ABSTRACT
Below-ground (bg) shoot emergence rates of Sonchus arvensis are dependent on temperature and root weight. However, it is unknown to what extent this is due to a root depletion rate that depends on initial root weight, or due to differences in resource allocation to fine root and bg shoot growth. To resolve this, we retrieved data from an experiment in which plants were grown in the dark at constant temperature (4°C, 8°C, and 18°C) and harvested prior to or at shoot emergence. A dynamic mass-balance model, in which biomass of the initial root was allocated to bg shoot and fine root daily growth, and where respiration took place from all tissues, was used. The relative depletion rate of root biomass (RDR; d−1) and fraction of the depleted biomass allocated to bg shoots (SFRR) were estimated and calibrated to observed biomass. The RDR increased with initial root weight and temperature and SFFR was highest for light roots and lowest for heaviest roots, whereas the rest was allocated to fine root biomass. The length-to-biomass ratio of bg shoots decreased with initial root weight. Under between-year weather variations (2004–2010), the reduction in root biomass during the coldest April–May was simulated to be over 12 days delayed compared with the warmest spring. The influence of biomass allocation on bg shoot elongation of heavier roots was thus stimulated by a larger fraction of root biomass being depleted, but counteracted by a smaller fraction of it allocated into bg shoot elongation, compared with lighter roots. The complexity of shoot emergence based on root depletion estimates may be a reason why predictions based on only an accumulated root weight-specific temperature sum, as proposed by a previous study, are expected to be less uncertain than those based on root depletion estimates.
Introduction
To obtain a mechanistic understanding of the life cycle of Sonchus arvensis L., we have studied the processes of shoot emergence (Anbari et al. Citation2011; Torssell et al. Citation2015), respiration during sprouting (Verwijst et al. Citation2013), and light and nitrogen competition during growth (Eckersten et al. Citation2010, Citation2011). An important determinant of the competitive ability of S. arvensis is shoot emergence (Brandsæter et al. Citation2010; Eckersten et al. Citation2011), which depends on several processes such as root depletion, respiration, and shoot elongation, which, in turn, depend on temperature and root biomass. To improve our understanding of the biomass dynamics during emergence, a simulation model for root depletion and dry matter allocation during shoot sprouting in the spring is used here to analyse observations on dry matter allocation, respiration, and below-ground (bg) shoot elongation in relation to temperature and initial root weight.
Most of the root system of S. arvensis is located in the ploughed horizon (Gruzdev & Tulikov Citation1966), but this weed has a weaker and less dense root system than other species, for example, Cirsium arvense (L.) Scop. (Jursik & Holec Citation2007). In the control of S. arvensis, attention has been paid to understanding the sprouting from the roots (Brandsæter et al. Citation2010) as related to dormancy, cold resistance, and build up and depletion of carbohydrate reserves. The roots are dormant only when the plant enters senescense during the later part of the growing season (Håkansson & Wallgren Citation1972a, Citation1972b; Fykse Citation1974 Grøndal et al. Citation2003; Brandsæter et al. Citation2010), and dormancy, therefore, is not regarded a pronounced feature of the life cycle during spring (Jursik & Holec Citation2007). The stored reserves consist of sugars, starch and inulin (Guncan Citation1973), which are gradually depleted during sprouting and growth. Weight loss due to respiration of the entire bg structure prior to shoot emergence is known to be strongly temperature dependent (Verwijst et al. Citation2013). Reserves are lowest at the flower budding stage, and replenished into roots towards the end of flowering and beginning of dormancy (Tulikov Citation1971; Fykse Citation1974, Citation1977). During emergence in spring, the root reserves are depleted and replenishment of the carbohydrate reserves in the roots can start after shoot emergence, which, in turn, depends on the depth at which bg shoots emerge from the roots.
Observations by Håkansson and Wallgren (Citation1972a, Citation1972b) and Gruzdev and Tulikov (Citation1966) conclude that shoots in the field commonly emerge from depths no deeper than 5–15 cm, and with increasing depth, the survival of sprouting shoots may decrease. Fykse (Citation1974) observed that S. arvensis emerged more rapidly and showed greater sprouting capacity at 15 cm depth than closer to the surface. However, in a previous experiment we found that shoot elongation was independent of depth (Torssell et al. Citation2015).
In the experiment by Torssell et al. (Citation2015), it was found that the time of shoot emergence at soil surface depended on the accumulated temperature sum, independent of the temperature regime for roots of a given initial weight. However, this sum was lower for heavier than lighter roots. This raised the questions to what degree this lower temperature requirement of the heavier roots could have been explained by a higher depletion rate from roots with a larger biomass, and if this rate was proportional to the root biomass (i.e. if the relative depletion rate [RDR] was constant), and further, how the depleted biomass was allocated between bg shoot and fine root biomass, and thereby may have influenced bg shoot elongation. The aim of this paper is therefore to estimate, from the same experimental material, parameters for root depletion rate, dry matter allocation, and bg shoot elongation and to derive the temperature influence on these processes. This is envisaged to provide information needed to evaluate the potential for predicting emergence time based on simulations of root depletion and the allocation of assimilates into bg shoot elongation.
Materials and methods
Observed plant data
Root runners of S. arvensis were taken from a root bank (for root collection and maintenance of the root bank, see Verwijst et al. Citation2013) and planted 30 June in 2009 in buckets (5 l) with moderately decomposed peat, fertilised (NPK) with 1 g N per bucket, and sealed with plastic and kept moist throughout the experiment. The buckets with root pieces of 5 and 10 cm length and thickness categories of fine (<2 mm), medium (2–4 mm) and thick (>4 mm) in diameter, respectively, planted at different depths (3, 10, and 17 cm), were placed in three dark chambers at constant temperature of 4°C, 8°C, and 18°C, respectively. The measured data were separated into three, not overlapping initial root weight classes with the mean initial root dry weights (RootDMInit) of 0.04425, 0.078836, and 0.162938 g. Concerning pretreatment, estimation of initial root weight, and further details of the experiment, see Torssell et al. (Citation2015). Harvest was performed five times per temperature treatment and comprised weight measurements of the retrieved roots (excluding fine roots) and bg shoots of 486 initially planted root fragments.
Biomass allocation model description
The change in old root dry matter from one day (t) to the next (t + 1) is the sum of losses by respiration and depletion into growth of bg shoots and fine roots. The old root biomass at day t + 1 is equal to(1) where Δt is one day. RRT is the fraction of root biomass lost by respiration and increases linearly with soil temperature T
(2) where aRR and bRR are coefficients (see below). The daily depletion of roots is proportional to its biomass according to the RDRTI, which depends on both initial root weight and temperature (denoted with subscripts I and T, respectively; see the Results section)
(3)
A fraction (SFRRI; 0–1) of the dry matter depleted from the roots is partitioned into bg-shoots but decreasing with its respiration according to(4) where SFRRI varies with the initial root weight (see the Results section). The remaining fraction of root depletion (1 − SFRRI) is allocated to fine roots. Considering also the respiration losses, which are calculated in analogy with root respiration, the fine root biomass is then:
(5)
Model application
Input parameters
The relative root depletion rate of each sample i(RDRObsi) was estimated analytically by integration of d(RootDM)/dt = −(RRT + RDRObsi) RootDM (combining Equations (1) and (3)) over time from t = ti to t = ti+1, which gives ln(RootDMti+1/RootDMti) = −(RRT + RDRObsi) (ti+1 – ti). Then, RDRObsi was determined analytically from the observed values of RootDM at t = ti and t = ti+1 as(6)
The linear regression function for daily relative respiration rates (RRT; Equation (2)), and its coefficients (aRR = 0.001126 d−1 and bRR = 0.000743 d−1 °C−1) were determined by Verwijst et al. (Citation2013) for the same dataset as used in our study, This made RRT equal to 0.0041, 0.0071, and 0.0145 d−1 for temperature treatments 4°C, 8°C, and 18°C, respectively.
Calibration
Using these estimates of RDRObsi and RRT as input to the simulations, the fraction of DM allocated to bg shoots (SFRRI) was calibrated by comparing simulated root and shoot weights with corresponding observed values (see the Results section). Model fit was analysed by means of the statistical relations between observed (O) and simulated (S) weights of the calibration targets (initial root weight and bg shoot biomass) in terms of the model efficiency (ModelEff = (Σ(O − OMean)2 − Σ(S − O)2)/Σ(O − OMean)2) and the relative error (RelError = 100(Σ((S − O)/O))/n). Also, linear regression between S and O was evaluated for the slope (target = 1) and the intercept (target = 0) of the line. In some cases, the additional criteria of the proportion of observed points above and below the predicted line were subjectively combined with the above criteria. The model and its optimisation routine were programmed in MATLAB-Simulink (The MathWork Inc., Citation2005) and in Microsoft Excel 2010. For the full set of parameter values, see .
Table 1. Parameter values used in the simulations (T = temperature), either estimated from experimental data or derived by calibration of simulated biomass to observed values.
Sensitivity simulations
For simulations of root depletion under field conditions, soil temperatures at 5 cm depth for April and May in 2006 and 2008 (the coldest and warmest spring during 2004–2010, respectively) were used, as observed at the Ultuna Meteorological Station (Karlsson & Fagerberg Citation1995) close to Uppsala (58.40°N, 17.39°E). Mean soil temperatures at 5 cm depth for April and May in 2008 were 5.6°C and 11.2°C, respectively, and in 2006 2.8°C and 9.3°C, respectively.
Results
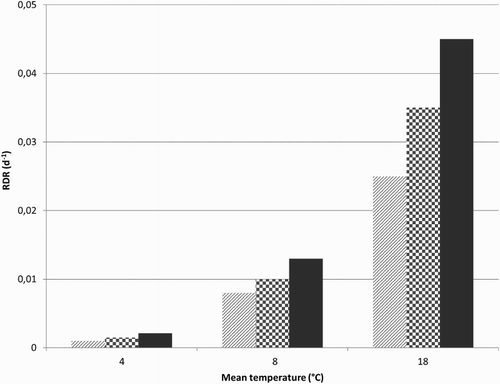
Both temperature and weight class had significant effects on the relative root depletion rate (RDRObs; Equation (6)) (). Within each weight class, there was a strong linear relationship between RDRObs and temperature, from which linear regressions were derived, to be used for calculating RDRTI for any temperature (T) between 4°C and 18°C(7) where aRDRI and bRDRI are −0.0058 d−1 and 0.0017 d−1 °C−1, −0.0086 d−1 and 0.0024 d−1 °C−1, and −0.0109 d−1 and 0.0031 d−1 °C−1 for light, medium, and heavy roots, respectively (n = 3 and R2 = 0.999−1.000).
Figure 1. Root relative depletion rate (RDRObs; d−1; Equation (6)) estimated for 4°C, 8°C, and 18°C and three root weight classes (light:hatched, medium:checkered, and heavy:solid).
Calibration of biomass partitioning
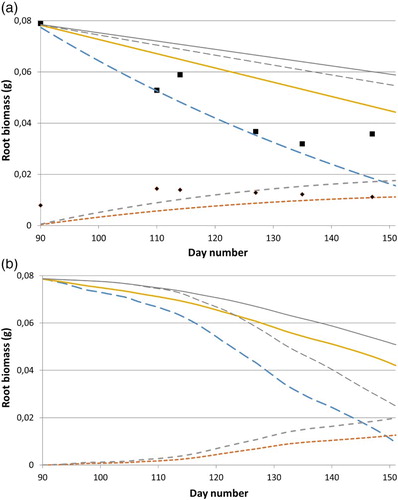
Using the RDRObs of , the fraction of depleted root biomass allocated to bg shoots (SFRRI) was determined by calibration of the model predictions (Equations (1)–(5)) to observed old root, bg shoot, and fine root biomass. It was found that SFRRI was not related to temperature, but decreased with initial root weight from 0.84 for the lightest root weight class, to 0.61 for medium weight roots, and down to 0.57 for the heaviest root weight class, that is, allocation to fine roots (1 − SFRRI) increased from 16% to 43% when the initial root weight was higher. The predictions of the decrease in biomass of the planted root fragments were essentially better than those of the increase in bg shoot biomass. The R2 values for roots ranged 0.20–0.45 and for bg shoots 0.03–0.30, and the number of samples per simulation was 24 and 17, respectively. An example of the agreement between simulated and observed values is given in (a).
Figure 2. Simulated biomass (g) of roots of weight class 2 (medium) during root depletion at (a) a constant temperature of 8°C and (b) for soil temperature at 5 cm depth at Ultuna during 2008. Thick lines are from above: total (solid line), old roots (dashed line), fine roots (smaller dashed), and bg-shoots (smallest dashed) biomass. Thin lines are corresponding values for total (solid line) and old roots (dashed line) biomass at (a) a constant temperature of 4°C and (b) year 2006. Points are observed values of old root (large points) and bg-shoot (small points) biomass for the constant temperature treatment at 8°C. x-axis is daynumber from January 1 (inputs to the simulation were: (a) initial start root weight = 0.079 g; SFRRI = 0.61; RRT = 0.71 d−1; RDRTI = RDRObs at 8°C = 0.0106 d−1; (b) the same except that RRT = f1(T) (Equation (2)) and RDRTI = f2(T) (Equation (7))).
Biomass partitioning dependence on temperature
Simulations of respiration, root depletion, and partitioning of dry matter to fine roots and bg shoots ((a)) show that respiration and depletion at a constant temperature of 8°C reduced the total biomass (old roots, bg shoot, and fine root) by almost 45% after 60 days. Of the remaining total biomass at this day, approximately 35% stayed in the initially planted root fragments (old roots) and the rest was allocated to shoot growth and fine root growth (about 25% and 40%, respectively). For the constant temperature of 4°C, the respiration losses were still fairly high, whereas the root depletion was small (see the two uppermost curves in (a)).
Under natural temperature variability, represented by temperatures during 2006 and 2008, the model predicted a weak bg shoot growth during April, but strong growth during May ((b)). At the end of May in 2008 (the warmest spring during 2004–2010), about 45% had been lost by respiration and 15% of the initial biomass remained in the old root. For 2006 (the coldest spring), the reduction in total biomass was about 12 days behind year 2008, and by the end of May about 30% of the initial biomass remained in the roots and about 35% had been lost as respiration.
Specific shoot length
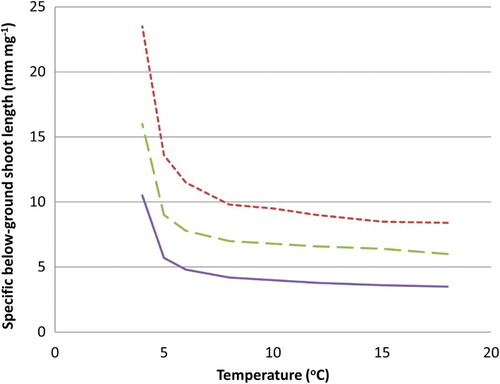
The bg shoot length-to-weight ratio (mm g−1, SSLTI, specific shoot length) was estimated as the ratio between values of bg shoot length as estimated by an empirical shoot elongation model developed by Torssell et al. (Citation2015), and the simulated bg shoot biomass in this study (ShootDM; Equation (4)). The model used for estimating the bg shoot length was Shootlengtht = aTI (t – to) + b, where aTI = cI × T + dI; where cI and dI varied with initial root weight class (see Torssell et al. Citation2015). SSLTI was dependent on root weight class and temperature and the shoots showed a drastic change in morphology at 6–8°C, below which the bg shoots tend to become increasingly thinner (). This tendency was more pronounced for roots from the light weight root class than for the heavy class.
Discussion
Heavier roots not only contributed to (i) more biomass to bg shoot growth in terms of being a larger source than lighter roots, but also (ii) depleted a larger fraction of their biomass (i.e. higher RDRTI). However, this was counteracted by (iii) a smaller fraction of this depletion to be allocated into bg shoot growth (i.e. lower SFRRI) (). Furthermore, (iv) shoot length per unit weight () was lower for shoots from heavy weight roots (i.e. lower specific shoot length SSLTI) than for bg shoots emerging from lighter roots.
The observed increased RDRTI with increasing root weight also agreed with results from the outdoor experiment performed by Anbari et al. (Citation2011). The increased depletion of heavier roots may be associated with the relatively larger proportion of stored carbohydrates and proteins versus lignified tissue; the higher the content of stored nutrition, the higher the depletion rate (Guncan Citation1973). Heavy roots apparently promote a rapid development of fine roots at the expense of allocation to shoots. It was found, when calibrating SFRRI, that the simulated bg shoot biomass had a larger prediction error, compared with the corresponding predictions of root biomass when calibrating the RDRTI. In a later, above-ground phase, shoots are known to display skewed distributions of shoot sizes, and a large size variation (Anbari et al. Citation2016). These skewed frequency distributions likely reflect the state of shoots prior to emergence, and that state may be a reason for the low fit of the shoot DM growth model based on depletion.
Temperature effects
The root reserves will decrease more efficiently (RDRTI is higher) if soil temperature increases, while shoots are still under ground, and decrease more for heavier than lighter roots (). This is in agreement with results of Verwijst et al. (Citation2013). Also, root morphology changes with temperature (), and at high temperatures the bg shoot length-to-biomass ratio (SSLTI) is low, whereas at low temperatures bg shoots are longer and lighter. Also, this effect is stronger for lighter than heavier roots, which might reflect a stress reaction.
Predicting emergence
When predicting the emergence time of above-ground shoots based on the rate of root depletion and allocation into bg shoot growth, all the information on root morphology and change in the rate of bg shoot growth rate with temperature gathered in this study would be needed. Therefore, the prediction of bg shoot elongation based on its growth rate is expected to be more complicated and more uncertain, than when based on the initial root weight alone, a method evaluated by Torssell et al. (Citation2015) for the same observed data as used in this study. In both methods, shoot elongation depends on temperature. In our case, the relative amount of biomass depleted from the roots, the fraction allocated into bg shoot growth, and the specific shoot length depended on temperature, whereas in the Torssell et al. (Citation2015) method, the bg shoot elongation dependency on temperature was invariant for a specific initial root size, resulting in fewer parameters needed to be determined. When we calculated emergence date of shoots from roots at 10 cm depth in Uppsala, in a similar way as did Torssell et al. (Citation2015), but based on root and shoot weight simulations, the emergence date during 2004–2010 varied more with temperature (between May 1 and 20) than the emergence calculations from Torssell et al. (Citation2015), which varied between May 9 and 22, but less with root weight class (). An increase of temperature by 2°C would cause growth in spring to start earlier than March 1 (not considered by Torssell et al. Citation2015). An increase by 2°C from January 1 would advance emergence by 17 days, instead of 10 days, indicating that this effect might be large.
Table 2. Time of emergence response to changes (Δ) of temperature and/or root weight.
Emergence predictions for weed control
Weed control strategies usually focus on disturbing the weed either during the root carbohydrate reserve storing period at the end of the growing season, or in the beginning of spring when the plants are close to their compensation point, that is, the stage when the plant biomass is at minimum (Håkansson Citation1969; Tavaziva Citation2012). The model developed in this study provides a method to predict the root depletion period in advance based on temperature conditions and root sizes, and suggests that root depletion occurs essentially earlier in case of a warmer climate. However, we conclude that the different temperature requirements for shoot emergence at the soil surface can not only be explained on the basis of inital root biomass. Instead, the positive effect of a large root biomass is amplified by the RDRTI increasing with the initial root biomass, but reduced by a decreased share of the depleted amount of the heavier roots being allocated to the bg shoots, which also have a smaller specific shoot length (SSLTI) than lighter roots. To further complicate the estimation of date of emergence, the temperature effects on RDRTI and SSLTI differed among heavy and light roots. Hence, predictions of bg shoot elongation based on root depletion and temperature-dependent respiration simulations would be difficult, because bg shoot growth was governed by both the relative rate of root depletion and respiration being temperature dependent, and because bg shoot growth is a less predictable process than root depletion. Furthermore, the specific shoot length was shown to be dependent on temperature and root weight. Consequently, predictions of shoot emergence date at soil surface based on root depletion estimates are complex, and can be a reason why predictions based on only an accumulated temperature sum specific for each initial root weight, as proposed by a previous study, are expected to be less uncertain than those based on root depletion estimates.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anbari S, Lundkvist A, Forkman J, Verwijst T. 2016. Population dynamics and nitrogen allocation of Sonchus arvensis L. Acta Agric Scand Sect B – Soil Plant Sci. 66:75–84.
- Anbari S, Lundkvist A, Verwijst T. 2011. Sprouting and shoot development of Sonchus arvensis in relation to initial root size. Weed Res. 51:142–150. doi: 10.1111/j.1365-3180.2010.00837.x
- Brandsæter LO, Fogelfors H, Fykse H, Graglia E, Jensen RK, Melander B, Salonen J, Vanhala P. 2010. Seasonal restrictions of bud growth on roots of Cirsium arvense and Sonchus arvensis and rhizomes of Elymus repens. Weed Res. 50:102–109. doi: 10.1111/j.1365-3180.2009.00756.x
- Eckersten H, Lundkvist A, Torssell B. 2010. Comparison of monocultures of perennial sow-thistle and spring barley in estimated shoot radiation-use and nitrogen-uptake efficiencies. Acta Agric Scand Sect B – Soil Plant Sci. 60:126–135.
- Eckersten H, Lundkvist A, Torssell B, Verwijst T. 2011. Modelling species competition in mixtures of perennial sow-thistle and spring barley based on shoot radiation use efficiency. Acta Agric Scand Sect B – Soil Plant Sci. 61:739–746.
- Fykse H. 1974. Research into Sonchus arvensis L. II. Distribution in Norway, growth and dormancy in comparison with similar species. Statens plantevern, Ugrasbiologisk avdeling, Særtrykk. 115:389–412.
- Fykse H. 1977. Research on Sonchus arvensis (L.), Cirsium arvense (L.) Scop. and Tussilago farfara L. Translocation of radioactive-labelled carbohydrates and MCPA. Meldinger fra Norges Landbrukshøgskole. 56:1–22.
- Gruzdev G, Tulikov A. 1966. Peculiarities of the vegetative reproduction of yellow sowthistle (Sonchus arvensis) and creeping thistle (Cirsium setosum M.B.). Izvestiya Timiryazevskoi Sel’skokhozyaistvennoi Akademii. 6:83–95.
- Grøndal H, Graglia E, Jensen RK. 2003. Root bud dormancy of Canada Thistle and Perennial Sowthistle? DJF Rapport. Markbrug. 89:357–358.
- Guncan A. 1973. Carbohydrate reserves of Sonchus arvensis rhizomes during a vegetative season and comparison with Convolvulus arvensis L. Mededelingen Fakulteit Landbouwwetenschappen Gent. 38:1011–1017.
- Håkansson S. 1969. Experiments with Sonchus arvensis L. I. Development and growth in response to burial and defoliation in different developmental stages. Ann Agric Coll Sweden. 35:989–1030.
- Håkansson S, Wallgren B. 1972a. Experiments with Sonchus arvensis L. 2. Reproduction, plant development and response to mechanical disturbance. Swed J Agric Res. 2:3–14.
- Håkansson S, Wallgren B. 1972b. Experiments with Sonchus arvensis L. 3. The development from reproductive roots cut into different lengths and planted at different depths, with and without competition from barley. Swed J Agric Res. 2:15–26.
- Jursik M, Holec J. 2007. Biology and control of sugar beet significant weeds: perennial sow-thistle (field sow-thistle) – Sonchus arvensis L. Listy Cukrovarnické a Reparské. 123:86–90.
- Karlsson S, Fagerberg B. 1995. Climate and bioclimate Station at Ultuna. Uppsala, Sweden: Swedish University of Agricultural Sciences, Department of Crop Production Science.
- The MathWork Inc. 2005. MATLAB version 7.1 with Simulink, release 14. Natick, MA: The MathWork Inc.
- Tavaziva VJ. 2012. Effects of competition on compensation point and phenological development in Sonchus arvensis L. [master thesis]. Uppsala: Swedish University of Agricultural Sciences. Available from: http://stud.epsilon.slu.se/4572/
- Torssell B, Eckersten H, Anbari S, Lundkvist A, Verwijst T. 2015. Modelling below-ground shoot elongation and emergence time of Sonchus arvensis shoots. Acta Agric Scand Sect B – Soil Plant Sci. 65:582–588.
- Tulikov AM. 1971. Investigation of the vegetative reproduction organs of rhizomatous weeds. Doklady TSKhA. 175:177–181.
- Verwijst T, Eckersten H, Anbari S, Lundkvist A, Torssell B. 2013. Weight loss in overwintering below-ground parts of Sonchus arvensis under current and temperature elevated climate scenarios in Sweden. Weed Res. 35:21–29. doi: 10.1111/wre.12001