Abstract
Potassium (K) is an essential macronutrient for plant growth and development. Plant growth and development can be seriously affected by K deficiency. However, plants with different K efficiencies behave differently. It is still not fully understood how plants with higher K efficiency could maintain better growth in a low K environment and what is the relationship between K recycling and photosynthesis metabolism. The aim of this study was to investigate whether the difference in K re-translocation and photosynthesis transportation can explain genotype differences in K efficiency between K-efficient genotype 103 and K-inefficient genotype 122. Results of this study showed that the dry matter accumulation of genotype 122 decreased much more than that of genotype 103 affected by K deficiency environment. Root growth of the two genotypes was inhibited by K deficiency, but genotype 122 was affected more than genotype 103. Using the K utilization index as an evaluation factor for K efficiency, it was found that genotype 103 was significantly higher than genotype 122. Potassium affected the K distribution in plants for both the genotypes. Potassium was distributed more to the stem and leafstalk in a normal K environment whereas it was more to the leaf and root in a low K environment, especially for genotype 103. Potassium also affected photosynthetic products’ distribution. The leaf of genotype 122 accumulated most of its photosynthetic product while genotype 103 had better ability to transport it into the root to maintain better growth under a K-deficient environment. Results of this study indicated that more K recycling into the root and more efficient transport of the photosynthetic product into the root contribute to better root growth and therefore increased tolerance to K deficiency.
Introduction
Potassium (K) is one of the macronutrients essential for plant growth and development. It is present in plant cells in high amounts (up to 10% of plant dry weight) and is absolutely required for plant growth (Chérel et al. Citation2014). Potassium plays an important role in a wide range of physiological processes, such as maintenance of electrical potential gradients across cell membranes, turgor generation, maintenance of anion–cation balances, activation of numerous enzymes, protein synthesis, and maintenance of photosynthesis and related processes (Marschner Citation2012). However, in intensive agricultural production systems, K has become a limiting element, particularly in coarse-textured or organic soils (Zörba et al. Citation2014), especially for cotton, which needs a large amount of K in its whole life; an average of mature cotton is estimated to contain between 110 and 250 kg K ha−1 (Bassett et al. Citation1970). Widespread K deficiency in cotton crop has occurred in many countries (Wang et al. Citation2012). It was reported that using K-efficient genotypes in combination with optimized soil fertilization is the perfect nutrient management strategy for stable and sustainable agriculture (Rengel & Damon Citation2008); furthermore, they also summarized eight areas in which breeders could intercede, including root morphology, formation of root hairs, root exudates, ability to release K from non-exchangeable pools, kinetics of K uptake, K translocation, K substitution and harvest. Among them, the first five traits are related to K uptake efficiency and the latter three traits reflect K utilization efficiency (Pettigrew Citation2008).
In this study, we selected two genotypes (K-efficient genotype 103 and K-inefficient genotype 122) that differ in K efficiency, from 86 cotton genotypes that belong to four different families including Stoneville, King, Coker and Deltapine (Jiang et al. Citation2005). A number of studies have been carried out on these two genotypes in this laboratory since 2001, including the pattern of absorption and distribution of K (Xia et al. Citation2011), root morphology (Hao et al. Citation2011) and physiological indicators related to the absorption and utilization of potassium (Jiang et al. Citation2011). A previous study on K utilization efficiency mechanism of the two cotton genotypes was focused on dry matter distribution, and it suggested that one of the mechanisms by which genotype 103 could maintain higher cotton yield was that it could transport more photosynthetic products into reproduction organs (Xia et al. Citation2011), but how the K-efficient genotype grows better in a K-deficient environment than the K-inefficient genotype and its relationship with the mineral element and photosynthesis metabolism are still not fully understood. The objects of this study were to study the agronomic differences between two genotypes in response to K deficiency, to investigate the relationship of better low K adaptation with K recycling and photosynthesis metabolism.
Material and methods
Plant materials
Two different K-efficient cotton genotypes, K-efficient genotype 103 and K-inefficient genotype 122 were selected from 86 cotton varieties. The seeds were provided by the Cotton Research Institute, Chinese Academy of Agricultural Sciences, and Huazhong Agricultural University, Wuhan, Hubei Province, China.
Plant growth and management
Uniform seeds were selected, and then soaked in warm water of 50–60°C for 6 h to fully absorb water. Seeds covered by moist gauze and were placed in a phytotron at 30°C to germinate. After the radicle grew up to 1 cm, seeds were transferred to absorbent gauze covered on a pot filled with distilled water. Seedlings with expanded cotyledon were transplanted into a 3-liter plastic bucket, of which the outer wall was painted black to block light. Each bucket had four seedlings and covered by a black polyethylene plate. All the plastic buckets were immersed in 1 mol L−1 HCl, and washed with distilled water prior to the experiment.
The seedlings were cultivated hydroponically with culture solution modified from Hoagland and Arnon (Citation1950), containing the following macronutrients in mmol L−1: NH4NO3, 3.00; Na2HPO4, 0.28; NaH2PO4, 0.83; CaCl2 • 2H2O, 2.45; MgSO4 • 7H2O, 2.03; Fe-EDTA, 0.07, and the following micronutrients in μmol L−1: H3BO3, 46.26; MnCl2 • 4H2O, 9.10; ZnSO4 • 7H2O, 0.77; CuSO4 • 5H2O, 0.32; Na2MoO4 • 4H2O, 0.37, while K was supplied as KCl at 2 mg L−1 as the low-K level (K1) and 20 mg L−1 as the adequate-K level (K2). The nutrient solution was a quarter of the strength at the first week and then turned to half of the strength at the second week. The culture solution was aerated for 20 min every four hours and renewed every week. The experiment had a completely randomized design with the two treatments replicated 4 times and each replication containing one plant. The plants were cultivated in a greenhouse at Huazhong Agricultural University, Wuhan, China.
Photosynthetic parameter measurement
At 30 days after initiation of the K deficiency treatment when the K-deficient symptoms were clearly visible, photosynthetic properties of the youngest fully expanded main-stem leaf (the 4th leaf from apex) were determined at 10:00–12:00 am with a Li-6400 (Li-COR, Lincoln, USA) at 25°C, 60% relative humidity, 500 μmol mol−1 CO2 concentration and 1200 μmol m−2 s−1 quantum flux.
Measurement of mineral elements
The plant samples were separated into roots, stem, leafstalk and leaves. Then the samples were dried at 60°C for at least 72 h. The dry weight of each part of the cotton was measured. Plant samples were then ground. The content of K was measured by burning a 0.2 g powder sample of all organs at 550◦C in porcelain crucibles for 5 hours. The ash from the burning was added to 10 mL 0.1 mol/L HCl and then the solution was filtered using a quantitative filter paper. The filtrate was collected in a 10 ml centrifuge tube, and K concentrations were measured by a flame spectrophotometer (FP 640, Shanghai Precision & Scientific Instrument Co.).
Determination of leaf sugars
Leaf and root total soluble sugar, sucrose and starch were extracted by 80% ethanol three times in 80°C water. The concentrations of these three carbohydrates were measured by the methods of García-Luis et al. (Citation2002)
Data analysis
The experiment was arranged in a completely randomized design with four replications. Statistical analysis was conducted and charts were prepared using SPSS software (IBM SPSS Statistics 20) and Origin pro. 8.6 (OriginLab Corporation, USA). Unless otherwise noted, results were given as mean ±standard deviation (SD). When a significant (P < .05) treatment effect was observed, the mean values were compared using the t-test (P < .05) and the LSD test (P < .05). Significant differences (P < .05) within each group are indicated by ‘*’ or by different lowercase letters (a, b, c and d).
Results
Plant growth and the potassium efficiency index of the two cotton genotypes
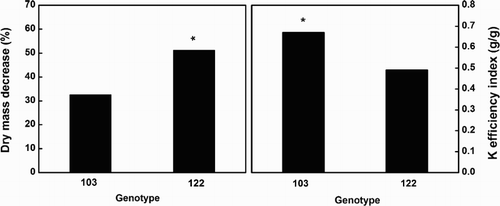
Potassium deficiency affected the growth of the two cotton genotypes remarkably. It can be seen from that genotype 122 gained more dry matter than genotype 103 under normal K treatment, whereas it followed the opposite pattern under low K treatment. Root growth was significantly different between the two cotton genotypes under a K-deficient environment. Root dry weight of genotype 103 was 35.7% higher than that of genotype 122, which was consistent with our previous research that genotype 103 had better root growth under a K-deficient environment.
Table 1. Dry weights (g per plant) of different parts of genotypes 103 and 122 under different potassium treatments (K1 as low potassium treatment, 2 mg L−1 KCl; K2 as control treatment 20 mg L−1 KCl).
shows that dry weight of genotype 122 was more seriously decreased by K deficiency compared to genotype 103, with 33.5% and 51.4%, respectively. Using the ratio of dry weight under K deficiency and that under normal K environment as the K utilization index (Damon & Rengel Citation2007), genotype 103 had a significantly higher K utilization efficiency than genotype 122.
Potassium concentration, accumulation and distribution in different plant parts
Low K treatment lowered the K concentration in all the parts of the two cotton genotypes (). There was no significant difference between each part of the two genotypes under the same K treatment. K concentrations of the root and leaf were higher in genotype 103 under low and normal K treatments.
Table 2. K concentration (%) of different parts of the two cotton genotypes under K1 (2 mg L−1 KCl; low K treatment) and K2 (20 mg L−1 KCl; control treatment) treatments.
K accumulation of each part of the two cotton genotypes had no significant difference, though the total K accumulation was higher in genotype 103 under normal K treatment and low K treatment ().
Table 3. K accumulation (mg per plant) of different parts of the two cotton genotypes under K1 (2 mg L−1 KCl; low K treatment) and K2 (20 mg L−1 KCl; control treatment) treatments.
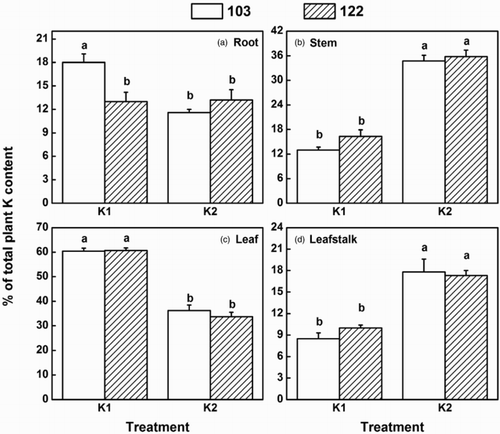
Potassium significantly influenced its distribution in plants. In a normal K environment, a larger proportion of K was allocated in the stem and leafstalk which was reported as a temporary bank for the mineral element, while in a K-deficient condition, more K was distributed to the root and leaf, which thrived and had a more vigorous demand for the nutrient. Moreover, the two cotton genotypes were significantly different in K distribution in the root under low K stress; i.e. the distribution of K to the root was much higher in genotype 103 than that of genotype 122 ().
Figure 2. Fractions (in percentages) of total plant potassium content in the (a) root, (b) stem, (c) leaf and (d) leafstalk of the two cotton genotypes under K1 (2 mg L−1 KCl; low K treatment) and K2 (20 mg L−1 KCl; control treatment) treatments. Values are means of four replicates ±standard error. Bars with different letters are significantly different at P < .05 by LSD.
Photosynthetic rate and carbohydrate transportation
There was no significant difference in NPn under normal K treatment for the two genotypes; however, the stomatal conductance, intercellular concentration of CO2 and transpiration rate that related to NPn were significantly lower in genotype 103 (). Under low K treatment, NPn of genotype 122 was significantly higher than that of genotype 103.
Table 4. Effects of potassium on parameters of photosynthetic gas exchange in two different K efficiency cotton genotypes under K1 (2 mg L−1 KCl; low K treatment) and K2 (20 mg L−1 KCl; control treatment) treatments.
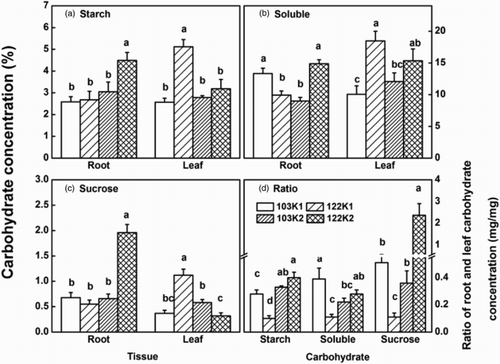
K deficiency dramatically affected the transportation and allocation of the photosynthesis product. The distribution patterns of the two genotypes were significantly different under different K conditions. The starch, soluble and sucrose concentrations of genotype 122 were significantly higher than those of genotype 103 under normal K treatment ((a–c)), and the ability to distribute carbohydrate to the root was better in genotype 122 than in genotype 103 ((d)). However, K deficiency caused a larger proportion of carbohydrate accumulation in the leaf of genotype 122, and the ability to transport carbohydrate to the root was significantly decreased ((d)). On the contrary, K deficiency increased the carbohydrate distribution to the root of genotype 103, which was very much stronger than genotype 122 under a low K condition.
Figure 3. (a) Starch, (b) soluble carbohydrate, (c) sucrose concentration and (d) ratio of root and leaf carbohydrate of the two cotton genotypes under K1 (2 mg L−1 KCl; low K treatment) and K2 (20 mg L−1 KCl; control treatment) treatments. Values are means of four replicates ±standard error. Bars with different letters are significantly different at P < .05 by LSD.
Discussion
Potassium deficiency and plant growth
Potassium is a major inorganic element for living cells and the most abundant cation in cytosol. A high K+ concentration is required for optimal protein synthesis and photosynthesis (Chérel et al. Citation2014). Genetic variation in nutrient efficiency may be attributed to two multifactorial components: (i) genotypes may differ in the efficiency with which the nutrients in the plant are utilized to produce yield (utilization efficiency) and/or (ii) they may differ in their effectiveness in absorbing nutrients from the soil (uptake efficiency) (Sattelmacher et al. Citation1994). In this study, genotype 103 had higher K efficiency ratio, and plant growth was less affected by low K stress compared with that of genotype 122 (). These results suggest that genotype 103 had higher K utilization efficiency and better adaptability in tolerating low K than genotype 122, as indicated in our previous study (Hao et al. Citation2015). As it is well known, the root is in charge of anchorage and uptake of water and nutrients from soil (Hodge et al. Citation2009). However, potassium deficiency limits root growth. Gruber et al. (Citation2013) reported that when K supply was reduced, a significant reduction of the root was observed under all treatments. In this study, root dry weights of the two genotypes were significantly reduced by low K stress; however, it could not be ignored that genotype 103 had a distinguished heavier root under low K treatment than that of genotype 122. This result indicates that genotype 103 had better root development to maintain plant growth in a low K environment.
Low K adaptation and mineral element distribution
Ions taken up by the roots can be used either for growth and accumulation in the root, or subjected to export via the xylem to the shoot (Peuke Citation2010). Also, it was reported that around half of the K+ transported in the xylem is recycled back to the phloem, most of which was incorporated into the root (Peuke Citation2010). It was reported that the cycling of mineral nutrients like K is required to cover the demand for growth of apical root zones (Marschnert et al. Citation1997). In this study, potassium significantly affected the distribution of K in plants. Potassium deficiency caused a lower distribution proportion of K into the roots ((a)). This result was in agreement with that reported by Lima Filho and Malavolta (Citation2003), who studied the potassium distribution of coffee plants. Their study showed that 54–63.8% of the total leaf K+ was remobilized in K+-sufficient plants, while 61.8–79.2% in K+-deficient ones. Our result also showed that under low K stress, genotype 103 could transport more K into the root (). Pettigrew (Citation2008) reported eight characteristics that high-nutrient-efficiency plants might have; one of them is the ability of K translocation. Considering the dominant role of K in root growth, in the long run, this might be the reason why genotype 103 had higher dry weight of root under low K treatment (), and therefore allowed the plants of genotype 103 to grow in a relatively normal status under the K-deficient condition.
Low K adaptation and photosynthesis transport
The primary roles of a macronutrient have been well documented and they are unlikely to fundamentally shift. However, many aspects of ‘macronutrition’ need further clarification especially in light of growing demands for sustainability in agriculture (Maathuis Citation2009). Potassium is not assimilated into organic matter but functions as a co-factor for enzymatic reactions and counter-ion for metabolite transport (both across membranes and over a long distance), translation (ribosomal function) and direct enzyme activation (of starch synthase, pyruvate kinase and many others (Amtmann & Armengaud Citation2009). It is well known that a K-deficient plant shows a significant accumulation of soluble sugars in the leaf (Amtmann et al. Citation2008). In this study, K deficiency caused an obvious accumulation of starch, sucrose and soluble sugars in the leaf of genotype 122 ((a–c)). Compared to normal K treatment, the concentrations of the three kinds of carbohydrate were affected a little under the K-deficient condition. Although genotype 122 had higher NPn in K-deficient circumstances, it seems it could not transport its photosynthetic product to the root efficiently ((d)). Sugars, amino acids and K+ are the dominant solutes and account for the majority of osmotic potential (Komor Citation2000). However, although the accumulation of sugars in leaves contributes further to the replacement of osmotic molecules for genotype 122, photosynthesis product transportation restricted by K deficiency resulted in worse root development, and eventually the K-deficient plant root generally showed a growth restriction (Zhao et al. Citation2001). Wang et al. (Citation2012) also reported that the K-inefficient cotton genotype had lower NPn and slower export of soluble sugars from the phloem (Wang et al. Citation2012). Results of this study indicated that better ability to transport sugars to the root under the K-deficient condition rather than NPn might be the reason why genotype 103 could adapt to the low K environment better than genotype 122. It was observed that there was an increased translocation of phloem-mobile carbohydrates to the root in N-deprived and P-deprived plants (Liu et al. Citation2009). Our study revealed that K-deficiency also could promote the translocation of photosynthetic products to root, but with genotype variation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amtmann A, Armengaud P. 2009. Effects of N, P, K and S on metabolism: new knowledge gained from multi-level analysis. Curr Opin Plant Biol. 12:275–283. doi: 10.1016/j.pbi.2009.04.014
- Amtmann A, Troufflard S, Armengaud P. 2008. The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant. 133:682–691. doi: 10.1111/j.1399-3054.2008.01075.x
- Bassett DM, Werkhoven CHE, Anderson WD. 1970. Dry matter production and nutrient uptake in irrigated cotton (Gossypium hirsutum). Agron J. 62:299–303. doi: 10.2134/agronj1970.00021962006200020037x
- Chérel I, Lefoulon C, Boeglin M, Sentenac H. 2014. Molecular mechanisms involved in plant adaptation to low K+ availability. J Exp Bot. 65:833–848. doi: 10.1093/jxb/ert402
- Damon P, Rengel Z. 2007. Wheat genotypes differ in potassium efficiency under glasshouse and field conditions. Crop Pasture Sci. 58:816–825. doi: 10.1071/AR06402
- García-Luis A, Oliveira MEM, Boedón Y, Sequeira DL, Tominaga S, Guardiola JL. 2002. Dry matter accumulation in citrus fruit is not limited by transport capacity of the pedicel. Ann Bot. 90:755–764. doi: 10.1093/aob/mcf257
- Gruber BD, Giehl RF, Friedel S, von Wirén N. 2013. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 163:161–179. doi: 10.1104/pp.113.218453
- Hao YS, Lei J, Wang QL, Wu LS, Jiang CC. 2015. Two typical K-efficiency cotton genotypes differ in potassium absorption kinetic parameters and patterns. Acta Agr Scand B-S P. 65:45–53.
- Hao YS, Jiang CC, Wang XL, Xia Y, Chen F. 2011. Differences of potassium efficiency characteristics and root morphology between two cotton genotypes. Acta Agron Sin. 37:2094–2098. doi: 10.3724/SP.J.1006.2011.02094
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Calif Agric Exp Sta Circ. 347:1–32.
- Hodge A, Berta G, Doussan C, Merchan F, Crespi M. 2009. Plant root growth, architecture and function. Plant Soil. 321:153–187. doi: 10.1007/s11104-009-9929-9
- Jiang CC, Gao XZ, Wang YH, Lu JW, Xu FS, Shi L. 2005. Potassium efficiency of various cotton genotypes and its nutritional mechanisms. Plant Nutr Fert Sci. 11:781–786.
- Jiang CC, Xia Y, Chen F, Lu JW, Wang YH. 2011. Plant growth, yield components, economic responses, and soil indigenous K uptake of two cotton genotypes with different K-efficiencies. Agr Sci China. 10:705–713. doi: 10.1016/S1671-2927(11)60053-9
- Komor E. 2000. Source physiology and assimilate transport: the interaction of sucrose metabolism, starch storage and phloem export in source leaves and the effects on sugar status in phloem. Aust J Plant Physiol. 27:497–505.
- Lima Filho OD, Malavolta E. 2003. Studies on mineral nutrition of the coffee plant (Coffea arabica L. cv. Catuaí Vermelho): LXIV. Remobilization and re-utilization of nitrogen and potassium by normal and deficient plants. Braz J Biol. 63:481–490. doi: 10.1590/S1519-69842003000300014
- Liu TY, Chang CY, Chiou TJ. 2009. The long-distance signaling of mineral macronutrients. Curr Opin Plant Biol. 12:312–319. doi: 10.1016/j.pbi.2009.04.004
- Maathuis FJ. 2009. Physiological functions of mineral macronutrients. Curr Opin Plant Biol. 12:250–258. doi: 10.1016/j.pbi.2009.04.003
- Marschner H. 2012. Mineral nutrition of higher plants. 3rd ed. London: Elsevier, p. 299–312.
- Marschner H, Kirkby EA, Engels C. 1997. Importance of cycling and recycling of mineral nutrients within plants for growth and development. Botanica Acta. 110:265–273. doi: 10.1111/j.1438-8677.1997.tb00639.x
- Pettigrew WT. 2008. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant. 133:670–681. doi: 10.1111/j.1399-3054.2008.01073.x
- Peuke AD. 2010. Correlations in concentrations, xylem and phloem flows, and partitioning of elements and ions in intact plants. A summary and statistical re-evaluation of modelling experiments in Ricinus communis. J Exp Bot. 61:635–655. doi: 10.1093/jxb/erp352
- Rengel Z, Damon PM. 2008. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol Plant. 133:624–636. doi: 10.1111/j.1399-3054.2008.01079.x
- Sattelmacher B, Horst WJ, Becker HC. 1994. Factors that contribute to genetic variation for nutrient efficiency of crop plants. Zeitschrift für Pflanzenernährung und Bodenkunde. 157:215–224. doi: 10.1002/jpln.19941570309
- Wang N, Hua H, Eneji AE, Li Z, Duan L, Tian X. 2012. Genotypic variations in photosynthetic and physiological adjustment to potassium deficiency in cotton (Gossypium hirsutum). J Photoch Photobio B. 110:1–8. doi: 10.1016/j.jphotobiol.2012.02.002
- Xia Y, Jiang CC, Chen F, Lu JW, Wang YH. 2011. Differences in growth and potassium-use efficiency of two cotton genotypes. Commun Soil Sci Plant Analys. 42:132–143. doi: 10.1080/00103624.2011.535064
- Zhao D, Oosterhuis DM, Bednarz CW. 2001. Influence of potassium deficiency on photosynthesis chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica. 39:103–109. doi: 10.1023/A:1012404204910
- Zörb C, Senbayram M, Peiter E. 2014. Potassium in agriculture – status and perspectives. J Plant Physiol. 171:656–669. doi: 10.1016/j.jplph.2013.08.008