Abstract
Three sweet potato cultivars (A40, A45 and 199062.1) were planted in three small-scale farms located under different agro-ecological zones of KwaZulu-Natal. The objective was to assess growth, physiological responses and yield of the sweet potato cultivars under low-input agricultural system and different environmental conditions. Sweet potato planted at Richards Bay (28°19'S; 32°06E), a coastal sandy soil location, recorded low stomatal conductance(SC; 102.2 m moles m−2 s−1) and chlorophyll content index (CCI; 29.4). This consequently resulted in reduced vine length, leaf number and branching of sweet potato plants. Environmental conditions in that location (Richards Bay) such as high evapotranspiration, high temperatures and low water retention capacity of sandy soils created drought stress condition. This caused reduction in photosynthetic activities and translocation to the harvestable plant parts. The other two locations (Deepdale at 28°01'S; 28°99'E and Umbumbulu at 29°98'S; 30°70'E) located further from the coast and characterized by clayey soils recorded higher SC and CCI. Branching and number of leaves were significantly influenced by locations and growing season while vine length varied with locations, indicating specific varietal adaptation. Biomass and storage root yield followed a similar trend as plant growth and physiology. Richards Bay recorded very low biomass and storage root yield (5.4 and 5.0 t ha−1) in both seasons while Deepdale recorded higher yields (42.0 t ha−1) during the first growing season. Yields reduced by 67% (13.6 t ha−1) in the second season. Storage root yields from Umbumbulu were stable in both growing seasons (29.4 and 28 t ha−1 during seasons one and two, respectively). Adding fertilizer only improved storage roots yield in Richards Bay, otherwise cultural practises were responsible for storage root yield increases in Deepdale and Umbumbulu. Orange-fleshed sweet potato cultivar A45 showed good environmental plasticity while cultivar 199062.1 responded well to fertilizer application. This indicated its suitability for use in food security programmes under low-input agriculture.
Introduction
African agriculture is characterized by frequent natural disasters such as droughts and floods while a large percentage of its population depend heavily on rain-fed agriculture. This is an indication of severe climate change, which has a negative impact on the economic, environmental and social status of the natives. Climate change disrupts the production of staple crops as it alters the normal growth environment thus leaving communities vulnerable to hunger and other human nutrition–related ailments. Rural communities are the most vulnerable to the negative impacts of climate change since they are resource constrained and practice rain-fed agriculture on marginal land (Hassan Citation2006). Conventional staple crops such as maize are currently struggling to survive under the current climatic conditions, creating a need for farmers to switch to other traditional starch crops such as sweet potato that are adapted to stress conditions.
In Sub-Saharan Africa, sweet potato is customarily grown by small-scale farmers for household consumption. Its cultivation is predominantly done in small plots by resource-constrained women farmers (CIP Citation1985; Ebregt et al. Citation2007; Agili et al. Citation2012) for food security, given their relatively high caloric and vitamin A content, especially the orange-fleshed varieties (Laurie et al. Citation2012). Sweet potato has a wide ecological adaptation and is drought tolerant with a short maturity period of three to five months (Agili et al. Citation2012; Laurie et al. Citation2012; Iheagwara Citation2013). It can be harvested piecemeal thus ensuring continuous food security throughout the season. The orange-fleshed varieties are very tasty and attractive to children (Kaguongo et al. Citation2012) and hence have the prospective of addressing vitamin A deficiencies of children (Laurie & Van Heerden Citation2012; Laurie et al. Citation2012). Commonly referred to as the ‘poor man's crop’, sweet potato has previously, received scant research attention. However, given the growing demand for food security crops in Sub-Saharan Africa, especially in the wake of climate change, the research bias has since shifted in favour of crops such as sweet potato (Agili et al. Citation2012).
Sweet potato can be produced with low-input requirements even on marginal soils and under adverse weather conditions (Oggema et al. Citation2007; Agili et al. Citation2012; Iheagwara Citation2013). It fits well into low-input agricultural system mainly practiced by small-scale farmers. The fact that it performs relatively well even under limited technologies (Laurie & Van Heerden Citation2012; Laurie et al. Citation2012) indicates a potential to increase productivity on small-scale farms in general. Efforts to breed sweet potato varieties suitable for small-scale conditions have been explored previously by the International Potato Centre (CIP) (see CIP Citation1985). South African institutions are also involved in this campaign and a number of their varieties are distributed mainly in the Southern African region (Laurie et al. Citation2004). Although some of these varieties were bred for resource-constrained farmers in South Africa (Laurie & Van Den Berg Citation2002), very few of them meet the ‘resource-constrained’ standards of production. Given the conditions under which a large proportion of small-scale farmers produce in Sub-Saharan Africa, it is not surprising that sweet potato has had a very low adoption rate among resource-constrained farmers. Therefore, in attempting to use sweet potato as a strategic crop to fight against food insecurity, especially in drought-prone areas, there is a need for Crop Scientists (in particular crop breeders, agronomists and plant pathologists) to introduce sweet potato varieties that are suitable for resource-constrained and small-scale production conditions.
A confirmation of whether the new varieties are suitable for wide adaptation under small-scale production is vital; thus, it would be imperative to explore sweet potato's wide ecological adaptation attribute. The province of KwaZulu-Natal (KZN) provides a diverse environment for sweet potato production. Its location in the eastern part of South Africa presents a climate ranging from extremely hot summers along the coast to heavy snow in the mountains in winter. It is a ‘world-in-one’ (Joubert Citation2012) with diverse soils and topography. The midlands are drier than the coast and can be very cold in winter (The Climate Group Citation2004; SouthAfrica.info Citation2012). Sweet potato production in this province is still at low levels, especially by small-scale farmers. A study by Kirsten et al. (Citation1998) reported that only 9.8% of the sample population were producing sweet potato at household levels in KZN. The low sweet potato production levels in the province could be attributed to the lack of awareness or lack of information regarding the potential benefits of the crop. Perhaps farmers regard sweet potato production as an unprofitable activity in their different agro-ecological conditions given the complex set of constrains they face in allocating land and labour resources across farm and off-farm activities (Doss Citation2003). It is also possible that their preference favours other crops other than sweet potato and thus could not adopt its production (Wale & Yallew Citation2007). Farmers were also reported to be less certain about the productivity of a new technology (variety) when using it for the first time (Kaguongo et al. Citation2012). To ensure the adoption of new sweet potato varieties, small-scale farmers should be involved from the grass-roots level. A study on adoption of orange-fleshed sweet potato varieties in Mozambique revealed that the intensity of extension services, availability of vines and community-level education programmes played a vital role (Mazuze Citation2005). It is envisaged, therefore, that introducing new sweet potato varieties at grass-roots level will have a positive adoption influence, increase adoption intensity and ultimately increase its production levels. Small-scale farmers will increase productivity in relation to yields achieved per unit area, improve food security both in terms of energy and micronutrient supplementation, increase affordability of other food crops and other utilities since farmers will be in a position to barter or sell the surplus and earn income. Additionally, farmers will readily accept and have a sense of ownership of these sweet potato cultivars thus ensuring continuous production of the crop.
This paper demonstrates the importance of bridging the gap between local breeding efforts in South Africa and the small-scale farmers or intended beneficiaries thus forging a relationship between these stakeholders with the aim of improving food security. Reports on evaluation of new sweet potato varieties in South Africa and other parts of Africa indicated that particular emphasis was mainly on the agronomy and storage root yield of the crop (Kulembeka et al. Citation2004; Shigwedha et al. Citation2004; Oggema et al. Citation2007; Laurie & Magoro Citation2008; Osiru et al. Citation2009). No empirical evidence has been availed relating to sweet potato's physiological responses to environmental factors and their effect on growth and yield in the different bio-resource groups of the KZN province. Moreover, there are very few reports of sweet potato evaluations done under resource-constrained, small-scale farmers’ conditions in South Africa (e.g. Laurie & Magoro Citation2008). Information about incorporation of farmers’ knowledge in the production of the crop is necessary. Most of the released sweet potato varieties have been evaluated on research farms (Laurie et al. Citation2004; Njoku et al. Citation2007; Egbe & Idoko Citation2009; Uwah et al. Citation2013) where the growth environment is supplemented with irrigation, fertilizers and pest and disease control. This article not only reports on the evaluation of locally bred sweet potato cultivars grown on resource-constrained small-scale farms but also provides information on the plant's physiological responses to growth environment and how it (environment) affects plant growth and yield of sweet potato. The objective of the study was, therefore, to assess growth, physiological responses and yield of locally bred sweet potato cultivars grown under low-input agricultural systems at different agro-ecological locations of KZN.
Materials and methods
Planting material
Three sweet potato cultivars (A40, A45 and 199062.1) were sourced from the University of KwaZulu-Natal's (UKZN) Plant Breeding Department. Two of them (A40 and A45) were bred at UKZN while the third (199062.1) was obtained from the International Potato Centre (CIP). Two of the sweet potato cultivars (A45 and 199062.1) are orange-fleshed, while the third (A40) is white-fleshed. Planting vines (30 cm long) were cut from the tip of mother plants, which were raised in a typical warm subtropical nursery (~18/33°C day/night temperatures and 60–80% relative humidity) (Modi Citation2007) at UKZN. Planting vines were defoliated to one top fully expanded leaf to reduce photosynthetic demand at crop establishment stage. Ridges (~30 cm high) were used for planting and a minimum of three vine nodes were inserted into the ridge at planting.
Site descriptions
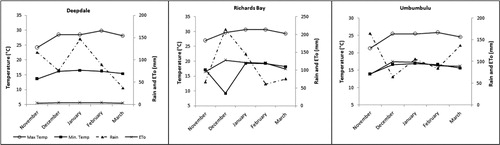
The study was carried out in three small-scale farms located in three different locations (Deepdale, Umbumbulu and Richards Bay) of KwaZulu-Natal, South Africa. These three locations were representatives of three distinct agro-ecologies (). Planting dates were both in November for summer seasons of 2012/13 (first growing season) and 2013/14 (second growing season).
Table 1. Experimental site description for Deepdale, Richards Bay and Umbumbulu.
Experimental design and agronomic practices
The experimental design was a randomized complete block design replicated three times. Two factors were considered – location and sweet potato cultivar. Experimental layout was similar across all three locations. The total plot area was 105 m2 with an individual plot size of 6 m2. Plant spacing was 1 m × 0.3 m and planting was done on ridges. Land preparation in Deepdale and Umbumbulu was initially done using a tractor-mounted mouldboard plough (one pass), after which ridges were prepared by hand in each plot, while in Richards Bay it was done using hand hoes. Local farmers provided assistance with guidance on how to plant sweet potato in their specific locations where the crop is normally planted on land that had been fallow for at least a year ().
For both seasons, soil samples were taken prior to planting to analyse for fertility and textural characteristics. According to the small-scale famers’ local knowledge, no fertilizer, pesticides or supplementary irrigation was required for the duration of crop growth at all experimental sites. A fertilized trial was only planted adjacent to the experimental plots during the second (2013/14) planting season for comparison purposes. Details of weather parameters (maximum and minimum temperatures, reference evapotranspiration [ETo] and rainfall) were obtained from the Agricultural Research Council – Institute for Soil, Climate and Water's network of automatic weather stations.
Data collection
In all the three sites, plants were allowed one month for establishment before data collection commenced. Plant growth and physiology data collected included vine length, leaf and branch number, stomatal conductance (SC) and chlorophyll content index (CCI). SC was measured using a steady-state leaf porometer (Model SC-1, Decagon Devices, USA) and CCI was measured using the CCM200 Plus chlorophyll meter (Opti-Sciences, USA). Measurements of SC and CCI were, respectively, taken from the abaxial and adaxial surfaces of the third youngest, fully expanded and fully exposed leaf.
Sweet potato plants were harvested at four months after planting. Using samples of four plants per plot, fresh mass of whole plant and below-ground biomass were measured; dry mass of the same was also measured to calculate harvest index (HI). Mass of marketable storage roots was recorded to compute total yield. Considered marketable storage roots were whole (undamaged) and weighed between 0.1 and 1.4 kg and without harvest wounds, pest and disease damage (Ossom and Rhykerd Citation2007).
Statistical analyses
Data were subjected to analysis of variance using GenStat® version 14 (VSN International, Hemel Hempstead, UK, 2011). Tukey's test was used to separate means at 5% level of significance.
Results
CCI and SC
Environmental conditions had a significant effect on plant ecophysiology. Measurements of SC were only taken during the 2012/13 growing season. Results of SC were strongly influenced (P ≤ .001) by locations (). Deepdale recorded significantly higher SC followed by Umbumbulu, then Richards Bay. The juvenile stage of the crop was characterized by high SC for all sweet potato cultivars at Deepdale. SC later decreased as the number of leaves and branches started to increase in the plants (vegetative growth stage). This period coincided with increased demand for water by the crop as there were more leaves transpiring, thus the decline in SC over time. At the other two locations (Umbumbulu and Richards Bay), SC was initially low and remained relatively stable throughout the growing season. Soil water deficit (ETo outstripped rainfall) was also high ( and ) in the different locations and SC is very sensitive to such conditions. Significant differences between sweet potato cultivars were also observed where line A40 recorded significantly higher (P ≤ .05) SC values than A45. Sweet potato cultivar 199062.1 was not significantly different from the two ().


CCI was also affected (P ≤ .001) by locations and cultivars. Significant interactions (P ≤ .05) were observed between locations and cultivars and between locations and seasons. Umbumbulu recorded the highest CCI followed by Deepdale then Richards Bay (). Sweet potato cultivar 199062.1 had significantly higher (P ≤ .001) CCI than A40 and A45 (). The high CCI recorded for sweet potato cultivar 199062.1 did not, however, translate to higher storage root yield as expected ( and ).
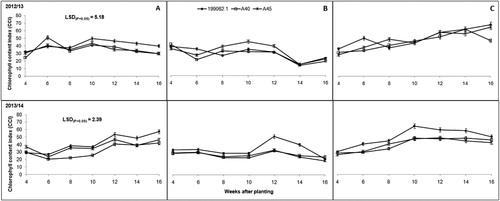
Table 2. Yield components of cultivars grown in three different locations of KwaZulu-Natal, South Africa, during 2012/13.
Table 3. Yield components of cultivars grown in three different locations of KwaZulu-Natal, South Africa, during the second growing season.
Plant growth
Plant branching and number of leaves were significantly (P ≤ .001) influenced by location, season and the interaction of the two (P ≤ .05). Vine length on the other hand varied significantly (P ≤ .001) across locations, among sweet potato cultivars and over the two seasons. The interaction of the latter and former was also significant (P ≤ .05). Cultivar 199062.1 was shorter than both A40 and A45 () mainly because it is a non-twining, semi-erect cultivar. The number of leaves however, was statistically similar across all sweet potato cultivars (). Growth parameters tended to increase with plant growth, with the exception of leaves, which later decreased due to leaf senescence and abscission as plants started ageing.
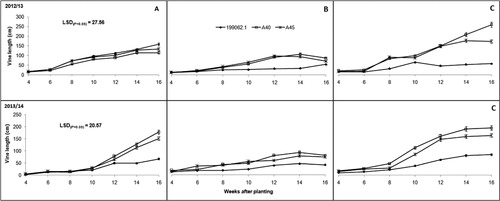
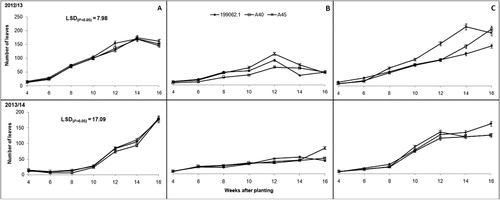
When comparing locations, Deepdale and Umbumbulu were more conducive for sweet potato growth (leaves, branches and vine length). Soil nutritional status in these two locations was also better than Richards Bay (). Plants grown in Richards Bay recorded poorest growth (significantly (P ≤ .05) shorter vines, fewer branches and low number of leaves) across all three cultivars when compared to the other locations. The first growing season showed superior plant growth when compared to the second growing season (–).
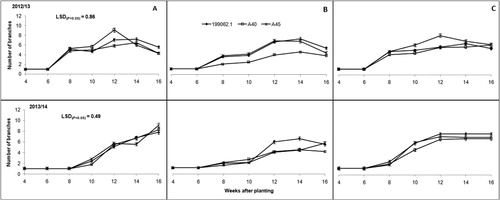
Table 4. Soil analysis results from the three experimental locations.
Biomass and storage root yield
Locations had a highly significant (P ≤ .001) effect on biomass and storage root yield. Seasons had a highly significant (P ≤ .001) effect on storage root yield. Umbumbulu and Deepdale showed highly significant (P ≤ .001) differences for both yield parameters when compared to Richards Bay ( and ). This was directly linked to the observed plant growth and physiological responses in these locations. No significant differences in biomass and storage root yield were observed among sweet potato cultivars ( and ). Correlations between biomass and storage roots yield showed that biomass contributed (r = .781; P ≤ .05) significantly to storage root yield. The first growing season recorded significantly higher (P ≤ .05) storage root yield than the second growing season at Deepdale. There were no significant differences in storage root yields from Richards Bay during both growing seasons. Umbumbulu storage root yield was constant in both growing seasons.
Deepdale recorded the highest storage root yield (42.0 t ha−1) during the first growing season and lower yield (13.6 t ha−1) during the second growing season. Yield reduction during the second season was 67%. Storage root yield from Umbumbulu and Richards Bay did not fluctuate that much during both seasons. Yield from Umbumbulu was 29.4 and 28.0 t ha−1 while it was 5.4 and 5.0 t ha−1 in Richards Bay during the first and second growing seasons, respectively. A greater percentage of storage roots from Richards Bay weighed less than 0.1 kg and thus were classified as non-marketable. Comparison of fertilized and non-fertilized sweet potato trials conducted during the second growing season () only showed significantly higher (P < .05) storage root yield (19.6 t ha−1) from fertilized trials at Richards Bay. Applying fertilizer increased storage roots yield by 76% in Richards Bay when compared to the non-fertilized crop. Even so, the yield was still lower than non-fertilized crops at Umbumbulu during that season. Fertilizer application at Deepdale and Umbumbulu did not increase storage root yield; in fact, the yield was lower than the non-fertilized crop.
Table 5. Fertilized and non-fertilized storage root yield from three different locations of KwaZulu-Natal during the second growing season
HI was significantly (P ≤ .05) affected by growing seasons, locations and cultivars. The first growing season recorded higher (P ≤ .05) HI than the second growing season. While all locations recorded no significant differences during the first growing season, Deepdale recorded the lowest (28.5%) HI during the second growing season. Umbumbulu and Richards Bay recorded similar HI values (52.0% and 43.0%, respectively) during the same season. Cultivar 199062.1 recorded a significantly higher (P ≤ .05) HI than the other two cultivars.
Discussion
Soil water deficit in Richards Bay may have forced the crop to adjust by closing its stomata thus reducing SC (), CCI (), growth parameters (–) and consequently storage root yield ( and ). The water deficit was probably caused by poor water-retaining capacity of the sandy soil (Zhao et al. Citation2016) in Richards Bay (). Such soils are more porous and characterized by very low field capacity and permanent wilting point (). Crops grown in such soils can easily experience periods of water deficit even if there has been significant rainfall as much of the water is drained away from the root zone. Moreover, stomatal responses are closely linked to soil water content than to leaf water status (Chaves et al. Citation2002). As a drought avoidance mechanism, plants close stomata to limit water loss through transpiration and also reduce intracellular carbon dioxide (CO2) availability (Mabhaudhi et al. Citation2013; Zhao et al. Citation2014). This behaviour affects the photosynthetic rate since CO2 is the chief substrate; hence, resulting in biomass reduction (Lawlor Citation2002; Blum Citation2009). This means that cultivar A45 is more sensitive to water/drought stress since it readily closes its stomata to reduce water loss through transpiration and limit CO2 uptake.
Decreases in chlorophyll content under drought stress conditions have been associated with oxidative stress and may be the result of pigment photo-oxidation and chlorophyll degradation (Jaleel et al. Citation2009; Anjum et al. Citation2011). The fact that SC and CCI were low in Richards Bay ( and ) implies that photosynthesis was both substrate and energy limited. Consequently, leaves showed rapid signs of chlorosis and later abscission. In the other locations, photosynthesis was not limited by neither chlorophyll degradation nor water deficit (the misty conditions characteristic of the agro-ecological zones where Umbumbulu and Deepdale are located may have reduced the length of exposure to water deficit) and CO2 flow. This implied that there was sufficient soil water to meet plant growth and evaporative demands ( and ). The clayey type of soils () in these locations may have enhanced soil water content since clay soils have good water retention capacity. Cultivar A40 recorded low CCI but high SC values during this season, suggesting that it might not respond to water stress by closing stomata to control water loss and limit CO2. Instead, it responded by chlorophyll degradation and photo-oxidation.
Plant growth parameters CCI and SC tended to have a link and tended to follow a particular trend. Low values of CCI and SC resulted in short vines and fewer leaves and branches (–). This was another indication of drought stress and plant coping mechanisms as explained by Chaves et al. (Citation2002) and Jaleel et al. (Citation2009). Vine length was influenced by both cultivar growth habit within a location and environmental variations across the three locations. This observation was contrary to reports by Oggema et al. (Citation2007) that growth habits of sweet potato plants were more related to varietal than environmental differences. Interesting observations were also made on the growth parameters. These cultivars tended to limit their vine extension, number of leaves and branching under harsh environmental conditions like Richards Bay (–). Reduced plant size (height/length, leaf area and leaf area index) was reported by Blum (Citation2005) as the major mechanism for moderating water use and reducing injury under drought stress conditions. These results, therefore, indicated that Richards Bay was characterized by water stress. Our observations of rainfall and ETo ( and ) did not concur with this trend. It is therefore suspected that the prevalent sandy soils and high temperatures in Richards Bay may have accelerated soil water loss through ETo and rapid infiltration in the sandy soils since it (soil) had very low clay content (<5%). It was further noted that ETo alone could not have caused such a high impact on soil water loss since it was relatively similar ( and ) across the locations in both seasons (with the exception of Deepdale during the first growing season).
The tendency of sweet potato cultivars to branch in one location and not branch in another but prefer to extend in length or increase leaf number can be attributed to specific environmental adaptation. This study observed that the three sweet potato cultivars exhibited very poor growth in Richards Bay, a coastal area with low elevation (30 m above sea level [a.s.l.]) and classified as a ‘Moist coast forest, thorn and palm-veld’ () (Smith Citation2006). Growth improved as altitude increased and the best plant growth was recorded in the mid-elevation location (Umbumbulu, 632 m a.s.l.) classified as a ‘Moist coast hinterland and ngongoni veld’. Vine growth decline in lowland soils with low fertility was reported by Hartemink et al. (Citation2000). The sweet potato cultivar A45 showed the greatest environmental plasticity with regards to showing wide adaptation to all three environments. It was able to grow, branch and produce leaves and higher yields across all locations. This study proves the concept of wide adaptation, indicating that adaptation has more geographical than environmental meaning and it reduces genetic diversity while increasing genetic vulnerability (Ceccarelli Citation1994).
Richards Bay's poor soil physical and chemical properties () and poor water-holding capacity renders crops grown on such soils prone to frequent episodes of water stress. To curb the situation, farmers planted on ridges and added compost at planting. The two agronomic practices were expected to conserve soil water (Everson et al. Citation2011) but no evidence of improved soil water conservation was observed. Instead, plants developed more fibrous roots to help scavenge for water and nutrients at the expense of developing storage roots. Under such stressful environments, even high-yielding cultivars will produce low yields (Blum Citation2005).
Soil available potassium (K) was higher at Deepdale () during the first growing season and was sufficient to meet the large demand for K reported by George et al. (Citation2002). This may explain the high storage root yield recorded from this location during that season. According to Bourke (Citation1985), storage K influences root yield by increasing dry matter allocation to the storage roots. The drastic reduction (of 67%) in storage root yield during the second growing season may have been caused by lower temperatures (minimum temperatures) experienced during planting and establishment period (November), which consequently delayed plant establishment and vegetative growth. The delayed plant growth stages further affected ‘storage roots filling’ such that harvesting (after 120 days) was conducted before the storage roots could reach maximum expansion. Dry matter allocation to the storage roots at Umbumbulu was not limited by K levels during both seasons. The constantly warm temperatures during planting period enhanced crop establishment and vegetative growth such that ‘root filling’ started on time thus allowing the crop to give higher yields (after 120 days). This observation emphasizes the vital role played by prevailing temperatures in sweet potato crop establishment and storage root development (DAFF Citation2011). According to Lebot (Citation2009), temperatures of 15°C or 35°C inhibit storage root development, which was the case at Deepdale.
Fertilizer application during 2013/14 growing season did not have an effect on storage root yield in the already fertile soils (at Umbumbulu and Deepdale) (). According to Mukhtar et al. (Citation2010), non-significant response of sweet potato to fertilizer application should be blamed on the innate quality of sweet potato and the fact that it colonizes easily on marginal soils. Cultural practices such as fallowing may have contributed to improved yields at Deepdale and Umbumbulu. This is an indication that small-scale farmers understand and appreciate the principles of low-input cropping systems. Ridging also contributed to good yields since it conserves soil water (Everson et al. Citation2011) and allows for root expansion. The yield achieved from this study creates a scenario where small-scale farmers would continue to ignore fertilizer recommendations under low-input cropping systems if they can still get the best yield and improved food security just from applying their cost-effective (Dawson et al. Citation2007) cultural practices alone. Their only concern would be the inability to produce sweet potato throughout the year since the crop failed completely under low temperature conditions. This means that there would be episodes of food insecurity during the year, but this can be avoided by applying simple sweet potato preservation techniques such as flour processing and crisping. Furthermore, they can sell the surplus and use the income generated from the sales to buy other food crops that have a long shelf-life.
From the observations of this study, it can be concluded that environmental conditions can suppress sweet potato varietal growth habits by determining plant growth patterns thus affecting subsequent yield. Environmental conditions such as high temperatures and ETo coupled with low water retention capacity of sandy soils creates a drought stress scenario which results in the plants responding by lowering SC and CCI thus decreasing photosynthetic rates and assimilation. Consequently, the number of leaves which are the main source of assimilates utilized for plant growth and increasing storage root dry matter allocation are decreased resulting in low yields.
Sweet potato cultivars selected for the study performed well under low-input cropping system in Deepdale and Umbumbulu. This suggests that the three sweet potato cultivars can be produced using cost-effective agricultural methods, a trait desirable to resource-constrained small-scale farmers and can improve food security status of these farmers. Application of good cultural and agronomic practices such as fallowing and ridging contributed to the increased yields. Ridging conserved water in the soil and allowing enough room for storage root expansion while fallowing improved the soil nutrient status.
Fertilizer application did not improve storage roots yield when applied to naturally fertile soils. The innate quality and ability of sweet potato to colonize easily on marginal soils encourages its (fertilizer) application only in continuously cropped and exhausted soils.
Sweet potato cultivar A45, an orange-fleshed variety, showed more environmental plasticity than the other sweet potato cultivars (A40 and 199062.1). This indicates that its adoption can contribute towards improved food and nutrition security for resource-constrained and small-scale farmers since it can also supplement for vitamin A deficiencies.
Acknowledgements
This research was funded by the University of KwaZulu-Natal in partnership with the Swedish International Development Cooperation Agency (Sida) and the Organization for Women in Science from the Developing World (OWSD).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Agili S, Nyende B, Ngamau K, Masinde P. 2012. Selection, yield, drought tolerance indices of orange-fleshed sweet potato (Ipomoea batatas Lam) hybrid clone. J Nutr Food Sci. 2:1–8.
- Anjum SA, Xie X, Wang L, Saleem MF, Man C, Lei W. 2011. Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res. 6:2026–2032.
- Blum A. 2005. Drought resistance, water-use efficiency and yield potential – are they compatible, dissonant, or mutually exclusive? Austr J Agric Res. 56:1159–1168.
- Blum A. 2009. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 112:119–123. 10.1016/j.fcr.2009.03.009
- Bourke RM. 1985. Influence of nitrogen and potassium fertilizer on growth of sweet potato (Ipomoea batatas) in Papua New Guinea. Field Crops Res. 12:363–375. 10.1016/0378-4290(85)90081-4
- Ceccarelli S. 1994. Specific adaptation and breeding for marginal conditions. Euphytica. 77:205–219. 10.1007/BF02262633
- Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C. 2002. How plants cope with water stress in the field: photosynthesis and growth. Ann Bot. 89:907–916. 10.1093/aob/mcf105
- CIP. 1985. Sweet potato in Sub Saharan Africa; [cited 2013 Oct 3.]. Available from: www.cipotatoorg/research/sweetpotato-in-africa
- DAFF (Department of Agriculture, Forest and Fisheries) South Africa. 2011. Sweet potato (Ipomoea batatas L.) production guide. Pretoria: Department of Agriculture, Forest and Fisheries, Plant Production Section; p. 1–20.
- Dawson JC, Murphy KM, Jones SS. 2007. Decentralized selection and participatory approaches in plant breeding for low-input systems. Euphytica. 122:143–154.
- Doss CR. 2003. Understanding farm level technology adoption: lessons learned from CIMMYT's micro survey in eastern Africa. Apartado: CIMMYT. Available from: www.cimmyt.org
- Ebregt E, Struik PC, Odongo B, Abidin PE. 2007. Piecemeal versus one time harvesting of sweet potato in north-eastern Uganda with special reference to pest damage. J Life Sci. 55:75–92.
- Egbe OM, Idoko JA. 2009. Agronomic assessment of some sweet potato varieties for intercropping with pigeon pea in Southern Guinea Savanna of Nigeria. ARPN J Agric Biol Sci. 4:23–32.
- Everson C, Everson TM, Modi AT, Csiwila D, Fanadzo M, Naiken V, Auerbach RMB, Moodle M, Mtshali S, Dladla R. 2011. Sustainable techniques and practices for water harvesting and conservation and their effective application in resource-poor agricultural production through participatory adaptive research. Report No. 1465/1/11. Pretoria: Water Research Commission; p. 47–88.
- George MS, Lu GQ, Zhou WJ. 2002. Genotypic variations for potassium uptake and utilization efficiency in sweet potato (Ipomoea batatas L.). Field Crops Res. 77:7–15. 10.1016/S0378-4290(02)00043-6
- Hartemink AE, Poloma S, Maino M, Powell KS, Egenae J, O'Sullivan JN. 2000. Yield decline of sweet potato in the humid lowlands of Papua New Guinea. Agric Ecosyst Environ. 79:259–269. 10.1016/S0167-8809(00)00139-0
- Hassan R. 2006. Climate change and African Agriculture. Policy No. 28. Assessing the impact of climate change on crop water use in South Africa. CEEPA discussion paper no. 28. CEEPA, University of Pretoria, Pretoria.
- Iheagwara MC. 2013. Isolation, modification and characterization of sweet potato (Ipomoea batatas L (Lam)) starch. J Food Process Technol. 4:1–6.
- Jaleel CA, Manivannan P, Wahid A, Farooq M, Al-Juburi HJ, Somasundaram R, Panneerselvam R. 2009. Drought stress in plants: a review on morphological characteristics and pigment composition. Int J Agric Biol. 11:100–105.
- Joubert R. 2012 Mar. Huge projects promised for KwaZulu-Natal. In: Van der Walt A, editor. Farmers Weekly Magazine; [cited 2013 Aug 9]. Available from: www.farmersweekly.co.za.
- Kaguongo W, Ortmann G, Wale E, Darroch M, Low J. 2012. Factors influencing adoption and intensity of adoption of orange-fleshed sweet potato varieties: evidence from an extension intervention in Nyanza and Western provinces, Kenya. Afr J Agric Res. 7:493–503.
- Kirsten J, Townsend R, Gibson C. 1998. Determining the contribution of agricultural production to household nutritional status in KwaZulu-Natal, South Africa. Develop South Afr. 15:573–587. 10.1080/03768359808440032
- Kulembeka HP, Rugutu CK, Kanju E, Chirimi B, Rwiza E, Amour R. 2004. The agronomic performance and acceptability of orange fleshed sweet potato varieties in the lake zone of Tanzania. Afr Crop Sci J. 12:229–240.
- Laurie SM, Magoro MD. 2008. Evaluation and release of new sweet potato varieties through farmer participatory selection. Afr J Agric Res. 10:672–676.
- Laurie SM, Van Den Berg AA, Magoro MD, Kgonyane MC. 2004. Breeding of sweet potato and evaluation of imported cultivars in South Africa. Afr Crop Sci J. 12:189–196.
- Laurie SM, Van Heerden SM. 2012. Consumer acceptability of four products made from beta-carotene-rich sweet potato. Afr J Food Sci. 6:96–103. 10.5897/AJFS12.014
- Laurie SM, Van Den Berg AA. 2002. A review of recent progress in breeding sweet potato in South Africa for resource-poor farmers. In: Nakatani N, Komaki K, editors. 12th Symposium for International Society for Tropical Root Crops (ISTRC); September 10–16; Tsukuba. Ibaraki: Cultio Corporation; p. 216–219.
- Laurie SM, Van Jaarsveldp J, Faber M, Philpott MF, Labuschegne ST. 2012. Trans-ß-carotene, selected mineral content and potential nutritional contribution of 12 sweetpotato varieties. J Food Comp Anal. 27:151–159.
- Lawlor DW. 2002. Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. Ann Bot. 89:871–885. 10.1093/aob/mcf110
- Lebot V. 2009. Tropical root and tuber crops; Cassava, Sweet potato, Yams and Aroids. Cambridge: CABI North American Office.
- Mabhaudhi T, Modi AT, Beletse YG. 2013. Growth, phonological and yield response of bambara groundnut (Vigna subterranean L. Verdc) landrace to imposed water stress: II. Rain shelter conditions. Water South Afr. 39:191–198.
- Mazuze FM. 2005. Analysis of adoption and production of orange-fleshed sweet potatoes: the case study of Gaza province in Mozambique [ thesis]. East Lansing (MI): Michigan State University.
- Modi AT. 2007. Growth temperature and plant age influence on nutritional quality of Amaranthus leaves and seed germination capacity. Water South Afr. 33:369–371.
- Mukhtar AA, Tanimu B, Arunah UL, Babaji BA. 2010. Evaluation of the agronomic characters of sweet potato varieties grown at varying levels of organic and inorganic fertilizer. World J Agric Sci. 6:370–373.
- Njoku SC, Muoneke CO, Okpara DA, Agbo FMO. 2007. Effect of intercropping of sweet potato and okra in an ultisol of south-eastern Nigeria. Afr J Biotechnol. 6:1650–1654.
- Oggema JN, Kinyua MG, Ouma JP, Owuoche JO. 2007. Agronomic performance of locally adapted sweet potato (Ipomoea batatas (L) Lam) cultivars derived from tissue culture regenerated plants. Afr J Biotechnol. 6:1418–1425.
- Osiru MO, Olanya OM, Adipala E, Kapinga R, Lemaga B. 2009. Yield stability analysis of Ipomoeabatatas L. Cultivars in diverse environments. Aust J Crop Sci. 3:213–220.
- Ossom EM, Rhykerd RL. 2007. Effect of lime on weed species diversity and yield of sweet potato (Ipomoea batatas (L.) Lam) in Swaziland. Int J Agric Biol. 9:755–758.
- Smith B. 2006. The farming handbook. Wageningen, Technical Centre for Agriculture and Rural Cooperation (CTA).
- Shigwedha NM, Braun BR, Laurie SM. 2004. Performance of sweet potato varieties in evaluation trials in northern Namibia. Afr Crop Sci. 12:223–228.
- SouthAfrica.info. 2012. KwaZulu-Natal province South Africa; [cited 2013 Aug 9]. Available from: www.southafrica.info
- The Climate Group. 2004. The province of KwaZulu-Natal; [cited 2013 Aug 9]. www.theclimategroup.org.
- Uwah DF, Udie UL, John NM, Ukoha GO. 2013. Growth and yield response of improved sweet potato (Ipomoea batatas (L.) Lam) varieties to different rates of potassium fertilizer in Calabar, Nigeria. J Agric Sci. 5:61–69.
- Wale E, Yallew A. 2007. Farmer's variety attributes preferences: implications for breeding priority setting and agricultural extension policy in Ethiopia. Afr Dev Rev. 19:379–396.
- Zhao HF, Zhao Y, Zhang C, Tao X, Xu XN. 2014. Growth, leaf gas exchange and chlorophyll fluorescence responses of two cultivars of Salix integra Thunb. To waterlogging stress. J Agric Sci Technol. 16:137–149.
