (Buchs, Switzerland), Sigma-Aldrich, and Junsei Chemical Co. (Japan), respectively.
The stock solutions of quercetin (1 mg mL−1) and quercetin glucosides (4 mg mL−1) were prepared in methanol. Those of amino acids and saccharides were prepared in water, with concentration 2.6 and 50 mg mL−1, respectively. All the solutions were stored at −20°С calibration standards were obtained by appropriate dilution of the stock solutions.
Samples and storage conditions
Yellow onions (A. cepa L. cv. Sunpower) were grown at the Mokpo Experiment Station of the National Institute of Crop Science of the Rural Development Administration (Muan, Republic of Korea). The onions harvested in July 2011 were cured in the field of sandy soil for 10 days under the sunlight, trimmed of leaves and roots and transported to the laboratory. The bulbs in a weight range of 160–220g with no visible defects were taken for the study. These bulbs (ca. 20 kg) were packed in open cardboard boxes in one layer, each bulb was weighed and labeled to measure weight lost during storage. One half of the onions were kept in a dark place in a storage room equipped with an air conditioning system at temperature 20–25°C. Humidity fluctuated according to weather conditions between 70 ± 10%. This experiment modeled ambient storage conditions. In the other experiment, onions were exposed to sunlight in a glasshouse with controlled temperature (20–25°C) and relative humidity (RH) maintained at 70 ± 10%. In each experiment, eights bulbs were randomly sampled for analysis at day 0 and then at an interval of every 2–4 weeks until the 30th week. The selected bulbs were cleaned by removing the outer dry skin, chopped into small pieces and were mixed thoroughly to obtain a representative sample.
In order to investigate the distribution of phytochemicals across the bulb, five bulbs stored in the dark were selected at week 0, 3, and 21. The dry skin was removed, the bulbs were cut longitudinally, and the scales were separated into the three categories: outer (first and second scales), middle (fourth and fifth), and inner (sixth and seventh). Scales of each category were chopped into small pieces and mixed thoroughly.
Dry matter (DM) content
The DM content in onion bulbs was determined by drying chopped samples of approximately 35 g in an oven with air circulation first at 80°C for 24 h and then at 105°C for 2 h. Every determination was made in triplicates.
Analysis of flavonoids
Flavonoids were extracted in triplicates according to the method of (Bonaccorsi et al. Citation2005) with slight modifications. Approximately 10 g of a chopped sample was left overnight in 100 mL of methanol at 4°C. Then the methanol extract was separated and the residue was homogenized with a blender in 100 mL of methanol for three min, followed by stirring on a magnetic stirrer for 1 h. The slurry was centrifuged at 10,000 rpm for 40 min at 4°C. The supernatant was removed, the residue was mixed with a new portion of methanol, and centrifugation was repeated. The combined methanolic fractions were evaporated on a rotary evaporator at 45°C to approximately 8 mL and were diluted up to 10 mL with methanol. If not used immediately, the extracts were stored at −20°C.
The HPLC analysis of the extracts was carried out using an Agilent 1100 chromatograph (Agilent, Palo Alto, CA, USA) equipped with a solvent delivery system, an auto-sampler, a DAD detector set at 360 nm, and a ChemStation data acquisition system. Flavonoids were separated on a Lichrospher 100 RP-18 (250 mm × 4.6 mm) column with particle size of 5 µm (Merck KGaA, Darmstadt, Germany) protected with a Phenomenex (USA) C18-type guard column. The column was maintained at 25°C. The mobile phase was made of 0.1% TFA in water (solvent A) and methanol (solvent B). A gradient elution program was set as follows: 0–10 min, 20% B; 10–15 min, 20–80% B; 15–22 min, 80–20% B. The flow rate was set at 0.8 mL min−1. The injection volume was 10 µL. Quercetin and its glucosidase were quantified through comparison with respective calibration standards. Chromatographic analysis was done in triplicate unless noted otherwise and the average peak areas were used in calculations.
Analysis of amino acids
Amino acids were extracted in triplicates according to the method reported by Rhodes et al. (Citation1986) with some modifications. Five grams of chopped onion tissues were mixed with 25 mL of acetonitrile and 15 mL of water and was left overnight at 4°C. Then 60 mL of chloroform was added followed by homogenization with a blender. Then the homogenate was left for 2 h at 4°C for phase separation. When the phase separation was accomplished, the aqueous layer was collected and centrifuged (10,000 rpm for 40 min at 4°C). The supernatant was collected, the residue was suspended in 40 mL of acetonitrile:water (25:15, v/v), stirred for 30 min, and centrifuged again. The two supernatant phases were pooled and concentrated on a rotary evaporator to approximately 8 mL. The concentrated solution was transferred to a measuring flask and was diluted up to the 10 mL with water. The extracts were stored at −20°C if not used immediately.
Chromatographic analysis was performed with the Agilent 1100 HPLC system described above at temperature 25°C on a Chromolith HR RP-18e monolithic column (100 mm × 4.6 mm) from Merck KGaA (Darmstadt, Germany). Amino acids were eluted with a linear gradient of 0–80% of water in methanol over 20 min with the flow rate set at 0.8 mL min−1. The injection volume was 10 µL. Chromatograms were recorded at a wavelength of 210 nm. The quantification of Phe and Trp was made through comparison with their respective calibration curves. Chromatographic analysis of each replicate sample was repeated twice, and the average peak areas were used in calculations.
Analysis of sugars
Sugars (fructose, glucose, and sucrose) were measured in the same acetonitrile-water extracts prepared for the analysis of amino acids, as described above. Twenty microliters of an extract was injected into a Zorbax Carbohydrate (150mm × 4.6 mm) column from Agilent (Palo Alto, CA, USA) protected with an Agilent NH2 pre-column. The sample was eluted with acetonitrile/water (75:25, v/v) as recommended by the manufacturer. The column temperature was maintained at 30°C and the flow rate was 1 mL min−1. The analysis was carried out on a Shimadzu (Kyoto, Japan) 10A-VP series chromatograph with a Rheodyne 7725i manual injector (Rheodyne, Cotati, CA, USA) with a 20 µL sample loop and a refractive index detector calibrated against standard solutions (2–25 mg mL−1) of respective sugars. Chromatograms were integrated using the Shimadzu Class-VP software. Each injection was repeated 2–3 times.
Statistical analysis
Results presented in graphs are means ± standard deviations for three replicate samples. Differences between mean values were assessed by the Student's t test with a significance level of p < 0.05. All statistical calculations were made using OriginPro 8.1 software (OriginLab; Northampton, MA, USA).
Results and discussion
The onion cultivar “Sunpower” was chosen for this study because of its high storability. From a marketing point of view, the later can be defined as the capability of the bulb to retain firmness and integrity of the outer layer and to persist to sprouting and rooting. Onset of sprouting is commonly preferred to characterize the storage life of onions because of its clear relationship to physiological processes in bulbs (Pak et al. Citation1995; Yasin & Bufler Citation2007; Chope et al. Citation2007a; Bufler Citation2009). In the present work, the development of inner sprouts was observed on longitudinally cut bulbs at the 10th week of storage, both with those stored in the dark room and in the glasshouse under sunlight. Visible sprouts appeared at week 22 (glasshouse) and week 26 (storage room) in a good agreement with the results reported by Nam et al. (Citation2011).
Sprouting is an indication of the emergence from dormancy. In the dormant phase, supposedly started near harvest (Miedema Citation1994; Yasin & Bufler Citation2007), many physiological processes are slowed down, although not ceased (Pak et al. Citation1995; Yasin & Bufler Citation2007; Brewster Citation2008). After the dormancy breaking, onion bulbs enter the regrowth phase that is accompanied by gradually increasing metabolic activity. Thus, the chemical composition of onions may be expected to change in a certain relation to the dormancy duration. In this paper, we provide the results of our observations on the chemical composition of “Sunpower” onions made over a long period of time encompassing both the dormancy and regrowth phases.
Weight loss and DM content
Bulb weight is an integral characteristic determined by a superposition of respiration and desiccation processes. It normally reduces during storage, although special measures (mitosis inhibitors, CA, low temperature) may decelerate the process (Hurst et al. Citation1985). In our experiments, bulb weight was steadily decreasing () that eventually resulted in almost a 30% loss of the initial weight. The DM content is another integral indicator that directly correlates to the nutritional value of crops. It depends on respiration and other metabolic processes causing changes in the organic matter of the bulbs. Chope et al. (Citation2007b, Citation2012) have reported that field site and cultivar but not storage mostly affect onion DM, although a DM loss can be prevented by the application of an ethylene-action inhibitors (Chope et al. Citation2007a). A similar conclusion was drawn by Kaack et al. (Citation2004), who observed a variation in DM from 9.2 to 14.4 g 100−1 g as a function of cultivar, whereas a six month storage at 5°C had no measurable effect on this quantity. On the contrary, Pak et al. (Citation1995) have observed an approximately 9% decrease in DM of onion bulbs cv. Hysam. Their study, however, was carried out at 16°C, a temperature that is optimal for sprouting (Miedema Citation1994), unlike the experiments of Kaack et al. (Citation2004).
Table 1. Changes in the dry matter (DM) content and corrected DM content for weight loss in onion bulbs kept in the storage room and in the glasshouse.
In the present study, no essential change in DM was found till week 10 under both storage conditions considered. Relatively strong DM fluctuations, correlative for the dark and daylight storage, were observed beginning from this time point (), corresponding to the breaking of dormancy as indicated by onset of inner sprouting. It should be noted that the period of dry weight fluctuations coincided with the rainfall season characterized by abrupt fluctuations in ambient humidity. , which demonstrates the DM plots corrected for the weight loss, provides additional clues on the mass balance of storage. It is seen that storage resulted in the degradation of one third of the organic matter of onions. The processes of conversion of organic substances to volatile compounds intensify at week 10 that coincides with the beginning of the latent period of the emergence from dormancy when inner buds were first seen. The initial descent in the DM content ends at week 18, somewhat earlier than visible sprouts appeared.
Flavonols
Quercetin (in forms of aglycone, mono-, di- and triglucosides) is the predominant flavonol in onion. Total quercetin content varies among different cultivars of one color within an order of magnitude, given other factors are invariable, (Patil et al. Citation1995a, Citation1995b; Mogren et al. Citation2007a) and within one cultivar depends on growth location and environmental conditions (Patil et al. Citation1995a, Citation1995b Rodríguez Galdón et al. Citation2010). Mogren et al. (Citation2007a) ascribed a yearly variation in quercetin concentration reported by many (Mogren et al. Citation2007a; Rodríguez Galdón et al. Citation2010) to a respective variation in global radiation during the growth period. Soil was reported to have a minor effect (Patil et al. Citation1995b). Curing may result in an increase of flavonols in onions, although this phenomenon is year and cultivar dependent (Mogren et al. Citation2006; Chope et al. Citation2012). Sunlight is believed to be an important factor causing the accumulation of flavonol compounds (Mogren et al. Citation2006).
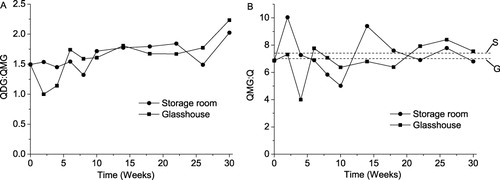
The effect of storage on flavonol content has been studied by many authors, with a dominant opinion of the effect being of minor importance (Price et al. Citation1997; Mogren et al. Citation2007b; Rodríguez Galdón et al. Citation2010). As an uncommon exception, Downes et al. (Citation2009) reported a steep increase in that value from ca. 0.1 to 0.4 (cv. Sherpa) or 0.9 (cv. Wellington) mg QE/g FW during the first six weeks of a cold storage, followed by a long period of slow fluctuations within ±30% with a weak tendency to decrease for cv. Sherpa. Individual quercetins, in general, followed the same pattern except 4-QG in cv. Wellington, whose concentration decreased during storage. Patil et al. (Citation1995a) observed inconsistent variations in the total quercetin content of “TG1015” onions stored for five months at temperatures of 5°C, 24°C, and 30°C. When the onions were stored in the CA (99% N2 + 1% O2 at 4.4°C), quercetin concentration gradually decreased by ca. 12%. The authors related their results to the phenomenon of sprouting as that was not observed only in CA storage. Our results () are in agreement with those of Patil with coauthors as both the sum of quercetins and the concentrations of the individual compounds were fluctuating while the sprouting, initially inner then apparent, took place during a larger part of the storage interval. Note that a period of increase in total quercetin content followed by a downward fluctuation after week 18 preceded to visible sprouting both in the storage room and in the glasshouse. Similarity between the two storage conditions becomes more noticeable when the ratios between different forms of quercetin are compared (presented in ). The ratio of QDG to QMG irregularly increases for both storage conditions considered. The ratios QMG to quercetin aglycone (Q) for each storage trial fluctuate around respective average values within ±18%. Considering a relative invariability in the concentration of QMG after week 10 (), one can deduce that the found increase in the QDG/QMG ratio is due to the accumulation of the diglucoside. Interestingly, this process was slow and relatively uniform during the period of inner bud development between week 10 and week 22 (). Thus, the synthesis of the diglucoside prevailed its hydrolysis during the later stages of storage. Similar findings have occasionally been reported in the literature. For example, the QDG/QMG ratio increased in the onions of the UK brown skinned cultivars Sherpa and Wellington during a 38 week storage if no treatment except curing was applied to the bulbs. Treatment with sprouting inhibitors made this dependence irregular; nevertheless the value of the ratio has been always significantly higher at the end than at start of storage (Downes et al. Citation2009). On the contrary, the QDG to QMG ratio was more or less invariable during storage in other UK grown onions: “Red Baron” and “Cross Bow”. The content of Q was decreasing with time till eventual disappearance after eight weeks, suggesting suppressed activity of glucosidases (Price et al. Citation1997). According to Rodríguez Galdón et al. (Citation2010) QDG/QMG may decrease, increase, or stay invariable depending on cultivar. A variety of patterns of flavonol composition at the late stage of storage is not surprising. Those are determined by an ensembled action of enzymes (Hirota et al. Citation1998; Takahama & Hirota Citation2000), whose activities, in turn, are defined by a number of factors from the genotype to the composition of soil.
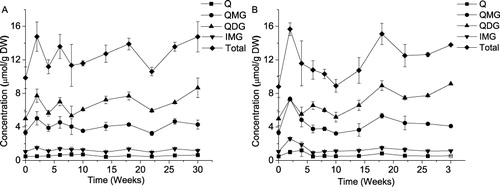
Quecetin glycoside content in fresh onion (week 0) decreases toward the center of the bulb (). The concentration of Q is larger in the innermost scales than in the outermost those. Since aglycone concentration is small, its presence does not contribute essentially to total quercetin content which is lower inside the bulb as compared to the exterior scales. The same regularity in the scale-to-scale distribution of the sum of quercetins has been reported by Beesk et al. (Citation2010), and Lee & Mitchell (Citation2011). Hirota et al. (Citation1998) measured flavonol contents in abaxial epidermis, adaxial epidermis, and mesophyll. In all the tissues, the amounts of the quercetin glucosides and Q decreased from outer to inner scales. Such a behavior of the aglycone is in conformity with the results of Beesk et al. (Citation2010) and Lee & Mitchell (Citation2011) but is in contradiction with the data in . As it is seen, the concentration of intact quercetin is higher in the innermost scales than in the first five scales (outer and middle). This tendency becomes more evident after 21 weeks of storage. The reason behind this contradiction is not clear. An explanation seems to lie in the distribution in different parts of onion of the enzymes involved in flavonol synthesis and conversion, which has not been comprehensively studied yet in spite of efforts made in this area (Hirota et al. Citation1998; Takahama & Hirota Citation2000). It is worth to point, however, to a positive relationship in the scales between quercetin and its early precursor in the phenylpropanoid pathway Phe observed in the present study (). Finally, note that a long storage does not change the pattern of flavonol distribution across the bulb qualitatively ().
Table 2. Effect of storage on the content of flavonols (μmol g−1 DW), amino acids (μmol g−1 DW) and carbohydrates (mmol g−1 DW) in different scales of onion.
Amino acids
There is scarce information on free amino acids (FAA) in onions except for S-derivatives of cysteine and gluthation studied intensively (Griffiths et al. Citation2002 and refs therein). Kuon and Bernard (Citation1963) measured FAA in four cultivars of American onions using an obsolete now paper chromatography technique. They found no large variation between the cultivars, with total FAA concentrations ranging from 0.47 to 0.56 mg g−1 FW. These data are in conflict with the results by Hansen (Citation2001), the studied onions grown in Scandinavia and found a ten-fold higher concentration of FAA than reported by Kuon and Bernard. One can suppose that different analytical methods employed rather than distinction in onion cultivars explain this disagreement in the results.
The fate of FAA in onions during storage is an even more obscure question. Hansen (Citation2001) seems to be the only researcher who investigated the problem. She monitored changes in the contents of 16 amino acids in typical Denmark onions “Hudiro” and “Hyton” during 48 weeks of a cold storage. The total amount of FAA was relatively constant (~ 60 mg g−1 DW) during the whole trial while the contents of individual amino acids changed according different trajectories depending on their biochemical functions. Concerning particular amino acids considered in the present study, Phe and Trp, the results shown in and are in accordance with those reported by Hansen. In both studies, the concentration of Phe eventually increases during storage (Hansen did not reveal the storage data for Trp). Moreover, the distributions of the amino acids across the bulb are identical, growing toward center the bulb. There is also agreement in the values of amino acid content (an order of 1 mg g−1 DW for both amino acids).
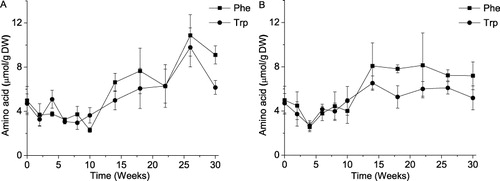
A closer look at the data in shows two regions on the plots, before and after week 10 when inner sprouts were first observed. During the first, obviously, dormant phase, Phe and Trp contents remained at almost the same level. After the tenth week, both amino acids experienced a sharp increase in concentration. The bulbs stored in the glasshouse maintained this higher level of concentration during the rest of the storage period. The amino acid content in the bulbs stored in the dark demonstrated a tendency to increase till week 26, camouflaged by a large experimental error. It dropped afterward. The final concentrations of respective amino acids in both storage conditions were the same within the experimental error.
Sugar content
Carbohydrates account for a major portion of the dry weight of onion bulbs and include glucose, fructose, sucrose, and fructooligosaccharides (fructans) with the degree of polymerization 3–12 (Darbyshire & Henry Citation1979; Salamal et al. Citation1990; Kaack et al. Citation2004). There are conflicting reports on the fate of sucrose and the monocarbohydrates in the whole bulbs during storage, as follows from a comprehensive summary compiled by Chope et al. (Citation2007b). It is seen that the temporal trajectories of individual saccharides depend on cultivar, storage temperature, and post-harvest treatment. It also may depend on growing conditions (Rutherford & Whittle Citation1982; Hansen Citation1999). In general, two types of behavior can be distinguished. According to the first one, the concentration of a sugar changes with storage time following a regular pattern such as a monotonous increase, decrease, or a stable behavior. Dependences with a peak or a well preceding and/or following a long period of a monotonous trend also fall in this category. Variation of total soluble sugars of onion bulbs cv. Rouge Amposta stored at 4°C, 10°C, and 20°C (Benkeblia et al. Citation2002) is an example of such a non-monotonous but regular behavior. The authors observed a peak of soluble sugars between the sixth and the tenth week of storage, which position was stable at any of the three investigated temperatures. They related this peak to the intensification of fructan hydrolysis before the initiation of sprouting. Another type of behavior consists in strong fluctuations of sugar content, with the amplitude and period of the fluctuations showing no regular pattern. Unlike the first category of dependences, this behavior hardly can be attributed to a known physiological process and suggests the existence of unrecognized, non-controlled but important influencing factors. Interesting that the type of behavior may be a function of temperature as in the study of Hurst et al. (Citation1985), when the sugar percentage monotonously decreased with time at 1°C but fluctuated at 4°C and 21°C.
The results in and demonstrate this second, fluctuating behavior, with the patterns being rather different from the dark and daylight storage. While the variations in sugar contents for the bulbs stored in the dark are characterized by alternation of abrupt decreases and increases between week 4 and week 12, only one peak is observed for all three sugars within the same experimental period for the bulbs stored under sunlight. Another distinction consists in the fact that the concentrations of glucose, fructose, and sucrose change in parallel in the dark storage, whereas in the bulbs stored in the glasshouse only the monosaccharides change in a correlative manner, with the sucrose level varying uncoordinately. A similar behavior – correlative trends for fructose and glucose was reported by Benkeblia et al. (Citation2004) for bulbs of cv. Tenshin stored at 10 and 20°C, by Chope et al. (Citation2007a) for bulbs of cv. SS1 stored at 4°C, 12°C, and 20°C. On the other hand, the concentration of fructose correlated to that of sucrose but not that of glucose in the study by Hansen (Citation1999). Kaack et al. (Citation2004) have reported no correlation between the contents of the three carbohydrates during a 6 month storage. Similar results were observed by Downes et al. (Citation2009). In the experiments of Salamal et al. (Citation1990), the direction of trends of carbohydrate concentration was a function of temperature and no essentially correlative behavior was observed. Chope et al. (Citation2012) have found that the sugar content in onions was primarily affected by post-harvest storage. The above short review does not contradict this finding with the reservation that the harvest level of sugars is determined by genotype and the geographical location of the plantation.
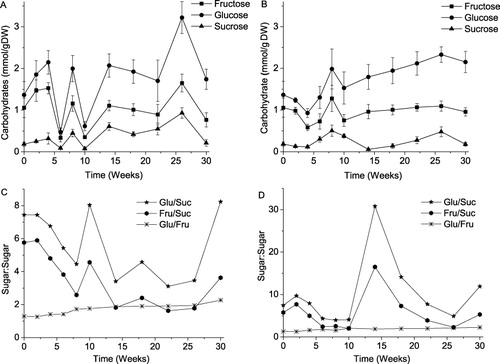
Elements of regularity are revealed in the plots of glucose to fructose and mono- to disaccharide ratios (, ). The former indicator increases slightly both under the dark and daylight storage conditions. The plots of the glucose (fructose) to sucrose ratios also demonstrate common patterns independent of storage conditions. In both storage trials, there are two declining parts of these plots. The first one is between weeks 2 and 8, the second one is between weeks 10 and 26 in the dark storage. The respective declining parts are observed within the intervals of weeks 2–10 and 14–26 in the daylight storage. Together with a distinctive (fluctuating) behavior of the individual sugars before the appearance of internal sprouts at week 10, it suggests mobilization of carbohydrate catabolism before the dormancy breaking in agreement with the hypothesis of Benkeblia et al. (Citation2002, Citation2004). The relation of the mono- to disaccharide ratio to the sprouting status was also discussed by Chope et al. (Citation2012).
As was mentioned, the distribution of saccharides over the bulb is not uniform. Commonly, researchers observed a decrease in fructose concentration toward the center of the bulb, whereas the sucrose level did not change markedly from scale to scale (Darbyshire & Henry Citation1978; Rutherford & Whittle Citation1982; Salamal et al. Citation1990; Hansen Citation1999; Yasin & Bufler Citation2007). Glucose usually demonstrated the same pattern as fructose except the work of Rutherford & Whittle (Citation1982), where it fluctuated within 10% of the average level. After the initiation of sprouting, fructan in the scales is hydrolyzed and the products of hydrolysis are transported to the bulb base to be used for growth processes (Pak et al. Citation1995). Therefore, a redistribution of sugars over the bulb during storage is expected and was documented by some authors (Rutherford & Whittle Citation1982; Pak et al. Citation1995). On the contrary, Yasin & Bufler (Citation2007) did not notice any qualitative change in the saccharide profile even after the onset of sprouting eight weeks after harvest. Our data mostly show variations within the experimental error (). Even so, a tendency to a change in a scale-to-scale saccharide distribution may be noticed after start of storage. This distribution becomes resembling a typical pattern with fructose and glucose decreasing toward the center of the bulb and sucrose varying insignificantly.
Storage room vs. glasshouse storage
Two storage modes – in a dark store room and in a glasshouse – considered in the present study differ in an illumination regime (dark against daylight). These storage regimes differently influence onion bulbs. The more distinctive effect was observed in variation of DM () and sugar content (). Effect of quercetin and amino acid contents was marginal ( and ). Rationalization of this phenomenon obviously relates to the physiological response of onions to environmental conditions. As the dormancy period recognized by the lack of inner sprouts had the same duration (ten weeks) in both storage conditions, one can conclude that signals coming from the environment were not strong enough to effect (maintain or release) the endodormant state. On the contrary, those signals were sufficient to effect the development of inner sprouts, which became visible four weeks earlier in the bulbs stored in the glasshouse. Note that an evident discrepancy between the two experimental series in the DM content happened only after the onset of inner sprouting. These observations are in line with the opinion of Brewster, who suggested based on a number of evidences (Brewster Citation2008 and Refs therein) that a temperature difference between a room temperature and the range of 25–30°C may be decisive in promoting sprout growth once the bulb dormancy is broken.
Another factor whose impact on physiological processes in stored onions is well documented is the humidity (Brewster Citation2008). A moderate RH of 65–70% is known to preserve bulb weight better than lower or higher humidity atmospheres. The equilibrium water content of onion skin, which influences the moisture transport to and from the edible part of the bulb (Brewster Citation2008), is an exponential function of RH, slowly increasing when RH < 75% and abruptly raising beyond this point. Salamal et al. (Citation1990) reported of no effect of humidity changing between 40% and 60% on sugar and organic acid contents in onions. It seems that humidity does not exert an essential influence on metabolic processes in the bulbs when RH is between 40% and 70%. It may not be true if humidity varies within a wide range extending beyond 70%. Thamizharasi & Narasimham (Citation1991), who studied the effect of naturally fluctuating humidity and temperature on different attributes of onions, came to a conclusion that fluctuations in storage conditions impact negatively the storage life of onion bulbs. They, however, were unable to ascertain whether temperature or humidity was a dominating factor. It is important to note that the mentioned fluctuations in experimental parameters per se could not cause the irregular variations in the chemical composition of onions observed in the present investigation. It follows from the fact that the period of fluctuations in storage conditions (hours to days) was manyfold less than that in the composition of onions (weeks). The lack of a tight control of storage conditions could however trigger some physiological mechanism, which contribution to the fluctuations in flavonols and sugars could be essential. For example, the accumulation of moisture in the outer scales of onion due to alternate cycles of the adsorption and desorption of water vapor, following fluctuating ambient temperature and humidity, would render them susceptible to microbial spoilage (Thamizharasi & Narasimham Citation1991).
Comparing the results of the present work with the literature data, one should take into account that the bulbs stored in the storage room were lacking of daylight illumination unlike the bulbs stored in the glasshouse. There is no much information on this issue in the literature. Our findings show that difference between ambient (with fluctuating humidity and controlled temperature in the dark) and non-ambient (controlled humidity and poorly controlled temperature, daylight) storage does not cause differences in the shapes of the time profiles of such general characteristics as the weight loss and the DM content but results in different variations of bulb's chemical composition.
“Sunpower” onions stored in ambient conditions lose in an average 25% of weight by the end of winter that adversely affects their marketability due to the loss of firmness and the deterioration of appearance. Glasshouse, which is another economical option for a domestic storage available to the Korean farmers, gives even worse bulb quality after overwinter storage due to earlier sprouting. In both storage trials, the chemical composition of bulbs varied significantly, demonstrating a fluctuating behavior in the case of sugars and flavonols. Fluctuation in the content of nutraceuticals is not an uncommon phenomenon in storage studies. It is probably explained by superposition of a multitude of metabolic processes involving the target compound (resulting in both accumulation and consumption) that proceed in the bulb with different rates. Despite an apparent irregular pattern of concentration-storage time dependencies, three sections in the plots may be revealed at a closer look. The first section corresponds to the rest phase including a period of metabolism mobilizations during the emergence from dormancy. It is followed by the long sprouting phase, which is initially hidden and eventually becomes apparent. The third section corresponds to the regrowth phase that encompasses the last four weeks of the trial. This phase is characterized by declining DM, sugar, and amino acid contents (well exposed in the dark storage) and by a relative increase in total flavonols content.
The two modes of storage considered in a conventional storage room and in a glasshouse – result in somewhat different changes in chemical composition of onion. This fact is explained by the different character and the amplitude of fluctuations of environmental conditions in a storage facility. Whether temperature, humidity, or daylight illumination plays a dominating role in the difference between these two storage modes is unclear. We may suppose that a temperature difference is the most influencing factor during the sprouting phase. This hypothesis explains our findings and agrees with the literature data.
Acknowledgment
This work was supported by Konkuk University research program.
References
- Beesk N, Perner H, Schwarz D, George E, Kroh LW, Rohn S. 2010. Distribution of quercetin-3,4′-O-diglucoside, quercetin-4′-O-monoglucoside, and quercetin in different parts of the onion bulb (Allium cepa L.) influenced by genotype. Food Chem. 122:566–571.10.1016/j.foodchem.2010.03.011
- Benkeblia N, Onodera S, Yoshihira T, Kosaka S, Shiomi N. 2004. Effect of temperature on soluble invertase activity, and glucose, fructose and sucrose status of onion bulbs (Allium cepa) in store. Int J Food Sci Nutr. 55:325–331.
- Benkeblia N, Selselet-Attou G. 1999. Effects of low temperatures on changes in oligosaccharides, phenolics and peroxidase in inner bud of onion (Allium cepa L.) during break of dormancy. Acta Agric Scand Sect B Soil and Plant Sci. 49:98–102.
- Benkeblia N, Varoquaux P, Shiomi N, Sakai H. 2002. Storage technology of onion bulbs c.v. rouge amposta: effects of irradiation, maleic hydrazide and carbamate isopropyl, N-phenyl (CIP) on respiration rate and carbohydrates. Int J Food Sci Tech. 37:169–175.10.1046/j.1365-2621.2002.00554.x
- Bonaccorsi P, Caristi C, Gargiulli C, Leuzzi U. 2005. Flavonol glucoside profile of southern Italian red onion (Allium cepa L.). J Agric Food Chem. 53:2733–2740.10.1021/jf048152r
- Bosch Serra AD, Currah L. 2002. Agronomy of onion. In: Currah HD, Rabinovich L, editors. Allium crop science: recent advances. Wallingford: CAB Int; p. 187–232.
- Brewster JL. 2008. Onions and other vegetable alliums. 2nd ed. Wallingford: CAB Int; p. 433.
- Bufler G. 2009. Exogenous ethylene inhibits sprout growth in onion bulbs. Ann Bot. 103:23–28.10.1093/aob/mcn203
- Chope GA, Cools K, Hammond JP, Thompson AJ, Terry LA. 2012. Physiological, biochemical and transcriptional analysis of onion bulbs during storage. Ann Bot. 109:819–831.10.1093/aob/mcr318
- Chope GA, Terry LA, White PJ. 2007a. The effect of 1-methylcyclopropene (1-MCP) on the physical and biochemical characteristics of onion cv. SS1 bulbs during storage. Postharvest Biol Technol. 44:131–140.10.1016/j.postharvbio.2006.11.012
- Chope GA, Terry LA, White PJ. 2007b. The effect of the transition between controlled atmosphere and regular atmosphere storage on bulbs of onion cultivars SS1, carlos and renate. Postharvest Biol Technol. 44:228–239.10.1016/j.postharvbio.2006.12.018
- Darbyshire B, Henry RJ. 1978. The distribution of fructans in onions. New Phytol. 81:29–34.
- Darbyshire B, Henry RJ. 1979. The associations of fructans with high percentage dry weight in onion cultivars suitable for dehydrating. J Sci Food Agric. 30:1035–1038.10.1002/jsfa.2740301103
- Dixon RA, Paiva NL. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell. 7:1085–1097.10.1105/tpc.7.7.1085
- Downes K, Chope GA, Terry LA. 2009. Effect of curing at different temperatures on biochemical composition of onion (Allium cepa L.) skin from three freshly cured and cold stored UK-grown onion cultivars. Postharvest Biol Technol. 54:80–86.10.1016/j.postharvbio.2009.05.005
- Griffiths G, Trueman L, Crowther T, Thomas B, Smith B. 2002. Onions-a global benefit to health. Phytother Res. 16:603–615.10.1002/ptr.1222
- Gubb IR, MacTavish HS. 2002. Onion pre– and post–harvest considerations. In: Currah HD, Rabinovich L, editors. Allium crop science: recent advances. Wallingford: CAB Int; p. 233–265.
- Hansen SL. 1999. Content and composition of dry matter in onion (Allium cepa L.) as influenced by developmental stage at time of harvest and long-term storage. Acta Agric Scand Sect B Soil and Plant Sci. 49:103–109.
- Hansen SL. 2001. Content of free amino acid in onion (Allium cepa L.) as influenced by the stage of development at the harvest and long-term storage. Acta Agric Scand Sect B Soil and Plant Sci. 51:77–83.
- Hirota S, Shimoda T, Takahama U. 1998. Tissue and spatial distribution of flavonol and peroxidase in onion bulbs and stability of flavonol glucosides during boiling of the scales. J Agric Food Chem. 46:3497–3502.10.1021/jf980294w
- Hurst WC, Shewfelt RL, Schuler GA. 1985. Shelf-life and quality changes in summer storage onions (Allium cepa). J Food Sci. 50:761–763.10.1111/j.1365-2621.1985.tb13791.x
- Kaack K, Christensen LP, Hansen SL, Grevsen K. 2004. Non-structural carbohydrates in processed soft fried onion (Allium cepa L.). Eur Food Res Technol. 218:372–379.10.1007/s00217-003-0869-y
- Kuon J, Bernhard RA. 1963. An examination of the free amino acids of the common onion (Allium cepa). J Food Sci. 28:298–304.10.1111/j.1365-2621.1963.tb00201.x
- Lee J, Mitchell AE. 2011. Quercetin and isorhamnetin glycosides in onion (Allium cepa L.): varietal comparison, physical distribution, coproduct evaluation, and long-term storage stability. J Agric Food Chem. 59:857–863.10.1021/jf1033587
- Miedema P. 1994. Bulb dormancy in onion. I. The effects of temperature and cultivar on sprouting and rooting. J Hortic Sci. 69:29–39.
- Mogren LM, Olsson ME, Gertsson UE. 2006. Quercetin content in field-cured onions (Allium cepa L.): effects of cultivar, lifting time, and nitrogen fertilizer level. J Agric Food Chem. 54:6185–6191.10.1021/jf060980s
- Mogren LM, Olsson ME, Gertsson UE. 2007a. Effects of cultivar, lifting time and nitrogen fertiliser level on quercetin content in onion (Allium cepa L.) at lifting. J Sci Food Agric. 87:470–476.10.1002/jsfa.2735
- Mogren LM, Olsson ME, Gertsson UE. 2007b. Quercetin content in stored onions (Allium cepa L.): effects of storage conditions, cultivar, lifting time and nitrogen fertiliser level. J Sci Food Agric. 87:1595–1602.10.1002/jsfa.2904
- Nam E, Cho DY, Lee ET, Kim CW, Han T, Yoon MK, Kim S. 2011. Bulb storability of red and yellow onion (Allium cepa L.) cultivars grown in Korea. Kor J Breed Sci. 43:126–132.
- Pak C, Van der Plas LHW, De Boer AD. 1995. Importance of dormancy and sink strength in sprouting of onions (Allium cepa) during storage. Physiol Plant. 94:277–283.
- Patil BS, Pike LM, Hamilton BK. 1995b. Changes in quercetine concentration in onion (Allium cepa L.) owing to location, growth stage and soil type. New Phytol. 130:349–355.10.1111/j.1469-8137.1995.tb01829.x
- Patil BS, Pike LM, Yoo KS. 1995a. Variation in the quercetin content in different colored onions (Allium cepa L.). J Amer Soc Hort Sci. 120:909–913.
- Price KR, Bacon JR, Rhodes MJC. 1997. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa). J Agric Food Chem. 45:938–942.10.1021/jf9605916
- Rhodes D, Deal L, Haworth P, Jamieson GC, Reuter CC, Ericson MC. 1986. Amino acid metabolism of Lemna minor L. Plant Physiol. 82:1057–1062.10.1104/pp.82.4.1057
- Rodríguez Galdón B, Peña-Méndez E, Havel J, Rodríguez Rodríguez EM, Romero CD. 2010. Cluster analysis and artificial neural networks multivariate classification of onion varieties. J Agric Food Chem. 58:11435–11440.
- Rutherford PP, Whittle R. 1982. The carbohydrate composition of onions during long term cold storage. J Hortic Sci. 57:349–356.
- Salamal AM, Hicks JR, Nock JF. 1990. Sugar and organic acid changes in stored onion bulbs treated with maleic hydrazide. Hortscience. 25:1625–1628.
- Takahama U, Hirota S. 2000. Deglucosidation of quercetin glucosides to the aglycone and formation of antifungal agents by peroxidase-dependent oxidation of quercetin on browning of onion scales. Plant Cell Physiol. 41:1021–1029.10.1093/pcp/pcd025
- Thamizharasi V, Narasimham P. 1991. Water vapour sorption and transmission by onion (Allium cepa L.) scale under different temperature and humidity conditions. Sci Hortic. 46:185–194.10.1016/0304-4238(91)90041-V
- Yasin HJ, Bufler G. 2007. Dormancy and sprouting in onion (Allium cepa L.) bulbs. I. Changes in carbohydrate metabolism. J Hort Sci Biotech. 82:89–96.
