ABSTRACT
Since the fate of nanoparticles after their release in the environment and their possible transfer in plants and subsequent impacts is still largely unknown, this paper evaluates the potential phytotoxic effects of up to 20% w/w TiO2 nanoparticles (nTiO2) on barley cultivated in hydroponics and agar media. The X-ray diffraction analysis confirmed that nTiO2 powder corresponds to anatase phase. On agar medium only high concentrations of nTiO2 (10% and 20% w/w) induced significant inhibition of shoot growth. However, hydroponics treatment with nTiO2 up to 1000 mg L−1 did not show any adverse effect on the shoot growth. In both experiments, (i) root growth inhibition effects became visible with increasing concentration of nTiO2, (ii) plants treated with nTiO2 showed no change in chlorophyll a and b content, even though the plants absorbed nTiO2, (iii) weight of biomass treated with nTiO2 was not significantly different compared to control. Therefore, we assume that transport of nTiO2 into the aerial parts is limited due to the presence of effective mechanical or physiological barriers in roots. Overall, it appears that early root growth is a relevant indicator of potential effects of nTiO2 exposure. Our results also indicate that synthesized nTiO2 are not significantly toxic to the barley when applied at the concentrations used in this work, even though plants absorb titanium.
Introduction
How toxic or beneficial the nanoparticles really are depends on many factors, such as how they are being made, what kind of chemicals have been used in the production, what type of coating nanoparticles possess, their crystallinity, size and morphology, specific surface area and reactivity, 3D spatial structure, aggregation/agglomeration state, etc. (Madhusudan Reddy et al. Citation2003; Yang et al. Citation2008). There are many ways to produce nanoparticles; manufactured nanoparticles may even differ from one preparation method and company to another. Titanium dioxide particles are produced and used in varying particle size fractions including fine (approximately 0.1–2.5 μm) and nanosize (<0.1 μm, primary particles) (Dankovic et al. Citation2007). Titanium dioxide nanoparticles (nTiO2) are manufactured worldwide in large quantities for use in a wide range of applications; they have been used in paints, coatings, plastics, papers, inks, medicines, pharmaceuticals, food products, cosmetics, toothpaste, sportswear, and others (Wolf et al. Citation2003; Wang et al. Citation2007; Skocaj et al. Citation2011; Larue et al. Citation2014). nTiO2 possess different physicochemical properties compared to their fine particle analogs, which might alter their bioactivity (Shi et al. Citation2013). Traditionally, TiO2 fine particles have been considered as poorly soluble, low-toxic (ACGIH Citation1992), and chemically inert. nTiO2 were considered medically harmless until recently. However, studies have revealed that nTiO2 are more toxic than fine particles (Zhao et al. Citation2009). People have encountered them for over one hundred years in a massive scale in the form of white pigment commonly used in paints. It is known that no burn occurs when the unprotected living tissue is exposed to an upper paint layer containing a high amount of the photocatalytically active particles, but so far it is not clear how the nTiO2 behave in the human body when penetrating it another way, for example, by inhalation or food. Nanoparticles from consumer products usually end up in the sewage sludge of wastewater treatment plants. Several million tons of dried-out sludge are subsequently mixed into agricultural soil each year. Therefore it is extremely important to know how much of these substances end up in plants, and what kind of transformations they subject to. Production of cereals has hundreds years of tradition in Slovakia and even today cereals are the most important crops in society; according to available data, in 2015, estimated spring barley (Hordeum vulgare L.) yield was 3.46 t/ha (Klikušovská et al. Citation2015). World production of barley is soon expected to climb to a 144.6Mt high (USDA Citation2015). Since the fate of nanoparticles after their release in the environment is still largely unknown and their possible transfer in plants and subsequent impacts have not been studied in detail (Larue et al. Citation2014), this paper specifically focuses on the potential toxic effect of nTiO2 on growth and germination of one of the world’s most widespread crops.
Materials and methods
Preparation of nTiO2
The thermal hydrolysis process was carried out using TiOSO4 (>29% Ti (as TiO2) basis, technical, Sigma–Aldrich), analytical grade ammonia solution (SLAVUS Ltd, Bratislava, Slovakia), and distilled water. In a standard experiment, 10 g of TiOSO4 was dissolved in 150 mL of distilled water at room temperature. The solution was then heated at 80°C for 1 h under constant stirring. Titanyl sulfate was precipitated by adding concentrated ammonia; drops of ammonia were added to adjust the pH to 7.0, the solution was then left under stirring for 1 h. The resulting white precipitate was washed three to four times with deionized water to remove impurities, dried at 60°C for 24 h, and obtained powder was then calcined in air at 300°C for 3 h (Ngamta et al. Citation2013).
Characterization of TiO2 nanoparticles
TEM
Before starting the experiment, the characterization of the morphology and particle size distribution of TiO2 phase crystals was conducted using a transmission electron microscope (TEM) JEOL 1200 EX (JEOL Ltd, Tokyo, Japan), operating at 120 kV. Powder samples were stirred in distilled water, then an appropriate amount of specimen was put on a carbon grid.
SEM
For the characterization of TiO2 in an aggregate form, a scanning electron microscope (SEM) QUANTA 450 FEG (FEI Company, USA) was used. The parameters for the optimized measurement were set at accelerating voltage 15 kV.
X-ray analysis
X-ray diffraction (XRD) measurements were carried out using Bruker D8 DISCOVER diffractometer equipped with X-ray tube with rotating Cu anode operating at 12 kW (40 kV, 300 mA). All measurements were performed in parallel beam geometry with parabolic Goebel mirror in the primary beam. The diffraction patterns in the angular range 20–80° of 2θ were recorded in grazing incidence set-up with the angle of incidence α = 1.5°. Parallel plate collimator with the angular acceptance 0.35° was inserted in the diffracted beam.
Preparation of nTiO2-amended culture medium – type I and barley cultivation
In all, 300 mg of agar and specified amount of nTiO2 was added to 25 mL of distilled water, to prepare 0–20% (m/m) nTiO2 suspensions. These suspensions were sterilized at 121°C for 15 min, and after removal from autoclave immediately poured into culture vessels and allowed to cool. Then, seven barley seeds per dish with different nTiO2 concentrations were transferred to culture vessels. During the first 24 h, the seeds were kept in the dark, then cultured for another five days in a 16 h light/8 h dark cycle at 25°C.
After completion of the cultivation process, plants were washed in distilled water, the lengths of the shoots and the longest root developed were measured and dry weight of plant biomass was recorded. Chlorophyll in leaves was extracted with N,N-dimethylformamide (Centralchem, Slovensko). The absorbance was read at 649 and 665 nm with a spectrophotometer (Optima SP-300). The total contents of chlorophyll a and b were calculated using the extinction coefficients and the equations by Ritchie (Citation2006).
Preparation of nTiO2-amended culture medium – type II and barley cultivation
The culture medium was prepared by mixing 200 mL of distilled water with 200 mL of Hoagland solution (Hoagland & Arnon Citation1950). Then, 350 mg of agar (Agar Agar Type I, HiMedia) was added; the solution was boiled and allowed to cool. Prepared nTiO2 at concentrations of 0, 100, 150, 200, 400, 600, and 1000 mg L−1 were then added to the medium. The suspension was sonicated for 30 min to ensure the uniform distribution of nanoparticles. The control experimental medium contained no nanoparticles.
Barley seeds, supplied by the Sempol, Bratislava, were sterilized in 10% sodium hypochlorite solution and germinated for three days in Petri dishes wrapped in plastic foil. After three days, seven seedlings (for each nTiO2 concentration) were embedded in a polystyrene holder and placed in a beaker, with roots immersed directly into the culture medium. Air was blown into the solution through a plastic tube with diameter of 0.5 mm connected with a compact laboratory peristaltic pump, type PLP-330 (Behr Labor-Technik, Germany), at a flow rate of 100 mL min−1. Besides providing air delivery to plant roots, the constant airflow prevented nanoparticles from settling at the bottom of the beaker.
Plants were cultured for another seven days in a 16 h light/8 h dark cycle at 25°C. After completion of the cultivation process, plants were washed in distilled water, the lengths of the shoots and the longest root developed were measured and both the wet and dry weights of plant biomass were recorded. Chlorophyll in leaves was extracted with N,N-dimethylformamide (Centralchem, Slovakia). The absorbance was read at 649 and 665 nm with a spectrophotometer (Optima SP-300). The total contents of chlorophyll a and b were calculated as mentioned previously.
Determination of titanium content
Ti was determined using ICP-MS (Perkin-Elmer Sciex Elan 6000) equipped with a cross-flow nebulizer and a Scott double-pass spray chamber. Isotope 50Ti was used for data acquisition; 103Rh was used as the internal standard. Plasma power: 1200 W.
Results and discussion
Characterization of nTiO2
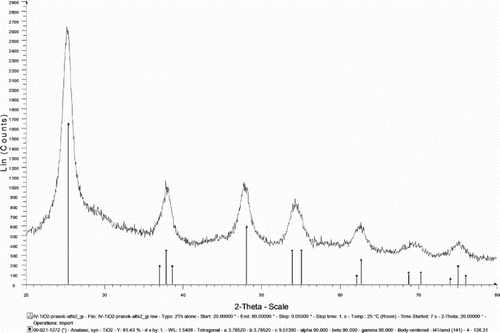
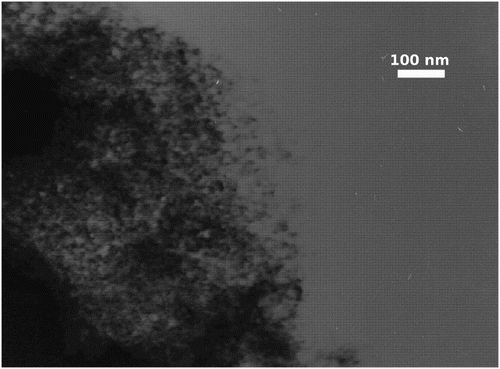
TiO2 exists in a large number of stable and metastable polymorphs, such as rutil, anatase, brookite, etc. Anatase and brookite, both metastable phases, transform into rutile, a stable phase, upon heating (Tobaldi Citation2012). XRD measurements reveal that the powder heated at 300°C corresponds to anatase (). According to SEM analysis, the synthesized anatase phase () forms particles in a wide range of size and morphology. The nanoparticles tend to aggregate; the diameters of TiO2 aggregates vary from several nanometers to several micrometers (50 µm), with a mean size of 5–10 nm (). Anatase-type TiO2 has a crystalline structure that corresponds to the tetragonal system with dipyramidal habit; it may also occur as rounded spherical grains (). Besides, aggregates also exhibit various types of overgrowth. TiO2 that occurs as aggregated material has its surface covered by adsorbed individual nanoparticles.
nTiO2-amended culture medium – type I
The results depicted in illustrate a statistically significant effect on the root growth even at the lowest nTiO2 concentrations (IC50 = 1.51%). The above-ground parts growth was significantly inhibited by 10% content nTiO2 (IC50 = 28.1%). Since the shoot system and the root system develop more or less parallel and they exert mutual influence, it is unlikely that a reduction of the aerial parts may only be attributed to cytotoxic effect of transported titanium. It is rather a physiological response to lower nutrient supply caused by the reduction in root length. The presence of an effective mechanical or physiological barrier is also confirmed by the analyses of physiological parameters – the contents of chlorophyll a and b in the aerial plant parts. Results in show that even at high concentrations, no significant effect of nTiO2 was detected. While the average weight values of dry shoot plant biomass in treatments with nTiO2 concentrations over 2.5 g L−1 were lower compared to the control experiment, the statistically significant adverse effects at the level of 0.05 manifested only at the highest concentration tested ((a)).
Figure 4. Average length of roots and shoots of barley (Hordeum vulgare L.) after six-day cultivation on agar medium (type I) under different nTiO2 concentrations (%). The lengths of both roots and shoots diminish with rising nTiO2 concentration. A significant change was observed at concentrations higher than 0.5% and 2.5% for roots and shoots, respectively. The error bars represent standard deviation from seven measurements (* statistically significant difference from control on significance level α = 0.05, ** statistically significant difference from control on significance level α = 0.01, *** statistically significant difference from control on significance level α = 0.001).
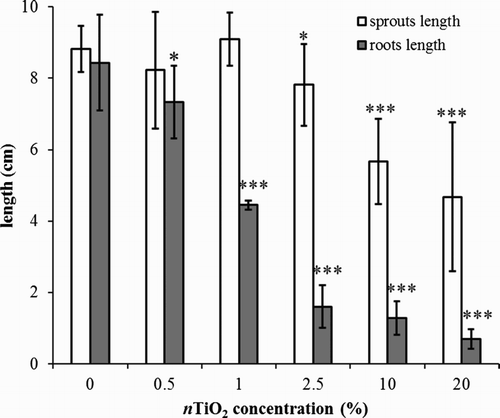
Table 1. Content of chlorophyll a and b, total and a:b ratio of leaf sections from the top of the first and second leaves of barley (Hordeum vulgare L.) after cultivation in agar medium (type I) or in hydroponics (type II).
Table 2. The dry weight of shoot biomass of barley (Hordeum vulgare L.) after cultivation in (a) agar medium (type I) or in (b) hydroponics (type II).
This experiment ought to be perceived as preliminary, conducted with a purpose to determine a concentration required to achieve 50% inhibition (IC50). From the collected data it was found that concentration of nTiO2 required to be effective in inhibiting growth and selected physiological functions was higher than expected. The release of nTiO2 was most probably hindered by the dense agar medium significantly affecting the particle transport to the plants. Assuming these extremely high concentration levels (≥10 g L−1) of nTiO2 are normally not common (have not been reported) in the environment, the concentration range up to 1000 mg L−1, which is usually applied in ecotoxicological studies of nanoparticles’ adverse effects on cultural plants, was selected for further evaluation in our hydroponic experiment. In consequence, in culture media – type I, the plant biomass was not further analyzed for Ti content by ICP-MS.
nTiO2-amended culture medium – type II
Many crop species are commonly used for phytotoxicity testing. Studies evaluating the effects of nanoparticles on plants have been mainly performed by root exposure on plants grown in hydroponics. There were some reported positive effects (Landa et al. Citation2012; Singh et al. Citation2012; Feizi et al. Citation2013; Song et al. Citation2013), negative effects (Asli & Neumann Citation2009; Ruffini Castiglione et al. Citation2011), or no effect (Seeger et al. Citation2009; Landa et al. Citation2012; Larue et al. Citation2012; Song et al. Citation2013) of nTiO2 on plant development.
For the purpose of our experiment, barley was grown in hydroponic culture for seven days, the plants were then removed from the beaker, weighted, and the lengths of roots and shoots were measured. Roots and shoots were analyzed for titanium content, and shoots were analyzed for total chlorophyll. Plants were then air-dried for one week and weighted again.
From the measured values, it is obvious that nTiO2 at the studied concentration range have no effect on the growth of aerial plant parts. ICP-MS analysis confirmed that there was no enhanced Ti translocation into aerial plant parts under nanoparticle treatments under 400 mg nTiO2 L−1 compared to the control (). On the contrary, the average roots’ length was less with the increase in nTiO2 concentrations. The roots at nTiO2 concentration of 400 mg L−1 reached only half of the length of roots in the control sample (). Elevated nTiO2 concentration did not significantly affect the plant weight. The contents of chlorophyll a and chlorophyll b under nTiO2 treatment are set out in . There were no significant changes for total chlorophyll in groups treated with different concentrations (Student’s t-test, at P ≤ .05). The ratio of chlorophyll a:b was relatively constant, varying from 3.91 to 4.63. The proportion of chlorophyll a was larger than that of chlorophyll b.
Figure 5. Average lengths of roots and shoots of barley (Hordeum vulgare L.) after seven-day cultivation in hydroponics (type II) under different nTiO2 concentrations (mg L−1). Only root length is affected when concentration of nTiO2 is above 150 mg L−1. The error bars represent standard deviation from seven measurements (* statistically significant difference from control on significance level α = 0.05, ** statistically significant difference from control on significance level α = 0.01, *** statistically significant difference from control on significance level α = 0.001).
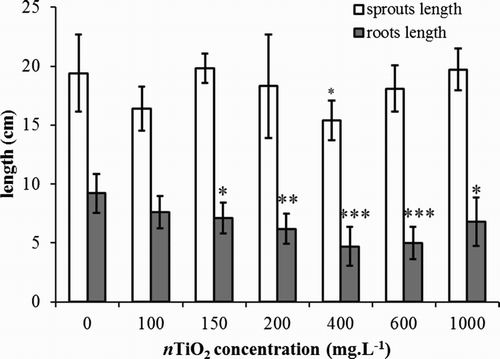
Table 3. Titanium concentrations (mg kg−1 d wt.) in roots and shoots of barley (Hordeum vulgare L.) cultivated for seven days in nTiO2-amended hydroponics (type II) and determined by ICP-MS.
Findings of the present study correspond with some of the results found in literature. The effect of nTiO2 on root growth was observed in several agronomic plant species at similar or lower nTiO2 concentrations. Andersen et al. (Citation2016) exposed 10 agronomic plant species to different concentrations of nTiO2 (0, 250, 500, and 1000 µg mL−1) to examine potential effects on germination and early seedling development. Six of ten species responded to nTiO2 through a change in average root length, with four of the six species showing decreased and two species showing increased root length. Regarding barley-related plants (Poaceae family), nTiO2 decreased average root length in oats, while the root length remained unaffected in ryegrass. Use of low concentrations (20–80 mg L−1) of nTiO2 enhanced fennel seed germination (Feizi et al. Citation2013) and caused an increase of root elongation during the first stages of development of wheat (50–100 mg L−1, Larue et al. Citation2012). Some other nanoparticles of metal oxides such as ZnO and Al2O3 behave in a similar way to nTiO2. There is therefore reason to believe that the photocatalytic activity occurring on the surface of metal oxides’ nanoparticles, including the production of reactive oxygen species (Larue et al. Citation2012; Andersen et al. Citation2016), can be one of the important processes that make these nanoparticles toxic. This fact was observed also by Ghosh et al. (Citation2010) who reported an interaction between nTiO2 and both plant and human cells where nTiO2 produced superoxide radicals inducing oxidative stress and lipid peroxidation. By accumulating in the cell wall surfaces of primary roots and reduction of cell wall pore size, nTiO2 may also interfere with water transport to some extent (Asli & Neumann Citation2009).
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Lucia Kořenkova completed her Masters´ and PhD degree at the Faculty of Natural Sciences, Comenius University in Bratislava, Slovakia, where she currently works as a researcher at the Institute of Laboratory Research on Geomaterials. Her research interests cover the areas of soil mapping and classification, crop-soil interactions, environmental investigation and nanoparticle research. She has published several papers in scientific journals and has given presentations at scientific and professional meetings.
Martin Šebesta is currently a PhD student at the Faculty of Natural Sciences, Comenius University in Bratislava, Slovakia. He researches nanoparticle transport in soils and their transport to plants and other soil organisms.
Martin Urík is a researcher at the Faculty of Natural Sciences, Comenius University in Bratislava, Slovakia. He has published several research articles on environmental geochemistry of potentially toxic metals and metalloids.
Marek Kolenčík is an associate professor at the Department of Geology and Soil Science, Faculty of Agrobiology and Food Resources at Slovak University of Agriculture in Nitra. He has published more than 20 research articles with more than 100 SCI citations on bioleaching, biohydrometallurgy and bionanotechnology research.
Gabriela Kratošová is a senior researcher at VŠB - Technical Univerzity of Ostrava, Nanotechnology Centre, Czech Republic. She is an electron microscopy specialist. She has published several articles on biosynthesis of nanoparticles, their characterization and application in catalysis and disinfection.
Marek Bujdoš earned his PhD degree in analytical chemistry. He is a researcher at the Institute of Laboratory Research on Geomaterials, Faculty of Natural Sciences, Comenius University in Bratislava, Slovakia. He works on developing new methods of atomic and mass spectrometry.
Ivo Vávra is currently a senior researcher at the department of Microelectronics and Sensors, Institute of Electrical Engineering of Slovak Academy of Sciences. His expertise lies in the analytical approach for the characterization of nanoparticles examined via transmission electron microscopy analysis. He has published many excellent articles on bio-nano-materials and their unique physical and chemical properties.
Edmund Dobročka is currently a senior scientist at the Institute of Electrical Engineering of Slovak Academy of Sciences. His research interest is focused on X-ray diffraction analyses of the structural properties of thin films with many relevant published articles on this topic.
ORCID
Martin Urík http://orcid.org/0000-0001-7998-7992
Additional information
Funding
References
- American Conference of Governmental Industrial Hygienists [ACGIH]. 1992. Threshold limit values and biological exposure indices for 1992–1993. Cincinnati (OH): American Conference of Governmental Industrial Hygienists.
- Andersen CP, King G, Plocher M, Storm M, Pokhrel LR, Johnson MG, Rygiewicz PT. 2016. Germination and early plant development of ten plant species exposed to TiO2 and CeO2 nanoparticles. Environ Toxicol Chem. doi:10.1002/etc.3374.
- Asli S, Neumann PM. 2009. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 32:577–584. doi: 10.1111/j.1365-3040.2009.01952.x
- Dankovic D, Kuempel E, Wheeler M. 2007. An approach to risk assessment for TiO2. Inhal Toxicol. 19:205–212. doi: 10.1080/08958370701497754
- Feizi H, Kamali M, Jafari L, Rezvani Moghaddam P. 2013. Phytotoxicity and stimulatory impacts of nanosized and bulk titanium dioxide on fennel (Foeniculum vulgare Mill). Chemosphere. 91:506–511. doi: 10.1016/j.chemosphere.2012.12.012
- Ghosh M, Bandyopadhyay M, Mukherjee A. 2010. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere. 81:1253–1262. doi: 10.1016/j.chemosphere.2010.09.022
- Hoagland DR, Arnon DI. 1950. Circular. California Agri Exp Station. 347:1–32.
- Klikušovská Z, Kusý D, Sviček M. 2015. Winter wheat, spring barley and oilseed rape crop and production estimates. Report to 15 July 2015. Soil Science and Conservation Research Institute, Bratislava, 24 p. (in Slovak).
- Landa P, Vankova R, Andrlova J, Hodek J, Marsik P, Storchova H, White JC, Vanek T. 2012. Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J Hazard Mater. 241-242:55–62. doi: 10.1016/j.jhazmat.2012.08.059
- Larue C, Castillo-Michel H, Sobanska S, Trcera N, Sorieul S, Cécillon L, Ouerdane L, Legros S, Sarre G. 2014. Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J Hazard Mater. 273:17–26. doi: 10.1016/j.jhazmat.2014.03.014
- Larue C, Laurette J, Herlin-Boime N, Khodja H, Fayard B, Flank AM, Brisset F, Carriere M. 2012. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): influence of diameter and crystal phase. Sci Total Environ. 431:197–208. doi: 10.1016/j.scitotenv.2012.04.073
- Madhusudan Reddy K, Manorama SV, Ramachandra Reddy A. 2003. Bandgap studies on anatase titanium dioxide nanoparticles. Mater Chem Phys. 78:239–245. doi: 10.1016/S0254-0584(02)00343-7
- Ngamta S, Boonprakob N, Wetchakun N, Ounnunkad K, Phanichphant S, Inceesungvorn B. 2013. A facile synthesis of nanocrystalline anatase TiO2 from TiOSO4 aqueous solution. Mater Lett. 105:76–79. doi: 10.1016/j.matlet.2013.04.064
- Ritchie RJ. 2006. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res. 89:27–41. doi: 10.1007/s11120-006-9065-9
- Ruffini Castiglione M, Giorgetti L, Geri C, Cremonini R. 2011. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J Nanopar Res. 13:2443–2449. doi: 10.1007/s11051-010-0135-8
- Seeger E, Baun A, Kastner M, Trapp S. 2009. Insignificant acute toxicity of TiO2 nanoparticles to willow trees. J Soils Sediments. 9:46–53. doi: 10.1007/s11368-008-0034-0
- Shi H, Magaye R, Castranova V, Zhao J. 2013. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 10:15. http://particleandfibretoxicology.biomedcentral.com/articles/10.1186/1743-8977-10-15 doi: 10.1186/1743-8977-10-15
- Singh D, Kumar S, Singh SC, Lal B, Singh NB. 2012. Applications of liquid assisted pulsed laser ablation synthesized TiO2 nanoparticles on germination, growth and biochemical parameters of Brassica oleracea var. Capitata. Sci Adv Mater. 4:522–531. doi: 10.1166/sam.2012.1313
- Skocaj M, Filipic M, Petkovic J, Novak S. 2011. Titanium dioxide in our everyday life; is it safe? Radiol Oncol. 45:227–247. doi: 10.2478/v10019-011-0037-0
- Song U, Shin M, Lee G, Roh J, Kim Y, Lee EJ. 2013. Functional analysis of TiO2 nanoparticle toxicity in three plant species. Biol Trace Elem Res. 155:93–103. doi: 10.1007/s12011-013-9765-x
- Tobaldi, DM. 2012. Titanium dioxide and rutile: an overview and its photocatalytic activity. In: Low J, editor. Rutile: properties, synthesis and applications. New York (US): Nova Publishers. Chapter 1; pp. 1–9.
- USDA. 2015. https://marketcheck.com.au/usda-wasde-oct-2015-limited-motivation/
- Wang JJ, Sanderson BJ, Wang H. 2007. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 628:99–106. doi: 10.1016/j.mrgentox.2006.12.003
- Wolf R, Matz H, Orion E, Lipozencic J. 2003. Sunscreens – the ultimate cosmetic. Acta Dermatovenerol Croat. 11:158–162.
- Yang HG, Sun CH, Qiao SZ, Zou J, Liu G, Smith SC, Cheng HM, Lu GQ. 2008. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature. 453:638–641. doi: 10.1038/nature06964
- Zhao J, Bowman L, Zhang X, Vallyathan V, Young SH, Castranova V, Ding M. 2009. Titanium dioxide (TiO2) nanoparticles induce JB6 cell apoptosis through activation of the caspase-8/Bid and mitochondrial pathways. J Toxicol Environ Health A. 72:1141–1149. doi: 10.1080/15287390903091764