ABSTRACT
Dill has multiple culinary and medicinal purposes and the use of their landraces into a plant breeding program, requires the analysis of their phenotypic diversity. In this study, 33 Greek dill landraces collected from diverse areas were evaluated using traits based on UPOV descriptor list. Phenotypic diversity was assessed using Shannon-Weaver diversity index (H΄) and non-linear principal component analysis. Grouping of landraces was further performed through hierarchical cluster analysis. The H' index ranged from 0.32 (stem waxiness) to 0.98 (density of foliage) with a mean value of 0.68 indicating a high level of phenotypic diversity. High H' values were recorded for the foliage width, stem color and anthocyanin coloration. Multivariate analysis revealed three common genetic groups: 1) North mainland Greece, 2) Aegean islands and 3) Central mainland Greece. The landraces’ heterogeneity was attributed to various traits linked to specific geographic origin, such as early time of flowering and high stem waxiness allied with the landraces originated from the Aegean islands. Greek dill landraces revealed useful variation on yield component traits related to fresh herb weight and to seed production, such as high number of leaves/plant and large diameter of main umbel that can be promptly exploited in breeding programs.
Introduction
The protection of plant genetic resources from genetic erosion has become a major priority, since the prevention of biodiversity loss is closely connected to global food safety (FAO Citation2010; Secretariat of the Convention of Biological Diversity Citation2010). Landraces are characterized by major heterogeneity and are among the most important genetic resources (Zeven Citation2002), for which the study of the phenotypic diversity is imperative to ensure their optimum use (Koutsika-Sotiriou et al. Citation2010; Terzopoulos & Bebeli Citation2010). The evaluation and use of local landraces in breeding programs as starting material is very important for plant breeders, providing new potential for future agriculture aiming to improve crop productivity and adaptability (Ulukan Citation2011). Recently, several studies were conducted on medicinal and aromatic plants using agro-morphological traits or molecular markers to describe their diversity (Solouki et al. Citation2008; Zaouali et al. Citation2012; Patel et al. Citation2015).
Dill (Anethum graveolens L.) is classified as an aromatic and medicinal plant belonging to Apiaceae (Umbelliferae) family (Solouki et al. Citation2012), which is native of Mediterranean and Central Asia. Dill was well known in Ancient Egypt and Ancient Greece (Quer Citation1981; Krymow Citation1989) and the name Anethum is derived from the Greek word ‘aneeson’ or ‘aneeton’ which means ‘strong smelling’ (Chung et al. Citation2012). It is widely cultivated in Europe, India and the United States (Suresh et al. Citation2013) for its leaves, seeds and its essential oil (Jana & Shekhawat Citation2010). A high degree of agro-morphological variation was observed in the Apiaceae family (Jiménez-Mejías & Vargas Citation2015) and different methods have been used recently for the evaluation of the genetic diversity within and among different Apiaceae species (Lopez et al. Citation2008; Iorizzo et al. Citation2013; Maghsoudi Kelardashti et al. Citation2015), employing also molecular markers in dill (Solouki et al. Citation2012; Suresh et al. Citation2013). The genetic diversity of different aromatic species was also studied using agro-morphological characters (Raghu et al. Citation2007; Dušek et al. Citation2010; Patel et al. Citation2015). These studies highlight the importance of certain traits in respect to the productivity of dill crop, such as plant height and number of leaves per plant on fresh herb weight (Karklelienė et al. Citation2014; Said-Al Ahl & Omer Citation2016) or the diameter and the number of umbels per plant on seed production (Holubowicz & Morozowska Citation2011). On the other hand, due to its multiple culinary and medicinal uses dill can constitute an important alternative crop with high added value for small scale farmers, providing additional economic input (Gao & Bergefurd Citation1998). Therefore, the assessment of phenotypic diversity of Greek dill landraces can reveal useful information on crop productivity associated traits.
Aim of the current study was the assessment of phenotypic diversity among 33 Greek dill landraces using a number of agro-morphological traits and the identification of those traits that contribute to heterogeneity and can be potentially exploited by the breeders for improving commercial crop and/or seed production. To our knowledge this is the first study of phenotypic diversity of Greek dill landraces from wide germplasm collection across the country.
Materials and methods
Plant material and experimental design
Thirty-three Greek dill (Anethum graveolens L.) landraces collected from diverse areas of Greece with different agro-climatic conditions were used for the study ( and ). The experiment was carried out in an open field at the Institute of Plant Breeding and Genetic Resources, Thermi-Thessaloniki during 2008, in a randomized complete block design with three replications. A total of 51 plants per landrace were grown in three blocks with a 50 cm from row to row distance and plants spaced 50 cm apart in-the-row.
Figure 1. (a) Representation of the 33 dill landraces and 26 agro-morphological traits in the first (explaining the 21.77% of variance) and second (explaining the 13.97% of variance) dimension obtained from the analysis of the morphological variables, using non-linear PCA. See accession codes and abbreviations in and . (b) Map of Greece with names of areas where dill landraces were collected and grouping of the landraces by non-linear PCA.
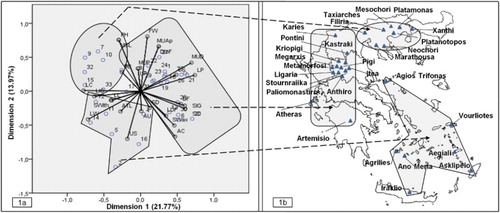
Table 1. Geographical data of the dill landraces studied.
Measurements
Twenty-two agro-morphological traits were recorded based on Dill descriptors (UPOV Citation1999) and four traits related to plant growth. These traits involved (i) six nominal measurements which included: anthocyanin coloration, attitude of leaves, leaf shape, leaf color, stem color and umbel shape, (ii) eight ordinal measurements which included density of foliage, stem intensity of green color, stem waxiness, leaf density of feathering and intensity of green color, time of appearance of the main umbel, time of beginning of flowering and foliage width and (iii) 12 continuous measurements included: number of primary branches, plant height, length of the main stem, stem diameter, leaf length, leaf width, main umbel: diameter and number of peduncles, average number of umbellets, number of umbels/plant, number of leaves/plant, width of stem.
Statistical analysis
All continuous traits were transformed to ordinal for the calculation of the Shannon–Weaver diversity index. More specifically, three discrete ranks were formed by dividing the range of continuous traits into three equal parts (Bechere et al. Citation1996; Pagnano & Gauvreau Citation2000). Finally, the frequency of each rank of the phenotypic classes for each character was computed. The numbers of phenotypic classes of each trait used for the calculation of the Shannon–Weaver diversity index, are listed in .
Table 2. Descriptors used for estimating agro-morphological trait diversity in dill landraces, their numbers of classes, and proportion (%) of occurrence of each class, and estimated phenotypic diversity index (H′) for each trait.
The Shannon–Weaver diversity index (H') was defined as
H' = H/Hmax,
lnpi, where n is the number of phenotypic classes for a character and pi is the % proportion of the total number of entries in the ith class.
Hmax = ln(n) in order to express the values of H' in the range of 0–1.
The diversity index was classified as low (0.10 ≤ H' ≤ 0.40), intermediate (0.40 ≤ H' ≤ 0.60) and high (H' ≥ 0.60) (Eticha et al. Citation2005).
Α non-linear (categorical) principal component analysis (PCA) with optimal scaling was applied (Linting et al. Citation2007), since data include simultaneously nominal, ordinal and quantitative variables (Linting et al. Citation2007; Abdi & Williams Citation2010) and resulted in reducing the observed variables to a smaller number of dimensions or uncorrelated new synthetic variables which describe most of the original variability. The dimensions selected were those having positive Cronbach’s alpha coefficient (Cronbach Citation1951) and eigenvalue at least 1 (Mengistu et al. Citation2015). Hierarchical cluster analysis (HCA) was carried out by using the resulted dimensions (new synthetic variables) selected from the non-linear PCA. Dendrogram of HCA was constructed using the unweighted pair group method with arithmetic mean analysis and the Euclidean distance as the genetic distance measure (Sneath & Sokal Citation1973). The above analyses were conducted using SPSS (version 15.0; SPSS, Chicago) package.
Results
Characterization of Greek dill landraces collection
Vegetative traits
More than the half of the studied Greek dill landraces, were characterized as ‘short’ regarding their plant height and the length of the main stem showing 57.6% and 66.7% of the plants in the whole collection respectively. Also, the majority of the Greek dill landraces were characterized with ‘small’ stem diameter (84.8%) and ‘low’ number of leaves/plant (81.8%) ().
A noticeable diversity was observed for the trait of leaf shape with the Greek dill landraces to be assigned among all the three different shape categories indicated in the official UPOV descriptor list. In particular, most of the accessions were equally dispersed between ‘triangular’ (48.5%) and ‘oblong’ (45.5%) leaf shape, while there was also a small portion of the landraces (6.0%) indicating the ‘rhomboidal’ type of leaf shape (). Green color intensity for stem and leaves did not follow the same pattern. More precisely, the majority of the landraces demonstrated a ‘medium’ green color intensity for the stem (78.8%) with some of the landraces characterized by ‘light’ green stem color (18.2%) and only few by ‘dark’ green stem color (3.0%). On the other hand, more than half of the landraces showed a ‘dark’ green leaf color (57.6%), followed by those with ‘medium’ green leaf color (39.4%), while there was a small portion of them (3.0%) characterized by ‘light’ green color (). In terms of stem waxiness all the landraces were characterized as ‘medium’ (21.2%) to ‘strong’ (78.8%), with none of them indicating ‘absence’ or ‘weak’ trend for this particular trait (). Similarly, the density of the leaf feathering was either ‘medium’ (63.6%), either ‘dense’ (36.4%), since there was no landrace characterized by ‘loose’ density of leaf feathering ().
Inflorescence traits
Most of the landraces were characterized with a ‘small’ diameter of the main umbel (75.8%) with ‘few’ number of peduncles (75.8%) and an umbel shape recorded as ‘flat-topped with straight rays’ (81.8%). Regarding the earliness of the landraces, most of them (72.7%) were characterized as ‘medium’ for both time of appearance of main umbel and number of days to flowering ().
Phenotypic diversity of Greek dill landrace collection
The Shannon–Weaver diversity index (H')
The diversity index (H') for each trait ranged from 0.32 to 0.98 with an average 0.68 (), indicating a high phenotypic diversity according to Eticha et al. (Citation2005). Many of the studied traits had H' ranging from 0.40 to 0.60, namely the stem intensity of green color, the stem diameter, the leaf length, the number of peduncles of the main umbel, the number of leaves/plant, the number of umbels/plant and the stem width while the majority of the traits had H' value higher than 0.61 showing high diversity ().
Landraces classification
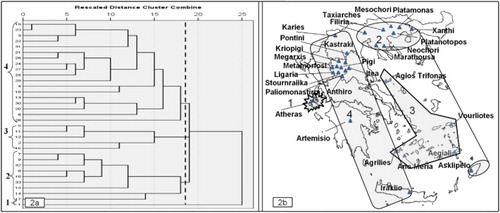
Non-linear PCA with optimal scaling, resulted in reducing the 26 agro-morphological traits to eight dimensions or uncorrelated new synthetic variables which represented the 83.4% of the original variability. The classification of the 33 Greek landraces according to the non-linear PCA, placed all of them into 3 groups (). The first group was consisted of eight landraces originated from North mainland Greece. The second group was consisted of seven landraces originated from seven different Aegean islands, that is, Samos, Amorgos, Milos, Skopelos, Folegandros, Rhodes and Crete. The third group was consisted of 18 landraces originated from Central mainland Greece. The HCA classified the 33 dill landraces into almost the same groups comparing to the non-linear PCA. More specifically the following groups were identified: (i) a group from North mainland Greece that included the same eight landraces plus the landrace RK-089/07 from Rhodes Island, (ii) a group that included five landraces originated from Aegean islands, (iii) a group identified in the Central mainland Greece that included the same 17 landraces and additionally included the HL-232/07 landrace from Iraklio and (iv) a group consisted only from the landrace IK-143/06 originated from Kefalonia (). The grouping of landraces with the two methods of multivariate analysis was depicted in the map of Greece according to the place of origin ((b) and (b)), where the similarities are clear.
Figure 2. (a) UPGMA Dendrogram of 33 dill landraces by applying HCA using the Euclidean distance. (b) Map of Greece with names of areas which dill landraces collected and grouping of the landraces by HCA.
Apart the general analysis of the landraces as a whole, a more detailed examination of the traits within the identified groups, revealed important information related to their productive characteristics. The three groups had similar ‘number of primary branches’ and ‘average number of umbellets’, however there were differentiated for specific characters. More specifically, the first group, North mainland Greece, included landraces characterized mainly by high ‘plant height’ and ‘length of main stem’ and lower ‘stem diameter’ by ∼15% in comparison to the other two groups. Also, ‘stem waxiness’ was characterized as ‘weak’ for the majority of the landraces (>75%) belonging to the above group, while the landraces belonging to the two other groups (Central mainland and Aegean Islands) were characterized with ‘medium’ stem waxiness (90% of the landraces). The landraces belonging to the second group, the Aegean islands, included landraces with a higher ‘number of peduncles’ and ‘number of umbels/plant’ by ∼30% comparing to the average respective values of the landraces classified to the two other groups. However, the ‘number of leaves/plant’ was lower by ∼40% comparing to the landraces which were classified in the group North mainland of Greece. Finally, most of the landraces originated from the islands were characterized as ‘early’ concerning the ‘time of appearance of main umbel’ (>85%) and the ‘number of days to flowering’ (>85%), while the majority of the landraces from the other two groups were characterized as ‘medium’ (more than 80% of the landraces) for the above mentioned characteristics. Finally, the landraces belonging to the third group, Central mainland of Greece, had 2.3 times higher ‘number of leaves/plant’ and a reduced ‘width of leaves’, by 40% comparing to the other two groups.
Discussion
The characterization results and the estimated H' values of the present study revealed a rich diversity of the collected Greek dill landraces (, and ). Non-linear PCA and HCA grouping methods have classified the 33 dill landraces into similar groups where three were in common: (a) Northern mainland Greece, (b) Aegean islands and (c) Central mainland Greece, as indicated in separate cycles of the dill landraces ((b) and (b)).
The studied Greek landraces showed variability for the most of the recorded traits confirming the genetic diversity that is captured and kept by farmers, which played an important role since they kept the initial diversity and through a long-term process of selecting and preserving their own seeds, they have contributed to expand it into landraces (Traka-Mavrona et al. Citation2002). This agricultural biodiversity is valuable and it could be exploited into breeding schemes aiming to produce cultivars adapted to the changing environment and emerging needs of the future agriculture (Xepapadeas et al. Citation2014).
The development and evolution of the landraces is also affected by agro-climatic conditions. This impact is constantly changing and there is increasing attention to the connection between climate change and the use and conservation of agricultural biodiversity (Bellon & van Etten Citation2014). The high topographic variability of Greece, favors diverse bioclimatic conditions, where the cultivated landraces develop different morphological characteristics. The results confirm the above, since the studied landraces were classified into groups, based on their agro-morphological characteristics, which had correlated with their geographical origin.
It is noteworthy that two out of the three geographic patterns, revealed through the clustering of dill landraces, ( and ) are coinciding with the two out of the total 13 distinct floristic regions of Greece, as these are designated according to Strid and Tan (Citation2002) in Flora Hellenica, that is, the North mainland of Greece (group 1) with the North-East Greece (NE) floristic region and Aegean islands (group 2) with the floristic region of the Island complex of Cyclades (Kik). The above enhancing the results of the study for a robust grouping according to the geographic origin of the landraces. In a similar study though, on a set of 37 Iranian dill landraces, Solouki et al. (Citation2012) reported that the genetic diversity revealed based on morphological traits and AFLP markers was not in accordance to the geographical region. Likewise, Suresh et al. (Citation2013) using a more diverse set of 135 dill accessions from different continents, concluded that the dendrogram derived by the application of RAPD markers showed little to no geographic structuring of the accessions.
In the current work, the second group included landraces originated from the Aegean islands, which consist isolation center for their native flora (Georghiou & Delipetrou Citation2010) and their dill landraces were characterized as ‘early’ and with higher ‘stem waxiness’ that is connected to the water economy under dry conditions (Hutmacher et al. Citation1990). Besides, in a recent technical study is reported that the highest value for Gaussen xerophytic index in Greece, was estimated for Cyclades complex Aegean islands, enhancing the thought for specific to drought adaptive traits of the dill landraces originated from the Aegean islands (Gouvas & Sakelariou Citation2011).
On the other hand, the accessions of the first group originated from the North mainland of Greece and especially East Macedonia and Thrace, which is characterized by prevalent environmental conditions in the region with the ample of water availability due to higher precipitation. This enables higher vegetative development and plant biomass comparing to the other two groups and it is probably connected with the higher ‘numbers of leaves/plants’ and ‘medium’ waxiness that is usually depended on increased water availability. Environment effects over time have been widely recognized by many authors as factors of paramount importance affecting the clustering patterns of the accessions during genetic diversity studies (Martins et al. Citation2006; Zhao et al. Citation2007; Solouki et al. Citation2008).
Beyond adaptive traits related to particular climatic conditions the study of Greek dill landraces revealed also useful variation that can be promptly exploited in crop improvement. Plant height and number of leaves per plant are always among the traits assessed in terms of productivity of dill crop, as these traits are directly related to the fresh herb weight (Karklelienė et al. Citation2014; Said-Al Ahl & Omer Citation2016). It was revealed that although few in proportion, some of the Greek dill landraces are characterized by ‘tall’ plant height and by ‘large’ number of leaves per plant that renders them as potential parents for useful genes for yield component traits, like KD-040/07, KD-235/07 and KD-272/07 regarding plant height and IK-143/06 and T-538/06 regarding number of leaves per plant. Furthermore, Holubowicz and Morozowska (Citation2011) emphasize on the problems during the seed production process of the dill crop and they conclude on the importance of the diameter of the main and primary umbels as well as on the importance of the position of the umbel in the seed stalk for producing sufficient and high quality of seeds. In respect to this, Greek dill landraces revealed also a useful variation for the seed production related traits, as T-349/06 and T-326/06 were characterized by ‘large’ diameter of the main umbel.
Conclusively, the results of our study showed a remarkable phenotypic variation for the Greek dill landraces that is promising for their use in the manipulation of value-added traits in dill commercial exploitation. Also, it was found a correlation of the agro-morphological characteristics with the geographical region of origin underlying the impact of the environment to the landraces.
Acknowledgements
The authors would like to acknowledge the staff of the Greek Genebank for the generous supply of the dill landraces accessions used in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Elissavet G. Ninou holds a PhD in Agronomy and Plant Breeding from Aristotle University of Thessaloniki. She is Postdoc researcher focusing on the evaluation and use of local landraces for sustainable growth. She has participated in many European projects and has published research articles on plant breeding and varieties' water use efficiency.
Ioannis G. Mylonas is an expert on plant breeding and biotic and abiotic stress resistance. He has published many researches in plant breeding and is currently working in life long learning projects of American Farm School. He has participated in many European projects related to plant breeding and has numerous published research articles.
Athanasios L. Tsivelikas is a Scientist at the International Center for Agricultural Research in the Dry Areas (ICARDA), Rabat, Morocco. He holds a PhD on Genetics and Plant Breeding from Aristotle University of Thessaloniki, Greece. His research is focusing on conservation of plant genetic resources and sustainable use of agrobiodiversity. He has published several research papers on promoting landraces diversity and sustainable agricultural systems.
Dr Parthenopi E. Ralli, belongs to the scientific staff of the Institute of Plant Breeding and Genetic Resources, of the Hellenic Agricultural Organization-Demeter since 2004. She holds a PhD in Plant Breeding and Agronomy from Aristotle University of Thessaloniki, Greece. She has experience on research related to plant genetic resources including collection, regeneration, characterization and evaluation with the use of agronomic traits and molecular techniques, ex situ and in situ conservation and documentation. She has been involved in several research European projects and working groups and committees and has publications in internationally referred journals, in Proceedings of International and National Conferences and in book chapters.
References
- Abdi H, Williams LJ. 2010. Principal component analysis. Wiley Interdiscip Rev. 2:433–459. doi: 10.1002/wics.101
- Bechere E, Belay G, Mitiku D, Merker A. 1996. Phenotypic diversity of tetraploid wheat landraces from north-central regions of Ethiopia. Hereditas. 124:165–172. doi: 10.1111/j.1601-5223.1996.00165.x
- Bellon MR, van Etten J. 2014. Climate change and on-farm conservation of crop landraces in centres of diversity. In: Jackson M, Ford-Lloyd B, Parry ML, editors. Plant genetic resources and climate change. Wallingford: CAB International; p. 137–150.
- Chung JW, Sundan S, Park JH, Lee GA, Sung JS, Lee SY, Baek HJ, Kim YG, Cho GT. 2012. Selection of RAPD markers for investigating genetic diversity in dill (Anethum graveolens L.) germplasm. J Korean Soc Int Agric. 24:463–469.
- Cronbach LJ. 1951. Coefficient alpha and the internal structure of tests. Psychometrika. 16:297–334. doi: 10.1007/BF02310555
- Dušek K, Dušková E, Smékalová K. 2010. Variability of morphological characters and active compound contents in Salvia verticillata L. in the Czech Republic. Czech J Genet Plant Breeding. 46:S85–S86. (Special issue).
- Eticha F, Bekele E, Belay G, Börner A. 2005. Phenotypic diversity in durum wheat collected from Bale and Wello regions of Ethiopia. Plant Genet Resour C. 3:35–43. doi: 10.1079/PGR200457
- Food and Agriculture Organization of the United Nations [FAO]. 2010. The second report on the state of the world’s plant genetic resources for food and agriculture. Rome: FAO.
- Gao G, Bergefurd B. 1998. Culinary herbs as alternative cash crops for small scale farmersn in southern Ohio. J Extension. 36:63–67.
- Georghiou K, Delipetrou P. 2010. Patterns and traits of the endemic plants of Greece. Bot J Linn Soc. 162:130–422. doi: 10.1111/j.1095-8339.2010.01025.x
- Gouvas M, Sakellariou N. 2011. Κλίμα και Δασική Βλάστηση της Ελλάδας [Climate and forest vegetation of Greece]. Athens: National Observatory of Athens, Technical Library; p. 106–107. Greek.
- Holubowicz R, Morozowska M. 2011. Effect of umbel position on dill (Anethum graveolens L.) plants growing in field stands on selected seed stalk features. Folia Hort. 23/2:157–163.
- Hutmacher RB, Steiner JJ, Ayars JE, Mantel AB, Vail SS. 1990. Response of seed carrot to various water regimes. I. Vegetative growth and plant water relations. J Am Soc Hortic Sci. 115:715–721.
- International Union for the Protection of New Varieties of Plants [UPOV]. 1999. Dill (Anethum graveolens L.). Guidelines for the conduct of tests for distinctness, uniformity and stability. TG/165/3. Geneva: UPOV.
- Iorizzo M, Senalik DA, Ellison SL, Grzebelus D, Cavagnaro PF, Allender CH, Brunet J, Spooner DM, van Deynze A, Simon PW. 2013. Genetic structure and domestication of carrot (Daucus carota subsp. sativus) (Apiaceae). Am J Bot. 100:930–938. doi: 10.3732/ajb.1300055
- Jana S, Shekhawat GS. 2010. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacogn Rev. 4:179–184. doi: 10.4103/0973-7847.70915
- Jiménez-Mejías P, Vargas P. 2015. Taxonomy of the tribe Apieae (Apiaceae) revisited as revealed by molecular phylogenies and morphological characters. Phytotaxa. 212:57–79. doi: 10.11646/phytotaxa.212.1.2
- Karklelienė R, Dambrauskienė E, Juškevičienė D, Radzevičius A, Rubinskienė M, Viškelis P. 2014. Productivity and nutritional value of dill and parsley. Hort Sci. (Prague), 41:131–137.
- Koutsika-Sotiriou M, Mylonas IG, Ninou E, Traka-Mavrona E. 2010. The cultivation revival of a landrace: Pedigree and analytical breeding. Euphytica. 176:15–24. doi: 10.1007/s10681-010-0206-z
- Krymow V. 1989. Healing plants of the Bible: History, lore, and meditations. Cincinnati, OH: St. Anthony Messenger Press; p. 234.
- Linting M, Meulman JJ, Groenen PJF, van der Kooij AJ. 2007. Nonlinear principal components analysis: Introduction and application. Psychol Methods. 12:336–368. doi: 10.1037/1082-989X.12.3.336
- Lopez PA, Widrlechner MP, Simon PW, Boylston SRTD, Isbell TA, Bailey TB, Gardner CA, Wilson LA. 2008. Assessing phenotypic, biochemical, and molecular diversity in coriander (Coriandrum sativum L.) germplasm. Genet Resour Crop Ev. 55:247–275. doi: 10.1007/s10722-007-9232-7
- Maghsoudi Kelardashti H, Rahimmalek M, Talebi M. 2015. Genetic diversity in Iranian fennel (Foeniculum vulgare Mill.) populations based on sequence related amplified polymorphism (SRAP) markers. J Agric Sci Tech. 17:1789–1803.
- Martins SR, Vences FJ, Saenz de Miera LE, Barrosa MR, Carnide V. 2006. RAPD analysis of genetic diversity among and within Portuguese landraces of common white bean (Phaseolus vulgaris L.). Sci Hortic. 108:133–142. doi: 10.1016/j.scienta.2006.01.031
- Mengistu DK, Kiros AY, Mario EP. 2015. Phenotypic diversity in Ethiopian durum wheat (Triticum turgidum var. durum) landraces. Crop J. 3:190–199. doi: 10.1016/j.cj.2015.04.003
- Pagnano M, Gauvreau K. 2000. Principles of Biostatistics. San Francisco, CA: Duxbury Press; p. 592.
- Patel RP, Kumar RR, Singh R, Singh RR, Rao BRR, Singh VR, Pankhuri G, Rashmi L, Lal RK. 2015. Study of genetic variability pattern and their possibility of exploitation in Ocimum germplasm. Ind Crops Prod. 66:119–122. doi: 10.1016/j.indcrop.2014.12.043
- Quer F. 1981. Plantas Medicinales, El Dioscorides Renovado. Barcelona: Editorial Labor, SA; p. 500.
- Raghu D, Senthil N, Saraswathi T, Raveendran M, Gnanam R, Wnjatachalam R, Shanmugassunduram P. 2007. Morphological and Simple Sequence Repeats (SSR) based Finger printing of South Indian Cassava Germplasm. Int J Integr Biol. 1:141–149.
- Said-Al Ahl HAH, Omer EA. 2016. Impact of cultivar and harvest time on growth, production and essential oil of Anethum graveolens cultivated in Egypt. Int J Pharm Pharm Sci. 8(4):54–60.
- Secretariat of the Convention on Biological Diversity. 2010. Global Biodiversity Outlook 3. Montreal: CBD; p. 94.
- Sneath PHA, Sokal RR. 1973. Numerical taxonomy. San Francisco: W.H. Freeman and Company; p. 573.
- Solouki M, Hoseini SB, Siahsar BA, Tavassoli A. 2012. Genetic diversity in dill (Anethum graveolens L.) populations on the basis of morphological traits and molecular markers. Afr J Biotechnol. 15:3649–3655.
- Solouki M, Mehdikhani H, Zeinali H, Emamjomeh AA. 2008. Study of genetic diversity in Chamomile (Matricaria chamomile) based on morphological and molecular markers. Sci Hortic. 117:281–287. doi: 10.1016/j.scienta.2008.03.029
- Strid A, Tan K. 2002. Flora Hellenica vol. 2. Ruggell: A.R.G. Gantner Verlag K.G.; p. X–XI.
- Suresh S, Chung JW, Sung JS, Cho GT, Park JH, Yoon MS, Kim CK, Baek HJ. 2013. Analysis of genetic diversity and population structure of 135 dill (Anethum graveolens L.) accessions using RAPD markers (Article). Genet Resour Crop Evol. 60:893–903. doi: 10.1007/s10722-012-9886-7
- Terzopoulos PJ, Bebeli PJ. 2010. Phenotypic diversity in Greek tomato (Solanum lycopersicum L.) landraces. Sci Hortic. 126:138–144. doi: 10.1016/j.scienta.2010.06.022
- Traka-Mavrona E, Tasios V, Palatos G, Koutsos TH, Stavropoulos N, Mellidis V. 2002. Περιγραφή, αξιολόγηση και αξιοποίηση εγχώριων ποικιλιών τομάτας άνυδρου τύπου που καλλιεργούνται σε ελληνικά νησιά [Description, evaluation and use of non-watering type landraces of tomatoes]. In: Proceeding of the 9th Greek Scientific Society of Genetics and Plant Breeding; 2002 Oct 30–Nov 1; Thessaloniki (Greece). Greek.
- Ulukan H. 2011. The use of plant genetic resources and biodiversity in classical plant breeding. Acta Agri. Scand Sect B. 61:97–104.
- Xepapadeas A, Ralli P, Kougea E, Spyrou S, Stavropoulos N, Tsiaousi V, Tsivelikas A. 2014. Valuing insurance services emerging from a gene bank: The case of the Greek Gene Bank. Ecol Econ. 97:140–149. doi: 10.1016/j.ecolecon.2013.11.012
- Zaouali Y, Chograni H, Trimech R, Boussaid M. 2012. Genetic diversity and population structure among Rosmarinus officinalis L. (Lamiaceae) varieties: var. typicus Batt. and var. troglodytorum Maire based on multiple traits. Ind Crops Prod. 38:166–176. doi: 10.1016/j.indcrop.2012.01.011
- Zeven AC. 2002. Traditional maintenance breeding of landraces: 2. Practical and theoretical consideration on maintenance of variation of landraces by farmers and gardeners. Euphytica. 123:147–158. doi: 10.1023/A:1014940623838
- Zhao W, Wang Y, Chen T, Jia G, Wang X, Qi J, Pang Y, Wang S, Li Z, Huang Y, et al. 2007. Genetic structure of mulberry from different ecotypes revealed by ISSRs in China: an implication for conservation of local mulberry varieties. Sci Hortic. 115:47–55. doi: 10.1016/j.scienta.2007.07.017
