ABSTRACT
To develop better mechanical management strategies, more information on the impact of root partitioning on generative reproduction of Sonchus arvensis L. is needed. Therefore, an outdoor experiment was performed in Sweden in 2008, to evaluate the effect of root fragmentation on generative reproduction of S. arvensis. Two artificial populations of S. arvensis with the same total root length per area but with different initial root lengths and different numbers of root fragments were planted. Cumulative numbers of flower receptacles which had shed mature seeds over the season were assessed. Changes in the number of seeds per flower receptacle and average seed weight were monitored over time during the late season. Plants from long root fragments produced more flower receptacles than plants from short ones. Per area, however, the number of mature flower receptacles did not differ. The number of seeds per flower receptacle and individual seed weight were not affected by initial root length for the first cohort of shoots which sprouted from the initially planted roots. A second cohort, from roots produced during the season, resulted, irrespective of its initial root length, in fewer flower receptacles per plant and per area, with less seeds per receptacle, but with the same average seed weight as the first cohort. The number of seeds per flower receptacle was higher in mid-September than earlier or later. Average seed weight slightly decreased over time. The weight of seeds produced in early September was inversely related to the number of seeds per receptacle, but this trade-off disappeared over time. Root fragmentation alone in pure populations of S. arvensis does not impede generative reproduction, but is likely to decrease input of seeds to the seed bank, when combined with crop competition.
Introduction
Perennial sow-thistle (Sonchus arvensis L.) is a deep-rooted perennial herb belonging to the family Asteraceae. The weed species is native to and common throughout Europe as far as the Western Urals. From Europe, it has been introduced to large parts of the US and Canada and it also exists in parts of northern and eastern Asia, Australia, and South America (Korsmo Citation1954; Håkansson Citation1969; Pegtel Citation1973). S. arvensis often occurs in annual crops in the northern parts of Europe and may cause substantial yield losses. During the last decades, the abundance of S. arvensis has increased in Scandinavia and Finland (Lundkvist Citation1998; Rydberg & Milberg Citation2000; Salonen et al. Citation2001; Vanhala et al. Citation2006), attributed to the increase in the practice of reduced tillage (Tørresen & Skuterud Citation2002; Salonen et al. Citation2013) and the expansion of organic farming (Salonen & Hyvönen Citation2002).
One of the characteristics of S. arvensis which makes it difficult to control is its fast-growing vegetatively reproducing root system (Lemna & Messersmith Citation1990). Mechanical control strategies such as repeated soil tillage applied with the aim of emptying root reserves through repeated sprouting can rather effectively control S. arvensis (Håkansson & Wallgren Citation1972). Due to relative tolerance of this species to many herbicides targeting dicotyledonous weeds in a growing crop (Lemna & Messersmith Citation1990; Fogelfors & Lundkvist Citation2008), combinations of mechanical and chemical control measures are often recommended to control S. arvensis (Lemna & Messersmith Citation1990).
Another trait of this species which may enhance its weediness is its potential of seed production. Flowers of S. arvensis are perfect, generally self-incompatible and pollinated by insects, for example, bees, hoverflies and blister beetles (Stevens Citation1924; Derscheid & Schultz Citation1960). Capitula contain 150–240 fertile flowers which produce different numbers of achenes depending upon environmental conditions and the availability of suitable pollinators (Stevens Citation1924). Sonchus arvensis can typically produce an average of 30 achenes per head and up to 55,000 achenes per m2 but competition with a crop can reduce the number of produced achenes substantially (Dorph-Petersen Citation1924). Derscheid and Schultz (Citation1960) found that 50 achenes were produced by artificially cross-pollinated heads from plants grown in the field and greenhouse but the number of achenes per head in natural populations varied from 20 to 40 or from 60 to 80 among years. In many perennial weeds, such as Cirsium arvense L. (Scop.) and Solidago canadensis L., sexual reproduction contributes towards the maintenance of a high genetic variability among the seeds, and also enables weed species to colonize new habitats by means of long-distance dispersal of seeds (Heimann & Cussans Citation1996; Dong et al. Citation2006).
Several studies have shown that differences in reproductive success among plant populations are due to differences in the number and size of the plants which constitute these populations. Factors which exert an influence on reproduction can be due to plant traits at an early stage of development, such as initial seed size (Giles Citation1990) and emergence time (Waller Citation1985) or originate from variation in the environment, for example, proximity of neighbours (Weiner Citation1982), soil density (Hartgerink & Bazzaz Citation1984), and irrigation (Klinkhamer et al. Citation1994).
Among induced environmental factors, soil disturbance plays an important role in changing sexual reproductive capacity of weedy plants. Its effect on reproductive success varies with the type of soil cultivation and species characteristics. In the perennial weed Acroptilon repens L., the number of seed heads produced by plants grown in a continuously disturbed area decreased substantially compared to those of plants grown in an undisturbed area (Kolören et al. Citation2005). Franke et al. (Citation2007) found that small plants of Phalaris minor (Retz.) grown in conventionally tilled plots had reduced numbers of seeds rather than lower individual seed weight when compared to larger plants grown in plots under direct sowing. Clements et al. (Citation1996) found that the number of seeds produced per unit area by Chenopodium album L. shoots was higher in a moldboard plough and the chisel plough system compared to ridge tillage and no tillage systems.
Despite the importance of seed as a major source of infestation, little is known about how various intensity of soil tillage affects reproductive capacity in perennial sow-thistle. S. arvensis is known to have a seed bank, with a seed longevity of up to three years (Chepil Citation1946). We performed an outdoor experiment in order to assess the effects of root fragmentation on seed production in S. arvensis. As general performance of perennial weeds is correlated to stored reserves in their roots (Zimdahl Citation2013), we hypothesized that: (i) longer root fragments will produce plants with a higher number of flower receptacles each of them containing more seeds, (ii) for a given total root length, the number of produced mature flower receptacles and the number of seeds will not be affected by root fragmentation and (iii) the first cohort of shoots, which can acquire resources over a longer time compared to the second cohort, will contribute more to generative reproduction than the second cohort of shoots. As seed mass in general is known to be a rather conservative trait (Harper et al. Citation1970), we hypothesized that (iv) average seed weight will not differ between plants originating from different root lengths or between shoot cohorts and (v) the number of seeds per flower receptacle and average weight per shed seed will be constant over time.
Materials and methods
The experiment
An outdoor box experiment was performed during 2008 at Ultuna, close to Uppsala, Sweden (59° 48′N, 17°39′E). The boxes were rectangular, 80 cm × 80 cm and 20 cm deep and filled with a soil (5% organic matter, 2–5% clay, and 1.5 mg NO3 per 100 g dry soil). The boxes were irrigated just before planting. The plant material used was perennial sow-thistle (S. arvensis L.) harvested from a plant bank kept at Ultuna. During 2006 and 2007, the plant bank of S. arvensis had been grown in buckets outdoors during May–October and was stored in a dark cold store during November–April. For more information, see Anbari et al. (Citation2011, Citation2016).
One day before planting, roots were first harvested and sorted into three thickness categories; fine (<2 mm), medium (2–4 mm), and thick (>4 mm) in diameter. They were then cut in 5 and 20 cm long pieces with at least two adventitious buds, but no sprouted buds, on each piece. At planting (29 May), roots were selected in such a way that all thickness categories were approximately equally represented in all boxes. The root pieces were planted and covered with 2–3 cm soil and the boxes were irrigated. Fertilization was performed on 14 July (10 g N m−2, Blomstra, Cederroth International AB, Falun, Sweden) and the soil was kept moist during the growing season.
Two root length traits (both with a total length of 1 m), 5 root pieces of 20 cm, and 20 root pieces of 5 cm, were comprised in a completely randomized design with 4 replicates for each trait, that is, 8 boxes. Emerging shoots were classified according to their origin, that is, whether they originated from the originally planted roots, or from roots developed during the season, defined here as the first and second shoot cohort, respectively.
For each of the root length traits and shoot cohorts, six samples, randomly chosen from the replicates, were taken at three sampling times (8, 17, and 26 September). Each sample was composed of three mature flower receptacles containing seeds just prior to shedding. All samples were oven dried for 24 h at 105°C. For each sample, the dry weight of the cleaned seeds was measured and the number of mature seeds was recorded. Plants were harvested during 2–8 October. The number of living plants and the number of flower receptacles that had produced mature seeds were counted per box for each of the generations and root length traits. Flower receptacles were considered to have produced mature seeds when their colours had changed from green to yellow.
Statistical analyses
To compute the total number of produced mature flower receptacles at final harvest, the number of flower receptacles that had been harvested from each box at the three sampling times was added to the number of flower receptacles that had produced mature seeds at the final harvest.
Number of seeds at harvest per flower receptacle was estimated by shoot cohort and box as the total number of collected seeds, summed over all sampling times, divided by the total number of collected flower receptacles.
Number of flower receptacles per unit area (m2) was calculated through division of the number of flower receptacles per box by the area of the box (0.64 m2).
Number of flower receptacles per plant was calculated through division of the number of flower receptacles per box by the number of living plants per box at harvest.
For each of the sampling times:
Number of seeds per flower receptacle was computed per shoot cohort, sampling date, and box though division of the total number of seeds by the number of sampled flower receptacles.
Number of seeds per box (0.64 m2) was calculated by multiplying the number of seeds per flower receptacle by the cumulative number of flower receptacles that had produced mature seeds at harvest.
Number of seeds per plant was computed by multiplying the number of seeds per flower receptacle by the cumulative number of flower receptacles per plant at the final harvest.
Number of seeds per unit area (m2) was obtained from the number of seeds per box after division by 0.64 m2.
Seed weight per plant was calculated by multiplying the number of seeds per plant with the average seed weight.
Seed weight per unit area (m2) was calculated by multiplying the number of seeds per unit area with the average seed weight.
In order to make the variance homogeneous, prior to the analysis of variance the number of flower receptacles per plant, the number of seeds per unit area, and seed weight per unit area were squared root transformed and the number of seeds per plant and seed weights per plant were log transformed.
To assess the impact of initial root length and shoot generation on seed number and seed weight per unit area and per plant, least squares means over the three sampling times were computed per initial root length and shoot cohort using a mixed model with fixed effects of root lengths, shoot cohorts, sampling times, their interactions, and random effects of boxes.
The effects of initial root length and shoot cohort on the number of flower receptacles per plant, the number of flower receptacles per unit area, the number of seeds at harvest per flower receptacle, and the average seed weight at harvest were evaluated using the mixed model including fixed effects of root lengths, shoot cohorts, their interactions, and random effects of boxes.
To evaluate changes in the number of seeds per flower receptacle and average seed weight over the three sampling times, repeated-measures analysis was performed using a mixed model containing fixed effects of initial root lengths, sampling times, shoot cohorts, all their interactions, and random effects of boxes. Sampling time was included as a repeated factor in the model. Observations from the same box were assumed to be correlated with a first-order autoregressive covariance structure.
Average seed weight was modelled as a function of the number of seeds per flower receptacle using a linear mixed model. This model also included fixed effects of sampling times, fixed effects of initial root lengths, all interactions, and random effects of shoot origin within replicates. All models were fitted using the mixed procedure of the SAS System (SAS Institute Citation2011).
Results
Number of flower receptacles at harvest
Both initial root length and shoot cohort significantly affected the number of produced flower receptacles per plant (). The number of flower receptacles per plant was higher for the first shoot cohort than for the second. The population originating from long root fragments produced more flower receptacles per plant in comparison to the short root population (). Also, a significant interaction for the number of flower receptacles per plant was found between initial root length and shoot cohort. Both shoot cohorts from plants originating from 20 cm root fragments produced more flower receptacles compared to the plants originating from 5 cm roots. The difference between the two root populations was, however, smaller for the second shoot cohort compared to the first shoot cohort (, ).
Figure 1. Number of produced flower receptacles per S. arvensis plant for the first and second cohort of shoots and for initial root fragment lengths of 5 cm and 20 cm. Whiskers indicate 95% confidence interval.
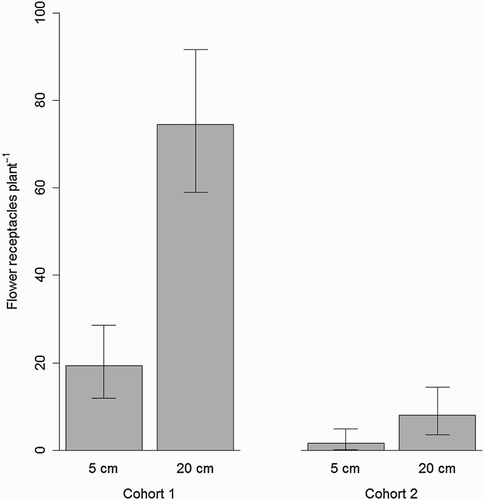
Table 1. ANOVA results, specifying the effects of initial root length (5 and 20 cm), origin of shoots (the first and second cohort) and their interaction on the production of: flower receptacles (plant−1 and m−2), number of seeds (flower receptacle−1, plant−1, and m−2), average seed weight (g seed−1), and total seed weight (plant−1, and m−2) in S. arvensis populations.
The number of flower receptacles per m2 was significantly higher for the first shoot cohort compared to the second shoot cohort: 579.69 ± 55.85 and 63.47 ± 55.84 for the first and second cohort, respectively. The figures after the plus-minus signs indicate 95% confidence intervals. No significant effects were observed of initial root length or interaction between initial root length and shoot cohort on the number of flower receptacles per unit area ().
Seed numbers and seed weights at harvest
Shoots from the first cohort produced a significantly higher number of seeds per flower receptacle than shoots from the second cohort: 64.30 ± 4.98 and 39.18 ± 4.98, for the first and second cohort, respectively. No significant effects of initial root length or of an interaction between initial root length and shoot cohort on the number of seeds per flower receptacle were found ().
The number of seeds produced per plant was significantly affected by both initial root length and shoot cohort, and there was no significant interaction (). For the first cohort of shoots, plants from long roots produced 3468 seeds plant−1 (95% confidence interval limits: 1998, 6020) while the second shoot cohort produced 283 seeds plant−1 (95% confidence interval limits: 162, 492). Corresponding values for the first and second shoot cohorts from plants from short roots were: 919 seeds plant−1 (95% confidence interval limits: 530, 1595), and 54 seeds plant−1 (95% confidence interval limits: 31, 95), respectively.
Shoot cohort had also a significant effect on seed number per unit area (). Shoots from the first cohort produced on average 30,391 (95% confidence limits: 27,275, 33,676) seeds m−2 whereas shoots from the second cohort produced 2307 (95% confidence limits: 1505, 3279) seeds m−2. No significant effects were observed of initial root length or its interaction with shoot cohort on the number of seeds per unit area ().
No significant effects of initial root length, shoot cohort, or their interactions were observed on average seed weight ().
Initial root length and shoot cohort strongly affected the total seed weight produced per plant (). The first shoot cohort from plants from long roots produced 1.98 g seeds plant−1 (95% confidence limits: 1.09, 3.57) while the second shoot cohort produced 0.17 g seeds plant−1 (95% confidence limits: 0.09, 0.30). Corresponding values for the first and second shoot cohorts from plants originating from short roots were: 0.51 g seeds plant−1 (95% confidence limits: 0.28, 0.92) and 0.03 g seeds plant−1 (95% confidence limits: 0.02, 0.06), respectively.
The weight of the total produced seeds per unit area (g m−2) was significantly affected by shoot cohort but not by initial root length or its interaction with shoot cohort (). In the first shoot cohort, seed weight per unit area was higher due to a larger number of seeds (16.7 g m−2, 95% confidence limits: 14.8, 18.9) and (0.86 g m−2, 95% confidence limits: 0.34, 1.49) for the first and second cohort, respectively).
Changes over time in the number of seeds and seed weight
The number of seeds per flower receptacle was significantly affected by shoot cohort, sampling time, and the interaction between shoot cohort and sampling time while initial root length had no significant effect (). Shoots from the first cohort produced significantly more seeds per flower receptacle as compared with shoots from the second cohort. Flower receptacles harvested at the second sampling time had the highest number of seeds ((a)). In shoots from the first cohort, the number of seeds per flower receptacle increased significantly until it reached a maximum level at the second sampling time and then decreased. In contrast, no change in the number of seeds per flower receptacle over time was observed in the second cohort of shoots ((a)).
Figure 2. Changes in (a) number of seeds produced per flower receptacle, and (b) average seed weight (mg seed−1) over three sampling times (1 = 8 September, 2 = 17 September, and 3 = 26 September) in shoots of first and second cohort of S. arvensis when averaged over root length traits (5 and 20 cm). Dotted line, open circle: first shoot cohort. Dashed line, filled circle: second shoot cohort. Whiskers indicate 95% confidence interval.
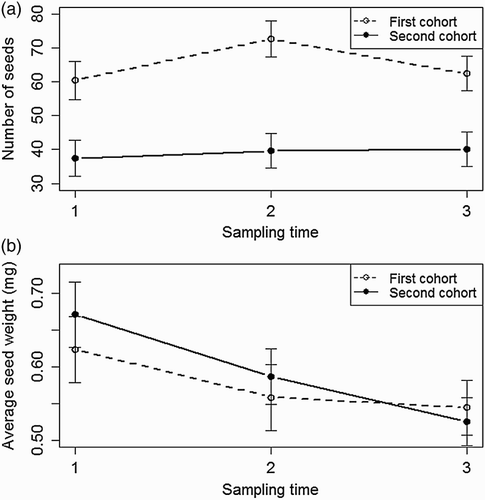
Table 2. Results of repeated-measures ANOVAs on number of seeds per flower receptacle and average seed weight (g seed−1) in S. arvensis populations. Initial root length (5 and 20 cm) and shoot cohort (the first and second) were used as the between-groups factors and sampling time (8, 17, and 26 September) as the within-group factor.
Average seed weight was significantly affected by sampling time (). Seeds had the highest average weights at the first sampling time. There was a slight tendency towards a decrease in average seed weight between the second and third sampling time but this difference was not statistically significant ((b)).
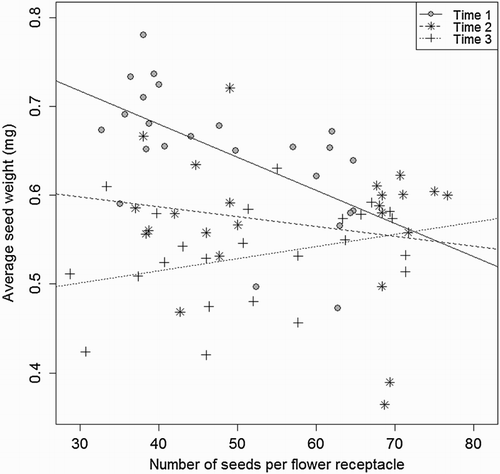
Average seed weight decreased with increasing number of seeds per flower receptacle at the first sampling time (d.f. = 60, t = −3.31, P = .0016). No significant linear relations between average seed weight and number of seeds per flower receptacle were found at the second (d.f. = 60, t = −1.18, P = .2408) and third (d.f. = 60, t = 1.38, P = .1718) sampling times ().
Figure 3. Average seed weight (mg seed−1) of S. arvensis as a function of number of seeds per flower receptacle at three sampling times (1 = 8 September, 2 = 17 September, and 3 = 26 September) when averaged over the two initial root lengths (5 and 20 cm). The slope (−0.00372) at sampling time 1 is significantly different from zero (P = 0016). Solid line, filled circle: sampling time 1, Dashed line, star = sampling time 2, Dotted line, cross: sampling time 3.
Discussion
In this study, we investigated the effects of root fragmentation and shoot cohort on the generative reproduction of S. arvensis. The underlying aim was to obtain more knowledge on how seed production of S. arvensis was affected by root fragmentation. A better understanding of the interaction between mechanical weed control measures and generative reproduction of S. arvensis is likely to improve the ability to design more efficient weed control strategies. We found that a higher degree of fragmentation of a given amount of roots per area resulted in plants with a lower generative capacity (, ), but also that the effects of fragment length and number were counteracting. While the plants growing from 20 cm long root fragments in terms of seed production had a much higher performance than plants growing from 5 cm long root fragments, the final differences in seed output per area between populations which differed in degree of root fragmentation were small in our experiment: plants originating from longer root fragments produced more mature flower receptacles per plant and with the same number of seeds per flower receptacle as compared with plants from shorter root fragments (, ). The first part of our first hypothesis was thereby supported while the second part was rejected.
Our second hypothesis, stating that for a given total root length, the production of mature flower receptacles and seeds will not be affected by root fragmentation, was supported (), due to the counteracting effect of fragment length and number. Hence, root fragmentation alone does not seem to be an efficient management measure to decrease input of seeds of S. arvensis to the seed bank, but neither a measure which increases seed output, which has been reported to occur in Rumex crispus L. (Cavers & Maun Citation1970). A similar result was obtained by Pye et al. (Citation2011), who found that fragmented roots of R. crispus produced more seeds than intact roots.
We also found support for our third hypothesis, which stated that the first cohort of shoots will contribute more to generative reproduction than the second cohort of shoots (). Average seed weight, regardless of root length and cohort, did not differ between plants originating from different root lengths or between shoot cohorts (), thereby giving support to our fourth hypothesis. Finally, we hypothesized that the number of seeds per flower receptacle and average weight per shed seed will be constant over time. As the number of seeds per receptacle peaked in mid-September, and average weight of the shed seeds decreased during September (, and ), we rejected both components of this hypothesis.
As weight per seed and number of seeds per flower receptacle were not affected by root length (), and plants from the longer root fragments produced more mature flower receptacles than plants grown from the shorter root fragments (), the plants originating from the larger root fragments also produced a larger number of seeds and a higher total seed weight per plant (). Our observed seed production per m2 was in the same order of magnitude as found by Dorph-Petersen (Citation1924) and number of seeds per flower receptacle in the same order of magnitude as reported by Derscheid and Schultz (Citation1960).
While a higher degree of root fragmentation resulted in more plants, fragmentation rather affected the number of modules (mature flower receptacles per plant, seeds per flower receptacle) than individual size or weight of these modules (, ). The receptacles of the second cohort contained less seeds per receptacle as compared with the first cohort (), indicating that there was a resource restriction or pollination restriction on the second cohort.
Among determining factors in success of self-incompatible flowers in the attraction of pollinators in animal-pollinated plants is the size and position of the flower receptacles and duration of flowering. Sato and Yahara (Citation1999) found that larger pollinators preferred the larger flowers of Impatiens hypophylla (Makino.) var. hypophylla within the same habitat of var. Microhypophylla which had smaller flower. In S. arvensis, average shoot height of the second cohort was much lower (Anbari et al. Citation2016) and thereby the flower receptacles had a lower position in the canopy. This is in line with the observations by Lortie and Aarssen (Citation1999) who found that taller plants of Verbascum thapsus L. had more opening flowers and attracted more pollinators. The first generation of S. arvensis shoots emerged on initial root pieces during the first part of June while the emergence of second generation shoots on newly developed roots took place in July. One of the reasons for fewer seeds per receptacle could be a shortened flowering duration due to delay in emergence time of the second generation shoots. This is in line with observations on Agilinis strictifolia (Benth.) by Dieringer (Citation1991) who found that large shoots flowered longer and produced more flowers than those of late emerging and small shoots which had their peak flowering later in the season. Baker and O'Dowd (Citation1982) also found that reduction in receptacle size of annual herb Hypochoeris glabra L. was associated with reduction in the number of produced seeds. While root fragmentation did not impact the number of mature flower receptacles produced per m2, Anbari et al. (Citation2011) found that the proportion of flower receptacles leading to mature seeds was lower in the population from short root fragments compared to from the long roots, and attributed this to differences in shoot emergence time. Anbari et al. (Citation2016) showed that shoots emerged on long root fragments of S. arvensis L. were taller compared to those of shoots appeared on short root fragments, which may contribute to pollination success.
On average, individual seed weight was not affected by cohort (), confirming that seed weight is a conservative character (cf. Choe et al. Citation1988). This was also confirmed by Weaver and Cavers (Citation1980) who found that individual seed weight did not vary with the size of R. crispus plants. However, we found some variation in individual seed weight and the number of seeds per flower receptacle over the seed shedding time (September) (, and ). The weight of seeds produced in early September was inversely related to the number of seeds per receptacle (). A similar observation was made by Primack (Citation1978) who found a negative correlation between weight per seed and seed number per capsule in the genus Plantago. In our experiment, these seeds were set during the first flowering, and may indicate a poor pollination of the first flowers. At a later stage, plants are producing many new flower receptacles which are likely to attract insects to such an extent that the majority of flowers per receptacle became pollinated. This likely caused the disappearance of the trade-off between seed weight and the number of seeds per receptacle over time.
The final differences in seed output per area between populations which differed in degree of root fragmentation were negligible in our experiment. However, given a number of S. arvensis individuals of different sizes at the start of a cropping season, a competitive crop is likely to hamper the growth of the weed plants, which thereby will produce fewer regenerative modules than when grown without a crop. As smaller individuals will produce less seeds, this will decrease input to the seed bank. S. arvensis is known to be sensitive to crop competition (Eckersten et al. Citation2011) and to size relative to the crop at the onset of competition. Thereby, crop competition also is likely to be more effective against smaller individuals of S. arvensis, and consequently, root fragmentation will furthermore decrease seed production, when acting together with a crop. Further investigation is required to assess the interaction of root fragmentation and crop competition on the regenerative production of S. arvensis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anbari S, Lundkvist A, Forkman J, Verwijst T. 2016. Population dynamics and nitrogen allocation of Sonchus arvensis L. Acta Agric Scand, Sect B – Soil & Plant Sci. 66:75–84.
- Anbari S, Lundkvist A, Verwijst T. 2011. Sprouting and shoot development of Sonchus arvensis in relation to initial root size. Weed Res. 51:142–150. doi: 10.1111/j.1365-3180.2010.00837.x
- Baker GA, O'Dowd DJ. 1982. Effects of parent plant density on the production of achene types in the annual Hypochoeris glabra. J Ecol. 70:201–215. doi: 10.2307/2259873
- Cavers PB, Maun MA. 1970. Rumex crispus. Abstracts of the meeting of the Weed Science Society of America. 8:15.
- Chepil WS. 1946. Germination of seeds. I. Longevity, periodicity of germination, and vitality of seeds in cultivated soil. Sci Agric. 26:307–346.
- Choe HS, Chu C, Koch G, Gorham J, Mooney HA. 1988. Seed weight and seed resources in relation to plant growth rate. Oecologia. 76:158–159. doi: 10.1007/BF00379615
- Clements DR, Benott DL, Murphy SD, Swanton CJ. 1996. Tillage effects on weed seed return and seed bank composition. Weed Sci. 44:314–322.
- Derscheid LA, Schultz RE. 1960. Achene development of Canada thistle and perennial sowthistle. Weeds. 8:55–62. doi: 10.2307/4040507
- Dieringer G. 1991. Variation in individual flowering time and reproductive success of Agalinis strictifolia (Scrophulariaceae). Am J Bot. 78:497–503. doi: 10.2307/2445259
- Dong M, Lu B-R, Zhang H-B, Chen J-K, Li B. 2006. Role of sexual reproduction in the spread of an invasive clonal plant Solidago canadensis revealed using intersimple sequence repeat markers. Plant Species Biol. 21:13–18. doi: 10.1111/j.1442-1984.2006.00146.x
- Dorph-Petersen K. 1924. Examinations of the occurrence and vitality of various weed seed species under different conditions, made at the Danish State Seed Testing Station during the years 1896–1923. Int Seed Test. Congress (Cambridge) Rep. 4:124–138.
- Eckersten H, Lundkvist A, Torssell B, Verwijst T. 2011. Modelling species competition in mixtures of perennial sow-thistle and spring barley based on shoot radiation use efficiency. Acta Agric Scand, Sect B – Soil & Plant Sci. 61:739–746.
- Fogelfors H, Lundkvist A. 2008. Selection in Cirsium arvense (L.) Scop. and Sonchus arvensis L.: susceptibility to MCPA on different types of farmland in Sweden. Agric Scand, Sect B – Soil & Plant Sci. 58:82–87.
- Franke AC, Singh S, McRoberts N, Nehra AS, Godara S, Malik RK, Marshall G. 2007. Phalaris minor seedbank studies: longevity, seedling emergence and seed production as affected by tillage regime. Weed Res. 47:73–83. doi: 10.1111/j.1365-3180.2007.00533.x
- Giles BE. 1990. The effects of variation in seed size on growth and reproduction in the wild barley Hordeum vulgare ssp. spontaneum. Heredity. 64:239–250. doi: 10.1038/hdy.1990.29
- Håkansson S. 1969. Experiments with Sonchus arvensis L. I. Development and growth in response to burial and defoliation in different developmental stages. Ann Agric Coll Sweden. 35:989–1030.
- Håkansson S, Wallgren B. 1972. Experiments with Sonchus arvensis L. III. The development from reproductive roots cut into different lengths and planted at different depths, with and without competition from barley. Swed J Agric Res. 2:15–26.
- Harper JL, Lovell PH, Moore KG. 1970. The shapes and sizes of seeds. Ann Rev Ecol Syst. 1:327–356. doi: 10.1146/annurev.es.01.110170.001551
- Hartgerink AP, Bazzaz FA. 1984. Seedling-scale environmental heterogeneity influences individual fitness and population structure. Ecology. 65:198–206. doi: 10.2307/1939471
- Heimann B, Cussans GW. 1996. The importance of seeds and sexual reproduction in the population biology of Cirsium arvense – a literature review. Weed Res. 36:493–503. doi: 10.1111/j.1365-3180.1996.tb01678.x
- Klinkhamer PGL, de Jong TJ, Nell HW. 1994. Limiting factors for seed production and phenotypic gender in the gynodioecious species Echium vulgare (Boraginaceae). Oikos. 71:469–478. doi: 10.2307/3545835
- Kolören O, Uygur S, Uygur FN, Schaffner U. 2005. Response of Acroptilon repens to simulated herbivory and soil disturbance. Ann Appl Biol. 147:101–107. doi: 10.1111/j.1744-7348.2005.00014.x
- Korsmo E. 1954. Ugras i nåtidens jordbruk. Oslo: AS Norsk landbruks forlag. 635 pp.
- Lemna WK, Messersmith CG. 1990. The biology of Canadian weeds. 94. Sonchus arvensis L. Can J Plant Sci. 70:509–532. doi: 10.4141/cjps90-060
- Lortie CJ, Aarssen LW. 1999. The advantage of being tall: higher flowers receive more pollen in Verbascum thapsus L. (Scrophulariaceae). Écoscience. 6:68–71.
- Lundkvist A. 1998. Weed control in ecological farming. A questionnaire. Växtskyddsnotiser. 62:23–26. (In Swedish with an English summary.)
- Pegtel DM. 1973. Aspects of ecotypic differentiation in the perennial sowthistle. Acta Hortic. 32:55–72. doi: 10.17660/ActaHortic.1973.32.5
- Primack RB. 1978. Regulation of seed yield in Plantago. J Ecol. 66:835–847. doi: 10.2307/2259299
- Pye A, Andersson L, Fogelfors H. 2011. Intense fragmentation and deep burial reduce emergence of Rumex crispus L. Acta Agric Scand, Sect B – Soil & Plant Sci. 61:431–437.
- Rydberg NT, Milberg P. 2000. A survey of weds in organic farming in Sweden. Biol Agric Hortic. 18:175–185. doi: 10.1080/01448765.2000.9754878
- Salonen J, Hyvönen T. 2002. Perennial weeds in conventional and organic cropping of spring cereals in Finland. Zeitschrift Pflanzenkrankheiten Pflanzenschutz. 18:519–525.
- Salonen J, Hyvönen T, Jalli H. 2001. Weed flora in organically grown spring cereals in Finland. Agric Food Sci Finland. 10:231–242.
- Salonen J, Hyvönen T, Kaseva J, Jalli H. 2013. Impact of changed cropping practices on weed occurrence in spring cereals in Finland – a comparison of surveys in 1997–1999 and 2007–2009. Weed Res. 53:110–120. doi: 10.1111/wre.12004
- SAS Institute. 2011. SAS/STAT 9.3 User's guide. Cary, NC: SAS Institute Inc.
- Sato H, Yahara T. 1999. Trade-offs between flower number and investment to a flower in selfing and outcrossing varieties of Impatiens hypophylla (Balsaminaceae). Am J Bot. 86:1699–1707. doi: 10.2307/2656668
- Stevens OA. 1924. Perennial sow thistle growth and reproduction. N D Agric Exp Stn Bull. 181:44 pp. North Dakota (US): North Dakota Agricultural Experiment Station (Fargo).
- Tørresen KS, Skuterud R. 2002. Plant protection in spring cereal production with reduced tillage. IV. Changes in the weed flora and weed seed bank. Crop Prot. 21:179–193. doi: 10.1016/S0261-2194(01)00081-3
- Vanhala P, Lötjönen T, Hurme T, Salonen J. 2006. Managing Sonchus arvensis using mechanical and cultural methods. Agric Food Sci Finland. 15:444–458. doi: 10.2137/145960606780061498
- Waller DM. 1985. The genesis of size hierarchies in seedling populations of Impatiens capensis Meerb. New Phytol. 100:243–260. doi: 10.1111/j.1469-8137.1985.tb02776.x
- Weaver SE, Cavers PB. 1980. Reproductive effort of two perennial weed species in different habitats. J Appl Ecol. 17:505–513. doi: 10.2307/2402345
- Weiner J. 1982. A neighborhood model of annual-plant interference. Ecology. 63:1237–1241. doi: 10.2307/1938849
- Zimdahl RL. 2013. Fundamentals of weed science. 4th ed. San Diego: Academic Press.
