ABSTRACT
During 2008–2011, model field experiments were carried out at the Joniškėlis Experimental Station of Lithuanian Research Centre for Agriculture and Forestry on a clay loam Endocalcaric Endogleyic Cambisol. The study was aimed to establish the comparison of various postharvest practices (mineral nitrogen fertiliser alone or together with a bioactivator Penergetic k, livestock slurry, red clover biomass and straw incorporation in the soil by a stubble cultivator at a 10 cm depth) on the acceleration of the initial (nine-month period) decomposition of winter wheat straw. During this period, straw mass decomposition intensity was 20.7–29.1%, carbon (C) concentration decreased by 6.5–22.8%, while an increase in nitrogen (N) by 1.1–2.2 times was observed. The highest straw decomposition rate was recorded when after straw incorporation autumn was warm and humid. That year straw mass C to N ratio (C/N) was 38–46. Under less-favourable autumn conditions, the highest decomposition of straw was achieved, having applied mineral N (with and without Penergetic) and livestock slurry and having incorporated the straw in the soil (C/N = 40–55). A slower decomposition rate was observed for the straw spread on the soil surface with mineral N addition or on undersown red clover.
Introduction
Short crop rotations, based on cereals and oilseed rape are most common in intensive farming systems, where biomass export from a farm prevails. Efforts to stabilise crop productivity result in an increased reliance on resource imports, which in turn aggravates management of their negative environmental impacts. Organic carbon (C) stocks in the soil are diminishing. Excess nitrogen (N) is accumulating in water, soil systems and the atmosphere (Roer et al. Citation2012). This contradicts the principles of sustainable agriculture. On the other hand, C and N cycles are fundamental components of ecosystem functioning and processes (Beier et al. Citation2008). Incorporation of cereal straw into the soil has become an important measure for soil fertility improvement in Lithuania. Moreover, large quantities of straw (up to 6 t ha−1) accumulate in crop production farms. Straw is natural, plant-derived organic manure, which is degraded and transformed into other organic compounds by soil microorganisms. Plant residues can be fully mineralised in the soil and increase CO2 emission and plant nutrient stocks; their mineralised products can be assimilated and immobilised in the biomass of microorganisms, or partly altered can be humified (Badia et al. Citation2013). During biochemical processes in the soil, changes occur in N availability, plant nitrogen-use efficiency and accumulation in the soil (Said-Pullicino et al. Citation2014). The above-mentioned processes are determined by the chemical composition of plant residues (Kriaučiūnienė et al. Citation2012), environmental conditions and agricultural management practices applied. The experience of Lithuanian and foreign researchers suggests that in order to accelerate straw decomposition and increase N immobilisation in the microbial biomass and soil organic compounds it is necessary to additionally incorporate mineral N (Mary et al. Citation1996; Janušauskaitė et al. Citation2013) and increase the contact with soil microorganisms, soil moisture and nutrients (Tarafdar et al. Citation2001). Soil organic C transformation and changes are the factors on which not only C sequestration but also many other soil quality indicators depend (Aguilera et al. Citation2013). Straw used as manure increases soil humus content (Badia et al. Citation2013; Bogužas et al. Citation2015), improves soil structure (Blanco-Canqui & Lal Citation2009) and its aggregate stability (Lenka & Lal Citation2013), crop yield and N accumulation in it (Malhi et al. Citation2011).
It is necessary to manage straw decomposition processes towards achieving the desired outcomes. This is especially relevant for heavy soils, whose intrinsic water, air and thermal regimes determine weaker microbiological activity and changes in biochemical processes (Janušauskaitė & Velykis Citation2010). With a more focus on environment-safe crop production technologies, mitigation of climate change effects and reduction of mineral N fertiliser use it is worthwhile to replace it with other more environment-friendly alternatives. The current study was aimed to determine the effects of N-rich organic and mineral fertilisers, bioactivator, as well straw incorporation and combination of these agricultural practices on the acceleration of initial straw decomposition during a postharvest period.
Material and methods
Experimental site and soil
Research was done in the northern part of Central Lithuania’s lowland (56°12′N, 24°20′E). The soil of the experimental site is a limnoglacial Endocalcaric Endogleyic Cambisol (Siltic, Drainic). The soil texture is clay loam (at a depth of 0–25 cm) on silty clay (at a depth of 26–76 cm) with deeper lying sandy loam (at a depth of 77–135 cm). The topsoil layer (0–25 cm) contained 270, 500 and 230 g kg−1 clay, silt and sand, respectively. The topsoil (0–25 cm) is close to neutral (pHKCL 6.3–6.5), medium in phosphorus (P2O5 146–169 mg kg−1 soil), high in potassium (K2O 221–260 mg kg−1 soil) and moderate in organic carbon (11.8–12.8 g kg−1 of soil) and moderate in total nitrogen (1.22–1.82 g kg−1 of soil). Lithuania has a climate mid-way between maritime and continental.
Experimental design
Field experiments were carried out at the Joniškėlis Experimental Station of the Lithuanian Research Centre for Agriculture and Forestry. Experiment was set up after the winter wheat harvesting in different periods: 2008–2009, 2009–2010 and 2010–2011. Decomposition of winter wheat straw was stimulated using the following measures: (1) stubble cultivation at 10 cm depth, straw removed from the field (SC); (2) N fertiliser spread on non-incorporated straw (NF); (3) N fertiliser spread and straw incorporated during stubble cultivation (NF+SC); (4) N fertiliser and bioactivator Penergetic k spread and straw incorporated during stubble cultivation (NF+Pk+SC); (5) straw spread on red clover (RC); and (6) livestock slurry spread and straw incorporated during stubble cultivation (LS+SC).
Establishment of model field experiments
Field experiments (plot size 5 m × 7 m) were conducted using a litter bag method in order to determine straw decomposition rate. Shortly after harvesting, winter wheat straw chopped by a combine harvester was evenly spread in the experimental plots. Mineral N (in the form of liquid urea; 10 kg N per tonne of straw) was applied in the fields of treatments 2, 3 and 4. At the same time the plots of treatment 4 were sprayed with a bioactivator Penergetic k at a rate of 300 g ha−1. Penergetic k promotes healthy fertile soil, aerobic processes, organic matter breakdown and root growth and is composed of the substances of patented composition present in a CaCO3+MgCO3 medium. Penergetic k works with the biological processes in nature to stimulate the microogranisms in the soil (Penergetic Products for Agriculture). In the plots of treatment 5, a red clover cultivar ‘Vyliai’ was undersown (at a seed rate of 18 kg ha−1) in winter wheat in spring after resumption of vegetation. In October, red clover fresh mass was chopped and together with straw incorporated into the soil during the autumn ploughing. The slurry content in the plots of treatment 6 was calculated according to the N content required for the decomposition of straw (according to the same rate as for N fertiliser). Stubble cultivation (treatment 1) and straw incorporation (treatments 3, 4 and 6) immediately after the N fertiliser, Penergetic k and animal slurry application, were performed by a universal stubble cultivator at a 10 cm depth. In the second half of October, the experimental field (all treatment plots) was ploughed by a mouldboard plough at a 25 cm depth. Samples of chopped straw (≈20 g) were analysed for dry matter (DM) content and chemical composition and then were placed in polychlorvinyl mesh bags 20 cm × 20 cm in size and a mesh diameter of ≈1.0 mm. The straw in the bags was treated with chemical and biological products in the same way as in the experimental plots (according to the experimental design). The bags were buried in the topsoil at a 0–10 cm depth or placed on the soil surface to simulate straw incorporation by stubble cultivation or spread on the soil. Bags with straw were incorporated into soil of all treatments 4 August 2008, 27 August 2009 and 10 August 2010. During the autumn ploughing the bags with straw were incorporated into a deeper 15–25 cm soil layer to simulate topsoil inversion and straw incorporation during ploughing. During the course of the initial straw decomposition process (nine months after incorporation) the bags with straw residue were removed early in spring and were analysed. In various experimental periods: 2008–2009, 2009–2010 and 2010–2011 the bags were kept in soil for 138, 136 and 143 days respectively, when the average daily temperature was >0°C. The experiment was set up in four replications.
Plant, microbial and soil analyses
Having removed the bags from the soil, all straw residues were thoroughly collected and weighed and analysed for DM content, chemical composition and counts of bacterial and fungal colonies. The DM content in straw was established by drying at 105°C. Straw of winter wheat was analysed for C – by the Dumas method, N – by the Kjeldahl method, phosphorus (P) and potassium (K) – using a spectrophotometer UV/VIS Cary 50 and an atomic absorptiometer AANALYST 200, respectively. DM change (%) per nine months was calculated as follows: 100 − (mass (DM) nine months after straw incorporation×100)/straw mass (DM) before incorporation. Quantification of fungal and bacterial cells on the straw was performed using a standard dilution spread-plating method. For heterothrophic bacteria quantification Soy Tryptic agar (TSA/10 agar, Biochemika) and malt extract agar (Liofilchem Diagnostici) were used for total fungal counts, and these media were acidified to inhibit bacterial growth. All inoculated plates were incubated at 22 ± 2°C temperature for 3–5 days. The total number of colony-forming units (CFUs) on the plate was expressed as the number of culturable bacterial and fungal cells per 1 g of dry straw. Mineral N concentration in the soil of the experimental plots at a 0–60 cm depth was determined in spring nine months after straw incorporation. The following methods were used for mineral N determination: nitrate N (N–NO3, mg kg−1) by the ionometric method and ammonia N (N–NH4, mg kg−1) by the spectrophotometric method.
Meteorological conditions
In 2008, the postharvest period of cereals was characterised by higher amount of rainfall at the beginning of straw decomposition ().
Table 1. Average monthly temperatures and precipitation.
During a month before and a month after straw incorporation (August 4) the rainfall was amounted to 64 and 108 mm. The autumn was little warmer than usual (+0.7°C). October was very warm and without negative temperatures (1.8°C warmer than normal). The average negative daily temperature settled in the second ten-day period of December. The winter of 2008–2009 was warmer and wetter compared to multi-annual average data. Positive average daily temperature was recorded in the last days of March 2009. April was 2.2°C warmer compared to multi-annual average. The study period of 2008–2009 is considered as favourable for straw decomposition.
In 2009, the straw was incorporated rather late (August 27). In this experimental period, the amount of rainfall during a month before and a month after straw incorporation was the lowest – 39 and 49 mm, respectively. In the autumn, the amount of rainfall was similar to that of multi-annual average. However, the conditions for straw decomposition in the autumn were little favourable due to the warm but dry September and moist but cold October. The negative daily average temperature settled in the second 10-day period of December. Winter of 2009–2010 was colder compared with multi-annual average. Positive average daily temperature was recorded in the middle of March 2010. Spring conditions were close to the usual. The period of 2009–2010 was less favourable for straw decomposition due to autumn of 2009 weather.
In 2010, the straw was incorporated on August 10. The amount of rainfall during a month before and a month after straw incorporation was 69 and 50 mm, respectively. The average daily temperature of August was 2.7°C higher compared to the multi-annual data. During September–October drier and cooler weather prevailed. Wintry weather started within the last days of November. The winter of 2010–2011 and March of 2011 were changeable. Positive daily temperature was recorded only at the beginning of April. April was warmer and drier than normal. Conditions for straw decomposition in this study period (2010–2011) were little favourable.
Statistical analysis
This paper presents the decomposition intensity (DIM) index, which represents the amount of straw mass (%), decomposed within a certain period time (nine months). DIM were calculated using Equation (1) as follows:(1) where Mstart – mass of straw dry matter (g) prior incorporation and Mend – mass of straw dry matter (g) at the end of experiment. The least significant differences in straw mass and chemical composition indicators, bacterial and fungal colony, mineral N contents between the treatments were established according to the LSD Fisher protected test. Differences were considered significant – at p < .05. Interrelationships among straw DM mass, chemical composition indicators, bacterial and fungal colony counts, mineral N were estimated by linear equations (y = a+bx, n = 24) and correlation coefficients (r). All statistical analyses were performed using the SELEKCIJA software package, STAT_ENG program vers. 1.55 and ANOVA adapted for EXCEL version 3.1 (Tarakanovas & Raudonius Citation2003).
Results and discussion
Variation of straw mass and chemical composition
The period after cereal harvesting until the beginning of the following vegetation season (August–April) is used for completing cereal cultivation technologies, that is, to promote initial straw decomposition and restore soil productivity. In our experiment with straw the soil received a large amount of organic matter (DM), C and K, while considerably lesser amounts of N and P. Such chemical composition of straw determined a wide C to N ratio (C/N).
The above-ground mass of red clover, used for straw decomposition, was rich in C, N and K. The C/N shows rapid clover mass decomposition; however, only after its incorporation late in the autumn ().
Table 2. The chemical characteristics of above-ground mass of red clover and slurry.
Livestock slurry additionally enriched the soil not only with N, but also with K (average 51.9 kg ha−1). The slurry contained very little organic matter and part of N (28.0–32.1%) was in the mineral form, therefore they were directly involved in the straw decomposition process. DM, C, N, P and K concentration in manures might be influenced by the intensity of cereal fertilisation, soil type (Ghaffar & Fan Citation2013) and year’s meteorological conditions (Orlova Citation2013).
Using the litter bag method it was established that during a nine-month period straw mass DIM was on average 20.7% in 2008–2009 experimental period, 29.1% in 2009–2010 and 22.4% in 2010–2011 ().
Table 3. Decomposition intensity of straw residue mass and its DM after nine months.
The data from experimental period 2008–2009 suggest that straw decomposed the slowest when spread on the RC crop. Compared with the above-mentioned treatment, significantly more rapid decomposition was recorded having applied LS+SC and NF+SC. In experimental period 2009–2010, no appreciable differences between the treatments with the addition of mineral N (NF+SC), slurry and RC for straw decomposition were established. However, in the NF+Pk+SC, NF+SC and LS+SC treatments the straw decomposed significantly more than in the NF or SC treatment. In experimental period 2010–2011 only DIM trends were established.
During straw decomposition the DM content was decreasing and after nine months accounted for 25.9–49.8% of the incorporated initial level (data not shown). In all experimental years, the highest DM content was in the NF treatment (high DM content was also in the RC in period 2009–2010); the lowest DM content was in the LS+SC treatment (low DM content was also in the NF+Pk+SC in period 2009–2010). The DM content in straw did not significantly differ between NF+SC and RC. DM content significantly decreased in experimental period 2009–2010 having used practices (NF+Pk+SC, LS+SC) promoting microbial activity (compared with SC). Another studies reported that the straw showed an initial weight loss of 30% during the first month (Christensen Citation1985) and 44% during the 2 months (Turk & Michelič Citation2013) after incorporation.
Release of C from straw was slower than the change of mass. Over a nine-month period, C concentration in the straw of experimental period 2008–2009 decreased by on average 10.7%, in 2009–2010 by 22.8%, and in 2010–2011 by 6.5%, compared with its content before straw incorporation (data not shown). The highest content of C in straw was in the treatments where straw had been spread on the RC (all periods) and on soil surface (2008–2009 and 2009–2010) ().
Table 4. The concentration of C and N in straw residues before incorporation and after nine months.
Kuang et al. (Citation2014) showed that the retention of residues on the soil surface retarded decomposition. The content of C in straw (2008–2009) significantly decreased by 18.1–23.4% having used other practices (SC, NF+SC, NF+Pk+SC and LS+SC), compared with NF. In experimental period 2009–2010 the above-mentioned practices under C content differed little and no significant differences between the treatments were established. In period 2010–2011 significantly lower C content in straw was identified in NF+SC and LS+SC treatments, where C content in straw decreased by 12.7% and 12.1%, respectively compared with treatment SC or by 20.0 and 19.4%, respectively, compared with treatment RC. Studies in Norway have shown that in field experiments carbon per year remained 49% of its initial amount, incorporated with straw (Henriksen & Breland Citation1999a). This process depends on the soil texture (Ghaffar & Fan Citation2013), soil moisture (Wu et al. Citation2011) and decomposition time (Kuang et al. Citation2014).
In straw, N and P are strongly bound in organic compounds, therefore with a rapid reduction in straw DM and C content, N and P concentrations increased (Henriksen & Breland Citation1999a). In our study, the N content in straw increased by 2.2 times (2008–2009), by 1.1 times (2009–2010) and by 1.7 times (2010–2011) (data not shown). In experimental periods 2009–2010 and 2010–2011, the highest N contents were found in the NF+SC and NF+Pk+SC treatments (). The differences in these treatments, compared with NF and RC treatments were significant. In 2008–2009, all practices were effective, compared with SC. Corbeels et al. (Citation2000) indicate that N fertiliser use for straw decomposition enhanced C mineralisation and N immobilisation compared to the treatment without added N. The effect of NF was ambiguous: in experimental periods 2009–2010 and 2010–2011 the N content in straw was the lowest and in 2008–2009 it was the highest and differed little from the straw incorporated in the NF+SC treatment. It can be concluded that mineral N efficacy for the decomposition of non-incorporated straw is determined by the weather conditions.
Changes in the straw P content were less consistent than those in N. The highest P content was in the straw of experimental period 2008–2009. The practices used for straw decomposition in this period practically did not differ in efficacy among the treatments ().
Table 5. The concentration of P and K in straw residues before incorporation and after nine months.
In experimental period 2009–2010, the P content in straw residues was significantly higher in the NF+SC, NF+Pk+SC and LS+SC treatments than in the SC, NF and RC treatments. The data of period 2010–2011 show that P concentration in straw changed in a similar way to that in period 2009–2010 (except NF+Pk+SC), only the differences between the treatments were less distinct. Data from Heuck et al. (Citation2015) suggested that mineralisation of organic phosphorus was driven by microbial carbon demand.
Unlike N and P, part of K from straw was lost during the initial nine months after its use as manure (). The greatest reduction in K concentration occurred in the straw of experiment period 2009–2010 in the NF and RC. In experiment periods 2008–2009 and 2009–2010, these differences were significant compared with other uses of straw. In all periods an inverse strong relationship (r = –0.64–0.78, p > .01) was established between K and C concentrations in straw residues. Literature sources suggest that for the straw of most crop species the highest contents of K are released during the first month after incorporation (Christensen Citation1985; Kuang et al. Citation2014). Wu et al. (Citation2011) demonstrated that the sequence of nutrient release rates was K > P > N ≈ C.
Bacterial and fungal counts in straw
Nine months after straw incorporation, the highest counts of bacterial colonies were identified in experimental period 2010–2011, where their number was several times higher than that in periods 2008–2009 and 2009–2010 ().
Table 6. The counts of bacterial and fungal colonies in straw residues nine months after straw incorporation.
The practices applied in experiment period 2008–2009 did not have significant effect on the bacterial counts in straw. Positive effects of Panergetic k manifested in period 2009–2010, where the bacterial count was significantly higher compared with the SC and LS+SC treatments. It is interesting to note that the stimulator used in combination with fertilisers enhances soils oxidation characteristics, increases its electrical conductivity and has an effect on vicissitude of mineral nitrogen in soil (Pekarskas et al. Citation2011). In experimental period 2010–2011, significantly higher bacterial count was detected in the treatment where straw decomposed the least in the autumn (NF), compared with other treatments.
Fungi are the most important contributors to the degradation of recalcitrant plant material (Marschner et al. Citation2011). In other studies, authors found that fungi amount was higher in the next year after straw incorporation (Janušauskaitė et al. Citation2013). The highest fungal counts were detected in the straw of experimental period 2009–2010; however, there were no significant differences between the treatments. In other experimental periods the straw contained twice as few fungi as in 2009–2010. There were significant differences between practices applied in 2008–2009. According to increasing fungal count, they ranked in the following order: RC, LS+SC < NF + Pk + SC < NF < NF + SC < SC. In experimental period 2010–2011, lower fungal count was detected having used slurry for straw decomposition (LS+SC), higher NF and NF+SC.
The relationship between bacteria, fungi and chemical composition indicators of straw residues was established only in experiment periods 2008–2009 and 2010–2011. In experiment period 2008–2009, there was determined a direct, weak but significant relationship between N concentration in straw and bacterial count (r = .456, p > .05). The relationship between fungal count and C concentration in straw was inverse and weak (r = –.443, p > .05). This showed that recalcitrant organic compounds were being decomposed. In period 2010–2011, the chemical composition indicators of straw residues influenced only bacterial changes. Bacterial counts increased with increasing straw residue DM (r = 0.613, p > .01), decreasing N concentration (r = –.450, p > .05) and increasing C/N (r = .495, p > .05).
Intensity of initial decomposition of straw
Straw decomposition depends on the intensity of productive soil moisture reserves, temperature (Wu et al. Citation2011; Kuang et al. Citation2014), microbial community (Henriksen & Breland Citation1999b) and straw particle size (Tarafdar et al. Citation2001). The impact of multiple freezing and thawing during winter, which caused an intermediate substrate mineralisation, on straw decomposition is undervalued (Lukas et al. Citation2013). In our study, the most favourable weather conditions after straw incorporation was in experimental period 2008–2009 and the less favourable in 2009–2010 and 2010–2011 (). Nine months after straw incorporation, the losses of straw mass (DM) directly depended on straw residue characteristics. However, these relationships differed between the periods. The data of period 2008–2008 indicated that when N had reached 7.7–9.8 g kg−1 and P 1.0–1.2 g kg−1 range in straw residue, these indicators did not have any direct effect on the reduction of straw mass.
In experimental periods 2009–2010 and 2010–2011, the contents of N and P in straw residues varied within a much wider range. In these experimental periods, with increasing N and P concentration in straw residues, the part of decomposed straw mass increased. The relationships between straw mass changes and N and P in straw residues were direct: in experimental period 2009–2010, with increasing N in straw from 5.7 to 10.3 g kg−1 and P from 0.5 to 1.0 g kg−1, straw mass losses during the research period increased by 22.4–29.3% (r = 0.69 and r = 0.89–0.55, p > .01 respectively); in 2010–2011, with increasing N content in straw from 6.0 to 8.3 g kg−1 and P from 0.7 to 0.9 g kg−1 straw mass losses increased by 19.5–25.2% (r = 0.69, p > .05 and r = 0.43, p > .05, respectively). Research findings suggest that soluble, readily decomposable organic carbon compounds that account for 7.2–18.9% of straw DM are the first to decompose (Ghaffar & Fan Citation2013). The other larger part of straw is composed of organic compounds that are harder to decompose – hemicellulose (34.04%), cellulose (45.58), and especially lignin (7.22%) (Le Guillou et al. Citation2011). These compounds decompose later (Kabuyah et al. Citation2012). Straw N and P are released much later when the C to N ratio was between 28 and 35 (Christensen Citation1985).
From incorporation to the beginning of the next vegetation the C/N in straw decreased on average by 58.7% (to 38–52) in experimental period 2008–2009, by 25.9% (to 40–79) in 2009–2010, and by 44.1% (to 49–76) in 2010–2011 compared with that before straw incorporation ().
Figure 1. The C/N in straw residues before incorporation and after nine months.
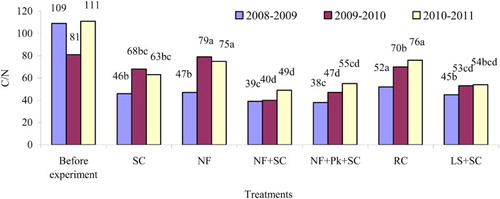
Lower C/N was established having used NF+SC and NF+Pk+SC for straw decomposition compared with other methods (significant in 2008–2009). Here, the C/N in period 2008–2009 was 39 and 38, in 2009–2010 was 40 and 47, and in 2010–2011 was 49 and 55. The significant slowest decomposition rate was observed in period 2008–2009 – RC, in 2009–2010 – NF and in 2010–2011 – NF and RC.
The greatest reduction in the C/N occurred in experimental period 2008–2009, where the variation of this ratio was influenced by the C and P concentration in straw residues, while N did not have significant effect. A strong direct correlation was established between straw C/N and C concentration (r = 0.70, p > .01). The relationship between C/N and P concentration was moderately strong and inverse (r = –0.50, p > .05). In periods 2009–2010 and 2010–2011, N had the greatest impact on the C/N. With increasing N, the C/N decreased (r = –0.92–0.86, p > .01). Similar but slightly weaker relationships were established between the C/N and P and K (in 2009–2010 they were stronger than that in 2010–2011). The C/N directly correlated with straw residue C (r = 0.56–0.64, p > .01). According to increasing C/N of straw residues and decreasing decomposition rate, the practices applied ranked in the following order: N fertiliser and straw incorporated by a stubble cultivator (39–49) < N fertiliser together with the bioactivator and straw incorporated by a stubble cultivator (38–55) < slurry and straw incorporated by a stubble cultivator (45–54) < stubble cultivation (46–68) < red clover (52–76); and N fertiliser spread on the soil and straw not incorporated (47–79).
Mineral N availability in soil is an important factor affecting plant residues decomposition under field conditions (Mary et al. Citation1996). Henriksen and Breland (Citation1999b) found that the concentrations of available N below 1.2% of straw dry matter significantly reduced the rate of carbon mineralisation from straw residues and the growth of total soil microbial biomass. In the initial stage of straw decomposition (in the autumn), microorganisms use soil, mineral and organic fertiliser N for straw decomposition and immobilisation of N takes place. Orlova (Citation2013) suggests that microorganisms incorporate up to 13% of fertiliser N into its biomass and up to 57% N into the composition of soil organic compounds. Our data indicate that the lowest N immobilisation occurred when the straw had been spread on the soil surface with N fertiliser (data from experimental periods 2009–2010 and 2010–2011). This happens because of the weak contact of microorganisms with straw and N. When straw is incorporated late in the autumn during ploughing, low temperature limits microbial N immobilisation and N losses are possible.
Experiments done abroad evidenced that after straw incorporation (in the autumn), nitrate content in the soil decreased and the C and N of microbial biomass increased within the first week of the experiment (Shindo & Nishio Citation2005). Straw use as manure helps to tighten N cycle (Said-Pullicio et al. Citation2014) and in this way reduce nitrate leaching in the autumn (Janušauskaitė et al. Citation2013). When the straw does not completely decompose in the autumn, mineral N concentration in the soil in spring can decrease due to N immobilisation. This is relevant in spring, when the demand for N is high. Our experimental data indicate that in period 2008–2009 significantly higher mineral N content in soil was recorded having used RC, NF, NF+Pk+SC, NF+SC for straw decomposition compared with other practices ().
Figure 2. Mineral N concentration in a 0–60 cm soil layer nine months after straw incorporation.
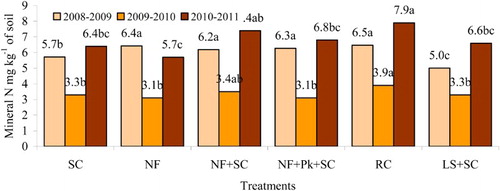
In other periods (2009–2010 and 2010–2011) significantly higher mineral N content in soil was recorded having used RC for straw decomposition compared with other treatment (except NF+SC). In RC treatment little decomposed straw in the autumn did not have negative effect on the crops grown in the spring, since N shortage was compensated for by nitrogen released during the decomposition of red clover mass. In all experiments, soil mineral N concentration consistently increased (5.8–14.4% compared to SC) also when NF+SC had been used for straw decomposition. In this case mineral N increase was stimulated by the release of N immobilised by microbes. These processes were weak in the treatment NF (except for the period 2008–2009). It can be argued that N immobilisation and remineralization processes intensity and duration in heavy soils are different. These processes take longer for straw decomposition under unfavourable conditions (Janušauskaitė et al. Citation2013). This was shown by the C/N of straw residues as well.
Acknowledgements
The paper presents research findings, obtained through the long-term research programmes ‘Productivity and sustainability of agricultural and forest soils’ and “Biopotential and quality of plants for multifunctional use” implemented by Lithuanian Research Centre for Agriculture and Forestry, to whom the authors acknowledge their gratitude.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Aušra Arlauskienė is a senior researcher at the Joniškėlis Experimental Station of the Lithuanian Research Centre for Agriculture and Forestry, Joniškėlis, Pasvalys district, Lithuania. She is a doctor, Agricultural Sciences, Agronomy. Her main research areas are plant diversification in crop rotation, legume plants, alternative crop cultivation and green manure using methods and plant residues decomposition models.
Aleksandras Velykis is a senior researcher at the Joniškėlis Experimental Station of the Lithuanian Research Centre for Agriculture and Forestry, Joniškėlis, Pasvalys district, Lithuania. He is a doctor, Agricultural Sciences, Agronomy. His main research areas are improvement of soil physical, chemical and biological properties, tillage systems on clayey soils, crop rotation systems, weed research, decomposition of soil organic matter and organic carbon accumulation processes.
Alvyra Šlepetienė is a head researcher and head of Chemical Research Laboratory at the Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry, Akademija, Kėdainiai district, Lithuania. She is a doctor (HP), Agricultural Sciences, Agronomy. Her main research area include application of modern analytical and bioanalytical methods to investigate the variation of chemical composition of the soil, especially soil humic substances, investigation of carbon sequestration and stabilization processes in agricultural and protected areas, chemical composition of forage and energy crops as influenced by different land management practices.
Dalia Janušauskaitė is a senior researcher at the Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry, Akademija, Kėdainiai district, Lithuania. She is a doctor, Agricultural Sciences, Agronomy. Her main research include the occurrence of soil micro-organisms and soil biological activity.
References
- Aguilera E, Lassaletta L, Gattinger A, Gimeno BS. 2013. Managing soil carbon for climate change mitigation and adaptation in Mediterranean cropping systems: a meta-analysis. Agric Ecosyst Environ. 168:25–36. doi: 10.1016/j.agee.2013.02.003
- Badía D, Martí C, Aguirre AJ. 2013. Straw management effects on CO2 efflux and C storage in different Mediterranean agricultural soils. Sci Total Environ. 465:233–239. doi: 10.1016/j.scitotenv.2013.04.006
- Beier C, Emmett BA, Peñuelas J, Schmidt IK, Tietema A, Estiarte M, Gundersen P, Llorens L, Riis-Nielsen T, Sowerby A, Gorissen A. 2008. Carbon and nitrogen cycles in European ecosystems respond differently to global warming. Sci Total Environ. 407:692–697. doi: 10.1016/j.scitotenv.2008.10.001
- Blanco-Canqui H, Lal R. 2009. Crop residue removal impacts on soil productivity and environmental quality. CRC Cr Rev Plant Sci. 28:139–163. doi: 10.1080/07352680902776507
- Bogužas A, Mikučionienė R, Šlepetienė A, Sinkevičienė A, Feiza V, Steponavičienė V, Adamavičienė A. 2015. Long-term effect of tillage systems, straw and green manure combinations on soil organic matter. Zemdirbyste-Agriculture. 102(3):243–250. doi: 10.13080/z-a.2015.102.031
- Christensen BT. 1985. Wheat and barley straw decomposition under field conditions: effect of soil type and plant cover on weight loss, nitrogen and potassium content. Soil Biol Biochem. 17(5):691–697. doi: 10.1016/0038-0717(85)90047-1
- Corbeels M, Hofman G, Van Cleemput O. 2000. Nitrogen cycling associated with the decomposition of sunflower stalks and wheat straw in a Vertisol. Plant Soil. 218(12):71–82. doi: 10.1023/A:1014904505716
- Ghaffar SH, Fan M. 2013. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenerg. 57:264–279. doi: 10.1016/j.biombioe.2013.07.015
- Henriksen TM, Breland TA. 1999a. Decomposition of crop residues in the field: evaluation of a simulation model developed from microcosm studies. Soil Biol Biochem. 31:1423–1434. doi: 10.1016/S0038-0717(99)00063-2
- Henriksen TM, Breland TA. 1999b. Nitrogen availability effects on carbon mineralization, fungal and bacterial growth, and enzyme activities during decomposition of wheat straw in soil. Soil Biol Biochem. 31:1121–1134. doi: 10.1016/S0038-0717(99)00030-9
- Heuck C, Weig A, Spohn M. 2015. Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol Biochem. 85:119–129. doi: 10.1016/j.soilbio.2015.02.029
- Janušauskaitė D, Arlauskienė A, Maikštėnienė S. 2013. Soil mineral nitrogen and microbial parameters as influenced by catch crops and straw management. Zemdirbyste-Agriculture. 100(1):9–18. doi: 10.13080/z-a.2013.100.002
- Janušauskaitė D, Velykis A. 2010. The influence of the expansion of winter crop proportion in the rotation structure on soil biological activity. Agron Res. 8(Special Issue 2):409–414.
- Kabuyah RNTM, van Dongen BE, Bewsher AD, Robinson CH. 2012. Decomposition of lignin in wheat straw in a sand-dune grassland. Soil Biol Biochem. 45:128–131. doi: 10.1016/j.soilbio.2011.10.014
- Kriaučiūnienė Z, Velička R, Raudonius S. 2012. The influemce of crop residues type on their decomposition rate in the soil: a litterbag study. Zemdirbyste-Agriculture. 99(3):227–236.
- Kuang E, Chi F, Jeng AS, Zhang J. 2014. A comparison of different methods of decomposing maize straw in China. Acta Agric Scand Sect B. 63(2):186–194.
- Le Guillou C, Angers DA, Leterme P, Menasseri-Aubry S. 2011. Differential and successive effects of residue quality and soil mineral N on water-stable aggregation during crop residues decomposition. Soil Biol Biochem. 43:1955–1960. doi: 10.1016/j.soilbio.2011.06.004
- Lenka NK, Lal R. 2013. Soil aggregation and greenhouse gas flux after 15 years of wheat straw and fertilizer management in a no-till system. Soil Till Res. 126:78–89. doi: 10.1016/j.still.2012.08.011
- Lukas S, Potthoff M, Dyckmans J, Joergensen RG. 2013. Microbial use of 15N-labelled maize residues affected by winter temperature scenarios. Soil Biol Biochem. 65:22–32. doi: 10.1016/j.soilbio.2013.05.008
- Malhi SS, Nyborg M, Solberg ED, Dyck MF, Puurveen D. 2011. Improving crop yield and N uptake with long-term straw retention in two contrasting soil types. Field Crop Res. 124:378–391. doi: 10.1016/j.fcr.2011.07.009
- Mary B, Recous S, Darwis D, Robin D. 1996. Interaction between decomposition of plant residues and nitrogen cycling in soil. Plant Soil. 181:71–82. doi: 10.1007/BF00011294
- Marschner P, Umar S, Bauman K. 2011. The microbial community composition changes rapidly in the early stages of decomposition of wheat residue. Soil Biol Biochem. 43:445–451. doi: 10.1016/j.soilbio.2010.11.015
- Orlova AP. 2013. Active organic matter as regulator of nitrogen and carbon transformation processes in soddy-podzolic soil [doctoral thesis]. Sant-Petersburg: State Scientific Institute the All Russia Research Institute for Agricultural Microbiology of the Russian Academy of Agricultural Sciences (in Russian).
- Pekarskas J, Vilkenyte L, Sileikiene D, Cesoniene L, Makarenko N. 2011. Effect of nitrogen fertilizers Provita and fermentator Penergetic k on winter wheat and soil quality. In: Cygas D, Froehner KD, editors. Environmental engineering. Proceedings of 8th International Conference; 2011 May 19–20. Vilnius (Lithuania): Vilnius Gediminas Technical University.
- Penergetic Products for Agriculture. [ cited 2016 May 6]. Available from: http://www.penergetic.ca/products.html.
- Roer AG, Korsaeth A, Henriksen TM, Michelsen O, Strømman AH. 2012. The influence of system boundaries on life cycle assessment of grain production in central southeast Norway. Agric Syst. 111:75–84. doi: 10.1016/j.agsy.2012.05.007
- Said-Pullicino D, Cucu MA, Sodano M, Birk JJ, Graser B, Celi L. 2014. Nitrogen immobilization in paddy soils as affected by redox conditions and rice straw incorporation. Geoderma. 228–229:44–53. doi: 10.1016/j.geoderma.2013.06.020
- Shindo H, Nishio T. 2005. Immobilization and remineralization of N following addition of wheat straw unto soil: determination of gross N transformation rates by 12N-ammonium isotope dilution technique. Soil Biol Biochem. l37:425–432. doi: 10.1016/j.soilbio.2004.07.027
- Tarafdar JC, Meena SC, Kathju S. 2001. Influence of straw size on activity and biomass of soil microorganisms during decomposition. Eur J Soil Biol. 37:157–160. doi: 10.1016/S1164-5563(01)01084-6
- Tarakanovas P, Raudonius S. 2003. Agronominių tyrimų duomenų statistinė analizė taikant kompiuterines programas ANOVA, STAT, SPLIT-PLOT iš paketo selekcija ir Irristat [Statistic analysis of agronomical research data with computer programs ANOVA, STAT, SPLIT-PLOT from packet selekcija and Irristat]. Akademija: Aleksandras Stulginskis University.
- Turk A, Michelič R. 2013. Wheat straw decomposition, N-mineralization and microbial biomass after 5 years of conservation tillage in Gleysol fields. Acta Agric Slov. 101(1):69–75. doi: 10.2478/acas-2013-0008
- Wu J, Guo X, Wang Y, Xu ZY, Zhang XL, Lu JW. 2011. Decomposition characteristics of rapeseed and wheat straw under different water regimes and straw incorporating models. J Food Agric Environ. 9(2):572–577.
