ABSTRACT
Earthworms and arbuscular mycorrhizal (AM) fungi are important ecosystem engineers that co-occur in soil and belong to different guilds. In this study, hyphosphere interactions between earthworms and AM fungi on soil nitrogen (N) and phosphorus (P) availability were investigated under field conditions with a modified chamber near the maize root. The experimental design consisted of root exclusion chambers that either allowed (30 μm pores) or precluded (0.45 μm pores) growth of AMF hyphae (AM factor), with or without earthworm addition (earthworm factor). Hyphal length density, soil and
content, soil available P concentration, urease and alkaline phosphatase activities were tested at maize VT (tassel), R1 (silking), R2 (blister), R3 (milk) and R4 (dough) stage. Results showed that earthworms did not impact hyphal length density in the hyphosphere. AM and AM + E treatments caused a decrease in available phosphorus, while the E treatment increased
and
content during the whole experiment period. AM and AM + E treatments could significantly increase soil alkaline phosphatase activities compared to non-AMF treatments, while E and AM + E treatment could significantly increase soil urease activities compared to non-Earthworm treatments. Hyphosphere regulation of earthworms and arbuscular mycorrhizal fungus on soil N and P availability might lead to greater nutrient uptake by plant. These interactions are important for utilization of soil biological fertilization in sustainable agro-ecosystems.
Introduction
As providers of key ecological services, soil organisms can have major impact on the ecosystem by altering the physical, chemical and biological nature of their soil environment, such as nitrogen and carbon cycling (Jeffries et al. Citation2003; Barrios Citation2007; van der Heijden et al. Citation2008). Soil organisms, such as earthworms and arbuscular mycorrhizal fungi (AMF), are often referred to by their benefits on their environment. Earthworms, for example, are referred to as ‘ecosystem engineers’ and AMF as ‘biofertilizers’ (Pellegrino et al. Citation2012). AMF are beneficial microbes that are fundamental for soil fertility (Pellegrino et al. Citation2011), as they lead to increased plant uptake of less mobile nutrients through an increased exploitation of the soil volume (Sorensen et al. Citation2008). AM fungi are able to extend their extensive hyphae from the P depletion zone to explore a greater soil volume for inorganic P sources, to take up inorganic N, and to transport N to host plants (Hodge & Fitter Citation2010; Smith & Smith Citation2011). On the other hand, through the burrowing, casting, and mixing of soil (Laossi et al. Citation2010), earthworms not only can promote plant growth (Milleret et al. Citation2009), but also influence the extent, connectivity and functionality of mycelial hyphal networks (mycelia) (Lawrence et al. Citation2003).
It is apparent that earthworms positively affect AMF hyphae in soil (Brown et al. Citation2000; Gormsen et al. Citation2004; Li et al. Citation2013). The positive effects of earthworms on AMF biomass occur through direct disruption of the hyphae and subsequent compensatory growth (Salem et al. Citation2013) or indirect modification of the chemical and physical soil conditions (Wurst et al. Citation2011). As the confounding interactions with plant roots cannot be ignored, the results therefore do not necessarily reflect direct interactions between AM fungi and earthworms. Most scientific studies that examine interactions between earthworms and mycorrhizal hyphae have been conducted in pots under greenhouse conditions; confirmatory results under more realistic field conditions could therefore be of high scientific value (Curry & Schmidt Citation2007). To date, the effects of earthworms on mycorrhizal hyphae that exclude root interference in field conditions remain unknown. Understanding the interactions between AMF hyphae and earthworms may allow for greater manipulation of the two groups of organisms to enhance N and P availability in soils. Contrary to AMF hyphae that cannot utilize cellulose and other complex carbon sources from soil in root exclusion compartments (Smith & Read Citation2008), earthworms can easily influence the turnover of soil organic matter (Laossi et al. Citation2010). Some AM fungus (Glomus intraradices) may have a low capability of utilizing phytate-P because it lacks phytase protein (Tisserant et al. Citation2012), while earthworms could enhance the phytate-P mineralization (Cao et al. Citation2015). According to evidence, combined effects of AM hyphae and earthworms can be dramatically different from their individual effects on nutrient activation (Wurst et al. Citation2011). These combined effects might play great roles during the reproductive stage of maize growth when the roots gradually age and lose the bioavailability of nutrients uptake (Ritchie et al. Citation1997; Grigeraa et al. Citation2007).
The aim of the present field study was to test the effects of indigenous AMF hyphae and earthworms on the soil’s available nutrients as well as the corresponding enzyme activities in chambers. We hypothesized that earthworms and indigenous AMF hyphae can positively interact with soil nutrients in the hyphosphere, which might lead to greater nutrient uptake by plant, particularly when the roots lose the capacity to absorb nutrients.
Materials and methods
Experimental site and design
The field study was carried out during the maize growing season from August to September 2013 at the Quzhou Experimental Station (36°5′ N, 115°0′ E) of China Agricultural University, located in Hebei Province on the North China Plain. The region has a typical continental monsoon climate, featuring warm and rainy summers and dry, cold winters. The average annual temperature is 13.1°C, precipitation is 556.2 mm, and the frost-free period is 210 days. A winter wheat/summer maize rotation system is the dominant crop cultivation system in this region. The experimental site has a loamy sand soil, containing, on a kilogram basis, 1.8 g total N, 13 g organic C, 7.72 mg available P (Olsen-P), and 33.6 mg available K (NH4OAc-K). The pH (H2O/soil, 2.5:1, v/v) is 8.44. The experiment was arranged in a continuous winter wheat/summer maize rotation system which had been cropped since 2008. For maize season a total of 185 kg N ha−1 as urea was applied: 45 kg N ha−1 incorporated with a field cultivator prior to planting, 80 and 60 kg N ha−1 at vegetative growth (June 25th and July 23th respectively). In addition, 45 kg ha−1 P as single superphosphate and 90 kg ha−1 K as K2SO4 were broadcasted before planting and incorporated. Maize (Zheng Dan 958) was planted on 12 June, with stand density was 75,000 plants ha−1.
A root exclusion device was used to inoculat vs. not inoculat with AM fungi, treat or not treat with earthworms. The experimental device was fabricated from PVC pipe sections with a diameter of 11 cm, a height of 5 cm and a wall thickness of 0.5 cm. The sides of each pipe were enclosed either with 30 μm pore size nylon mesh which allowed AM fungal hyphae to pass through but prohibited root growth, or a 0.45 μm pore size nylon mesh through which fungal hyphae or roots could not be passed. Each chamber was filled with approximately 450 g bulk soil. Four treatments were set up representing all the combinations of AMF hyphae and earthworms in the chambers: (1) CK, control with neither hyphae nor earthworms in the chambers; (2) E, with only earthworm in the chambers (3) AM, with only AMF hyphae in the chambers;(4) AM + E, both earthworms and hyphae were present in the chambers.
The soil that was used to fill the chambers was collected from the top 20 cm of the soil profile on 28 July. Field moist soil was passed through a 2 mm mesh sieve to remove plant residues and sterilized by autoclaving at 121°C for 2 h. After that, 10 ml of a soil filtrate (0.45 μm pore size) was mixed into the chambers to provide a comparable soil microbial community to the indigenous one. Earthworms (Aporrectodea trapezoides Duges (Lumbricidae)) obtained in the proximity to the field were washed with distilled water and kept in sterilized glass vessels for 24 h to minimize the number of naturally occurring mycorrhizal propagules associated with their surfaces or gut contents. Two earthworms (per chamber) with similar fresh weight (1.5 ± 0.1 g) were added to the E and AM + E treatments at the beginning of the study which is close to naturel densities of approximately 30 worms/m2 in agrosystems.
The chambers were buried so that the upper edge was 10 cm below the soil surface at the flowering stage of corn (2 August). The experimental design was a randomized complete block design with two factors, AM and earthworms blocked four times each so that a sequential harvest was possible over four instances making a total of 64 chambers. 16 chambers (four treatments and sampled 4 times) were buried in each blocks and each chamber was randomly buried (the distance between the four adjacent holes was 25 cm). Four chambers (CK, E, AM and AM + E) were carefully inserted into four adjacent holes around a maize individual and covered by soil. Sixteen chambers (CK, E, AM and AM + E) were removed from each plot 10 days after installation at grain filling and maturity stage respectively (12 and 22 August and 1 and 11 September).
Sample and analysis
The chambers were transported on ice to the laboratory. The soil was sieved through a 2 mm sieve to remove earthworms and visible organic residues, thoroughly mixed, and prepared for further analysis. The soil was subsequently treated as follows: for inorganic N extraction it was stored at −20°C; for soil moisture content, Hyphal length density, urease, phosphatase, and Olsen P analysis it was air dried.
Hyphal length density (HLD) was determined by using an aqueous extraction and a membrane filter technique modified after Jakobsen et al. (Citation1992). Briefly, three replicates of a 4 g soil sample were first dispersed in a sodiumhexametaphosphate solution (35 g l−1) and shaken for 30 s (rotation rate was 180 r min−1, end-over-end). After 30 min, the suspension was decanted quantitatively through a 400 μm sieve to retain hyphae, roots and organic matter, transferred with 200 ml of deionized water into a 250 ml flask and shaken vigorously by hand for 5 s. After 1 min, 4 × 1 ml aliquots (10 s interval) were taken and pipetted onto Millipore RAWG02500 membranes (Millipore, Bedford, MA, USA). The filter was finally stained in 0.05% Trypan Blue. HLD was estimated with a gridline intersect method at 250× magnification (Newman Citation1966).
Soil exchangeable and
were extracted with a 2 M KCl buffer (1 g soil 6 ml−1 solution), and the filtrates were analyzed using a Bran + Luebbe Auto Analyzer 3 (Bran + Luebbe, Norderstedt, Germany) (Liu et al. Citation2008). Soil available P was extracted with 0.5 mol L−1 NaHCO3 and determined: 1 g of air-dried soil and 20 ml extraction solution (0.5 M NaHCO3) (pH 8.5) were mixed by shaking at 200 r min−1 for 30 min and then P concentrations in the suspension (Olsen solution) were measured spectrophotometrically at 880 nm using the phosphomolybdate method (Murphy & Riley Citation1962).
Urease activity was determined as proposed by Kandeler and Gerber (Citation1988). Soil (10 g) was treated with 10 ml urea (10%, w/v), 20 ml citrate buffer (1 M, pH 6.7), and 1.5 ml methylbenzene and kept at room temperature for 15 min. The sample was then incubated at 37°C for 24 h under shaking. The solution was filtered, and about 1.0 ml of the filtrate was mixed with 10 ml distilled water, 4 ml sodium phenolate hydroxide, and 3.0 ml sodium hypochlorite. The optical density was determined 20 min later by a UV-2450 Spectrophotometer at 578 nm. Urease activity was expressed as milligrams per gram dry soil released in 24 h. Ure = a × V × n × m−1 (a: the concentration of NH3-N obtained by standard curve, V: color liquid volume, n: share ratio, m: soil dry weight).
Alkaline (pH 10) phosphatase activity was determined according to the procedure of Tabatabai (Citation1982), with minor modifications. Four ml of a modified universal buffer (MUB, pH 9.0) and 1 ml 25-mM sodium p-nitrophenyl phosphate in MUB were added to 1.0 g soil and then incubated at 37°C. After 60 min, the reaction was stopped by adding 4 ml 0.5-M NaOH, and the sample was kept at room temperature for 20 min. The optical density of the solution was determined at 420 nm and by using 1% p-nitrophenol as the standard. The alkaline phosphatase activity was expressed as the number of micrograms per gram (PNP) per gram dry soil released in 1 h.
Statistics and data analyses
Data on all parameters/response variables (e.g. HLD, earthworms biomass, soil available P, soil inorganic nitrogen, soil alkaline phosphatase and urease activities etc.) were subjected to analysis of variance using SPSS software package version 13.0 (SPSS Institute, Inc., Cary, NC, USA). Fisher’s least significant difference test was used to test for significant differences between treatment means at the 5% level. A two-way analysis of variance (ANOVA) was carried-out to assess the effects of AMF and earthworms. Relationships between treatments were also tested through Pearson’s correlation analyses.
A redundancy analysis (RDA) implemented with the Canoco version 4.5 software package further examined the relationship between the experimental factors and the nutrient status of the soil. Significance of the RDA relied on Monte Carlo permutation tests that were conducted using 499 random permutations. Significance was assumed when p < 0.05 (Li et al. Citation2012b).
Results
Hyphal length density and earthworms in the hyphosphere
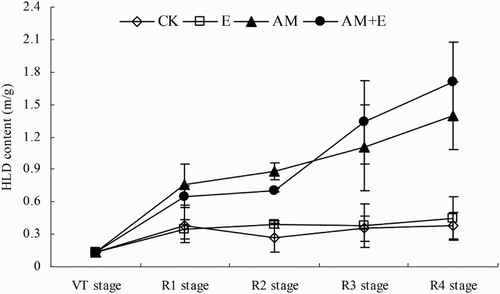
At the end of the experiment almost no mycorrhizal hyphae were detected in the non-AMF-treated samples (CK and E). The hyphal length density (HLD) of chambers in CK and E treatment remained from the VT stage to R4 stage which might have been due to non-AMF fungal hyphae. The HLD in AM and AM + E treatments were significantly greater than in the treatments without hyphae (CK and E treatments; p < 0.01) in the chambers. The HLD increased from 0.73 ± 0.19 to 1.28 ± 0.31 m g−1 when only AM hyphae was present, and increased from 0.67 ± 0.11 to 1.66 ± 0.36 m g−1 when hyphae and earthworms were both present during the course of the experiment. The HLD in AM and AM + E treatments increased gradually during the course of the experiment. Total hyphal length density increased nearly two times in the AM and AM + E treatments from the R1 stage to R4 stage of corn ().
Figure 1. Dynamic change of AMF biomass (HLD) in CK, E, AM and AM + E treatments from VT to R4 stage. Bars represent means ± SEs (n = 4). CK stands for the chamber without earthworm and hypahe addition; E for the chamber with earthworms; AM for the chamber with indigenous hyphae access; AM + E for the chamber with earthworms and indigenous hyphae access.
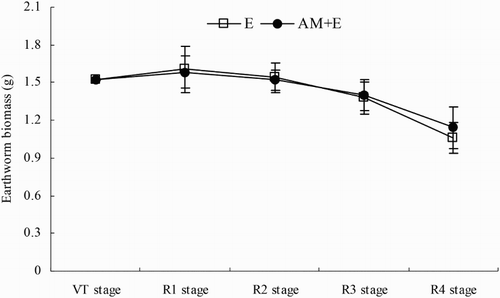
In each of sample stage, the two initially added earthworms were recovered. The earthworm biomass in the E and AM + E treatments were significant decreased from the VT to R4 stage, decreasing on average by 25% during the course of the experiment. The two treatments showed similar tendencies but there was no significant different between them ().
Available phosphorus and inorganic nitrogen
The available phosphorus in the AM and AM + E treatments was significantly lower than in treatments without hyphae in the chambers (CK and E treatments; p < 0.05). The chambers without AMF treatments saw decreases in available phosphorus by 14.8% and 17.1% during the course of the experiment, but the available phosphorus decreased sharply by 62.2% and 47.7% in AM and AM + E treatments, respectively. The difference in available phosphorus in the chambers was not significant in the CK and E treatments (except for the R3 stage). The available phosphorus in AM and AM + E treatments decreased gradually from the VT stage to R4 stage, and there was no significant difference except for in the R4 stage of corn ().
Table 1. Dynamic change of inorganic nitrogen and available phosphorus in the chambers.
Compared to the CK treatment, the E treatment significantly increased and
content (p < 0.05; ), while AM treatment decreased these components, especially at the R2 and R4 stage. When both earthworms and hyphae were present, the
and
content were slightly lower, indicating that inorganic nitrogen (especially ammonium N) mineralized by earthworms was assimilated by mycorrhizal hyphae. A two-factor analysis of variance indicated significant interactive effects in inorganic nitrogen (p < 0.01; ).
Table 2. Variance analysis of mycorrhizal hyphae, earthworms and sampling stages.
Soil alkaline phosphatase and urease activities
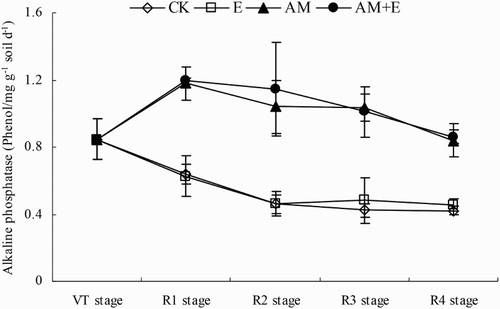
The alkaline phosphatase activities in AM and AM + E treatments were significantly greater than the non-AMF treatments (CK and E) (p < 0.01). The alkaline phosphatase activities in both AM and AM + E treatments increased firstly from the VT stage to R1 stage, and then decreased gradually at the R4 stage. But there was no significant different between these two treatments. On the contrary, the alkaline phosphatase activities in the non-AMF treatments (CK and E treatments) decreased instantly from the VT to R4 stage, and there was also no significant different between them ().
Figure 3. Dynamic change of alkaline phosphatase activities in CK, E, AM and AM + E treatments from VT to R4 stage. Bars represent means ± SEs (n = 4). CK stands for the chamber without earthworm and hypahe addition; E for the chamber with earthworms; AM for the chamber with indigenous hyphae access; AM + E for the chamber with earthworms and indigenous hyphae access.
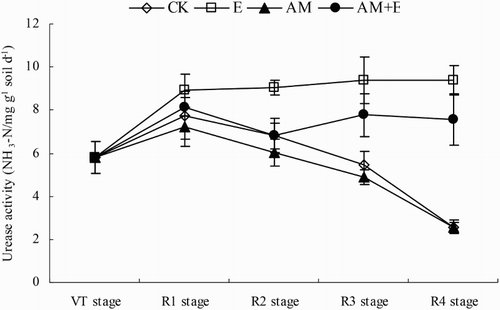
The urease activities in the non-Earthworm treatments (CK and AM) decreased continuously as time progressed, while the urease activities in the Earthworm treatments (E and AM + E) remained stable and even had a slight upward trend. The urease activities in E treatment were always significantly higher than the other treatments (p < 0.05), while the urease activities in AM + E treatment were significantly higher than the non-Earthworm treatments (CK and AM) on R3 and R4 stage ().
Figure 4. Dynamic change of urease activities in CK, E, AM and AM + E treatments from VT to R4 stage. Bars represent means ± SEs (n = 4). CK stands for the chamber without earthworm and hypahe addition; E for the chamber with earthworms; AM for the chamber with indigenous hyphae access; AM + E for the chamber with earthworms and indigenous hyphae access.
RDA analysis of soil nutrients and enzymes activities in the chambers
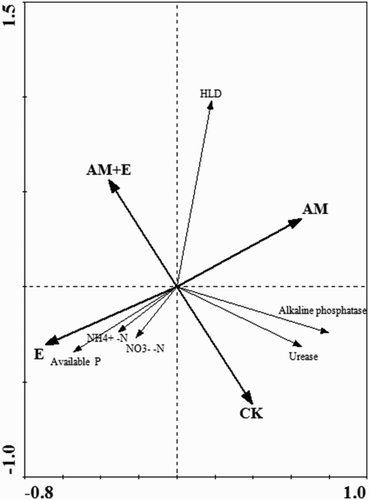
The RDA showed that the presence of earthworms and AMF had a significant influence on soil nutrient depletion and corresponding enzymes activities in the chambers. Inorganic nitrogen ( and
content) and available P were positively affected by the presence of earthworms, while soil nutrients depletion were mainly affected by HLD. The presence of earthworms and HLD affected both soil urease and alkaline phosphatase activities were both affected by the present of earthworms and HLD, and they had a significant positive relationship with the corresponding soil nutrients. Hyphae length density in chambers, on the other hand, was not affected by the present of earthworms or not ().
Figure 5. Independent and interactive action of earthworms and hyphae on the properties of the soil available nutrients and the corresponding enzymes activities in the chambers, harvested at four stages in ordination diagrams from RDA. The coordinate from the first two ordination axes explained 52.5% of the variance. The significance (according to Monte Carlo permutation tests) of all canonical axes was P = 0.003, indicating that the presence of soil organisms had a significant influence on the soil properties, HLD content and enzymes activities in the chambers. CK stands for the chambers without hyphae pass through and earthworms addition; E for the chambers with earthworms addition; AM for the chambers with hyphae pass through; AM + E for the chambers with both hyphae and earthworms.
Discussion
Effect of earthworms on mycorrhizal hyphae in hyphosphere soil
Earthworms and AMF are two taxonomically dissimilar groups of soil organisms that have pronounced effects on soil fertility (Aghababaei et al. Citation2014). The effects of earthworm and AM fungi interactions on plant performance have been further studied. The results are commonly species-specific and vary from an increased plant growth and nutrient uptake to no interactive effects (Zaller et al. Citation2011). In non-compartmentalized systems, root activity can mask the magnitude of effects that either earthworms or AMF have (Tuffen et al. Citation2002; Xiang & Li Citation2014). In the present study, we established hyphosphere compartments (chambers covered with 30 μm mesh), excluded access to the roots, and added earthworms to investigate earthworms-hyphae interactions under field settings. The HLD in AM and AM + E treatments was significantly greater than in treatments without hyphae in the chambers (), indicating that the 0.45 μm nylon mesh effectively restricted hyphal penetration into the chambers, while the 30 μm nylon mesh allowed hyphae to pass though (Xiang & Li Citation2014). The hyphal length density in the chambers increased gradually from the VT stage to R4 stage of corn, which was not significant affected by the addition of earthworms (, ). The hyphal length density was not affected by the presence of earthworms. These results were in agreement with the findings of Yang et al. (Citation2015), which showed that earthworms did not have significant effects on AMF biomass. AMF can transfer plant-derived C-rich compounds to the attached soil through extensive extraradical hyphae, providing them to microbes (Zhang et al. Citation2016). But earthworms can release various enzymes to decompose organic matter, and in doing so can provide the AMF hyphae with inorganic nutrients (Hodge Citation2014). Therefore, earthworms selectively feeded on the AMF hyphae but may also provide some benefits to the fungi. This finding can largely be explained by the fact that the effects of damage on fungal mycelia and support for fungal mycelia were offset by one other (Eisenhauer et al. Citation2009).
The experiment showed that the earthworms biomass decreased with the reduction of soil moisture content in chambers with time, which might directly affect earthworms activities. The changes in soil moisture content were mainly enacted by rainfall. In the past, high soil moisture has been shown to be favorable to earthworms (Birkas et al. Citation2010). The water content of the drilosphere affects both C and N dynamics which can also affect the feeding behavior of earthworms (Amador et al. Citation2005). So the dominating factor on the dynamic changes and effects of earthworms biomass was soil moisture content.
Earthworms-hyphae interactions on soil nutrients availabilities and enzymes activities
In the present study, the available phosphorus in AM and AM + E treatments decreased significantly during the reproductive period in the chambers. When both hyphae and earthworms were present the alkaline phosphatase activity was highest while available-P was the lowest ( and ), indicating that the AM fungus-earthworms interaction promoted the mineralization of soil P. This was evidenced by the capture of soil P in the hyphal soil, which had a linear relationship with HLD and soil acid phosphatase activities (). These results imply that the alkaline phosphatase produced by hyphae might play an important role in the mineralization of soil P. The dynamic changes of HLD from VT to R4 were regulated by maize. The reduction in available P inside the chambers from VT to R4 stage was likely due to hyphal-mediated P uptake (Grigeraa et al. Citation2007). Compared with the AM treatment, however, no further P depletion occurred in the AM + E treatment probably because (1) a large proportion of extraradical hyphae excited in hyphosphere to complete P uptake and (2) P mineralization by earthworms offset the damage to hyphae by disrupting. That means that earthworms, on the one hand, promoted soil P availabilities, and on the other hand, they reduced mycorrhizal functions due to soil physical disturbance from burrowing, casting, or selective feeding on hyphae and spores (Li et al. Citation2012a).
Soil organisms are known to influence ecosystem processes and influence plant growth by changing soil nutrients cycling and enhancing nutrients uptake (Wardle Citation2006). Earthworms generally promote N availability for plants, while AMF increase P availability (Tian et al. Citation2011). Thus, earthworms and AMF can increase plant nutrient supply via different but complementary mechanisms (Li et al. Citation2012a). In the present study, earthworms significantly increased and
content (p < 0.05; ), while AM treatment decreased them especially at R2 and R4 stage. There was also a significant interaction between hyphae and earthworms in inorganic nitrogen (). Chambers that allowed only access to AM hyphae were as efficient as roots. The facilitation effect was highly relevant for inorganic nitrogen uptake by fungal mycelium. Indicating that hyphae acted as a route of transfer of available soil nutrients, their extraradical mycelia might increase the nutrient uptake capacity of the roots by extending the soil volume exploited (Grigeraa et al. Citation2007). A previous work had shown that the AMF hyphae assisted maize in the uptake of 15N mineralized from wheat straw by earthworms in the hyphosphere (Li et al. Citation2013). The interaction between earthworms and mycorrhizal hyphae might have been due to inorganic nitrogen mineralized from organic matter by the earthworms and transferred from the chambers by the hyphae. The inorganic nitrogen (especially ammonium N) mineralized by earthworms was facilitated by mycorrhizal hyphae delivery.
Soil enzymes are important indicators of soil biochemical processes because they are involved in the dynamics of soil nutrient cycling and nutrient availability (Aira et al. Citation2005; Aghababaei et al. Citation2014). It has been shown that AM fungi can increase soil neutral phosphatase and alkaline phosphatase activities by altering the root exudation pattern or fungal exudates (Zhang et al. Citation2011). In the present study, the activity of urease in chambers significantly increased in the present of earthworms (p < 0.05), confirming what has already been reported (Don et al. Citation2008; Tao et al. Citation2009). Consistent with the previous studies (Li et al. Citation2012b; Aghababaei et al. Citation2014), earthworms-hyphae have significant interactions with soil urease activities, but the alkaline phosphatase activities were mainly dominated by hyphae, rather than affected by earthworms. The previous studies showed that earthworms and AMF complement one another in influencing plant growth by affecting soil nutrient availability under greenhouse conditions; the interactive effects were not significant, however, in field conditions.
Maize is known to have high nutrients requirements during reproductive stages (Karlen et al. Citation1988), and root biomass gradually declines after tasseling (Plenet Citation1995). Meanwhile, there was a high N, P demand for maize at this stage. Maize may be more dependent on earthworms for nutrient activation and AM hyphae for nutrient acquisition. Consistent with our hypothesis, inorganic nitrogen and available P were positively affected by the presence of earthworms, while soil nutrients depletion were mainly affected by hyphae (). These results indicate that earthworms-hyphae interaction can conjointly promote nutrients availability in the soil and might lead to greater nutrient uptake by plants in the hyphosphere. A more focused experiment must be designed in order to better understand the underlying mechanisms and disentangle these complex interactions.
Acknowledgments
We are grateful to Dr. Stavros D. Veresoglou for valuable comments on an earlier version of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Huan Li, Ph.D., is a researcher at College of Resources and Environment, Qingdao Agricultural University, Qingdao, China. He published articles on Biology and Fertility of Soils and Acta Agriculturae Scandinavica Section B - Plant Soil Science, focusing on plant nutrition, soil biological fertility and Mycorrhiza-earthworm interaction.
Dan Xiang, Ph.D., is a researcher at College of Resources and Environment, Qingdao Agricultural University, Qingdao, China. She published articles on New Phytologist, Acta Agriculturae Scandinavica Section B - Plant Soil Science, and Microbial Ecology, focusing on microbial molecular ecology and microbial biogeography.
Chong Wang, Ph.D., is a researcher at College of Resources and Environment, China Agricultural University, Beijing, China. He published articles on Biology and Fertility of Soils, Pedobiologia, Microbial Pathogenesis and European Journal of Soil Biology, focusing on plant nutrition, soil biology and soil health and earthworm ecology.
Additional information
Funding
References
- Aghababaei F, Raiesi F, Hosseinpur A. 2014. The combined effects of earthworms and arbuscular mycorrhizal fungi on microbial biomass and enzyme activities in a calcareous soil spiked with cadmium. Appl Soil Ecol. 75:33–42. doi:10.1016/j.apsoil.2013.10.006.
- Aira M, Monroy F, Domínguez J. 2005. Ageing effects on nitrogen dynamics and enzyme activities in casts of Apprrectodea caliginosa (Lumbricidae). Pedobiologia. 49:467–473. doi:10.1016/j.pedobi.2005.07.003.
- Amador JA, Görres JH, Savin MC. 2005. Role of soil water content in the carbon and nitrogen dynamics of Lumbricus terrestris L. burrow soil. Appl Soil Ecol. 28:15–22. doi:10.1016/j.apsoil.2004.06.009.
- Barrios E. 2007. Soil biota, ecosystem services and land productivity. Ecol Econ. 64:269–285. doi:10.1016/j.ecolecon.2007.03.004.
- Birkas M, Bottlik L, Stingli A, Gyuricza C, Jolánkai M. 2010. Effect of soil physical state on the earthworms in Hungary. Appl Environ Soil Sci. 1–7. doi:10.1155/2010/830853.
- Brown GG, Barois I, Lavelle P. 2000. Regulation of soil organic matter dynamics and microbial activity in the drilosphere and the role of interactions with other edaphic functional domains. Eur J Soil Biol. 36:177–198. doi:10.1016/S1164-5563(00)01062-1.
- Cao J, Huang Y, Wang C. 2015. Rhizosphere interactions between earthworms (Eisenia fetida) and arbuscular mycorrhizal fungus (Funneliformis mosseae) promote utilization efficiency of phytate phosphorus in maize. Appl Soil Ecol. 94:30–39. doi:10.1016/j.apsoil.2015.05.001.
- Curry JP, Schmidt O. 2007. The feeding ecology of earthworms – a review. Pedobiologia. 50:463–477. doi:10.1016/j.pedobi.2006.09.001.
- Don A, Steinberg B, Schöning I, Pritsch K, Joschko M, Gleixner G, Schulze ED. 2008. Organic carbon sequestration in earthworm burrows. Soil Biol Biochem. 40:1803–1812. doi:10.1016/j.soilbio.2008.03.003.
- Eisenhauer N, König S, Sabais ACW, Renker C, Buscot F, Scheu S. 2009. Impacts of earthworms and arbuscular mycorrhizal fungi (Glomus intraradices) on plant performance are not interrelated. Soil Biol Biochem. 41:561–567. doi: 10.1016/j.soilbio.2008.12.017
- Gormsen D, Olsson PA, Hedlund K. 2004. The influence of collembolans and earthworms on AM fungal mycelium. Appl Soil Ecol. 27:211–220. doi:10.1016/j.apsoil.2004.06.001.
- Grigeraa MS, Drijbera RA, Shores-Morrowa RH, Wienhold BJ. 2007. Distribution of the arbuscular mycorrhizal biomarker C16:1cis11 among neutral, glyco and phospholipids extracted from soil during the reproductive growth of corn. Soil Biol Biochem. 39:1589–1596. doi:10.1016/j.soilbio.2007.01.009.
- Hodge A. 2014. Interactions between arbuscular mycorrhizal fungi and organic material substrates. Adv Appl Microbiol. 89:47–99. doi: 10.1016/B978-0-12-800259-9.00002-0
- Hodge A, Fitter AH. 2010. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. P Natl Acad Sci USA. 107:13754–13759. doi: 10.1073/pnas.1005874107
- Jakobsen I, Abbott LK, Robson AD. 1992. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol. 120:371–380. doi: 10.1111/j.1469-8137.1992.tb01077.x
- Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM. 2003. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fert Soils. 37:1–16.
- Kandeler E, Gerber H. 1988. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fert Soils. 6:68–72. doi:10.1007/BF00257924.
- Karlen DL, Flannery RL, Sadler EJ. 1988. Aerial accumulation and partition of nutrients by corn. Agron J. 80:232–242. doi: 10.2134/agronj1988.00021962008000020018x
- Laossi KR, Ginot A, Noguera DC, Blouin M, Barot S. 2010. Earthworm effects on plant growth do not necessarily decrease with soil fertility. Plant and Soil. 328:109–118. doi:10.1007/s11104-009-0086-y.
- Lawrence B, Fisk MC, Fahey TJ, Suarez ER. 2003. Influence of nonnative earthworms on mycorrhizal colonization of sugar maple (Acer saccharum). New Phytol. 157:145–153. doi:10.1046/j.1469-8137.2003.00649.x.
- Li H, Li XL, Dou ZX, Zhang JL, Wang C. 2012a. Earthworm (Aporrectodea trapezoides)–mycorrhiza (Glomus intraradices) interaction and nitrogen and phosphorus uptake by maize. Biol Fert Soils. 48:75–85. doi: 10.1007/s00374-011-0610-0
- Li H, Wang C, Li XL, Christie P, Dou ZX, Zhang JL, Xiang D. 2013. Impact of the earthworm Aporrectodea trapezoides and the arbuscular mycorrhizal fungus Glomus intraradices on 15N uptake by maize from wheat straw. Biol Fert Soils. 49:263–279. doi:10.1007/s00374-012-0716-z.
- Li H, Xiang D, Wang C, Li XL, Lou Y. 2012b. Effects of epigeic earthworm (Eisenia fetida) and arbuscular mycorrhizal fungus (Glomus intraradices) on enzyme activities of a sterilized soil–sand mixture and nutrient uptake by maize. Biol Fert Soils. 48:879–887. doi: 10.1007/s00374-012-0679-0
- Liu YL, Zhang B, Li CL, Hu F, Velde B. 2008. Long-term fertilization influences on clay mineral composition and ammonium adsorption in a rice paddy soil. Soil Sci Soc Am J. 72:1580–1590. doi:10.2136/sssaj2007.0040.
- Milleret R, Renée-Claire LB, Jean-Michel G. 2009. Root, mycorrhiza and earthworm interactions: their effects on soil structuring processes, plant and soil nutrient concentrations and plant biomass. Plant Soil. 316:1–12. doi:10.1007/s11104-008-9753-7.
- Murphy J, Riley JP. 1962. A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta. 27:31–36. doi:10.1016/S0003-2670(00)88444-5.
- Newman EI. 1966. A method of estimating total length of root in a sample. J Appl Ecol. 3:139–145. doi: 10.2307/2401670
- Pellegrino E, Bedini S, Avio L, Bonari E, Giovannetti M. 2011. Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biol Biochem. 43:367–376. doi:10.1016/j.soilbio.2010.11.002.
- Pellegrino E, Turrini A, Gamper HA, Cafa G, Bonari E, Young PW, Giovannetti M. 2012. Establishment, persistence and effectiveness of arbuscular mycorrhizal fungal inoculants in the field revealed using molecular genetic tracing and measurement of yield components. New Phytol. 194:810–822. doi:10.1111/j.1469-8137.2012.04090.x.
- Plenet D. 1995. Fonctionnement des cultures de mais sous contrainte azotee. Determination et application d’un indice de nutrition. Dr. These Academie de Nancy-Metz, Institut National Polytechnique de Lorraine.
- Ritchie S.W., Hanaway J.J., Benson G.O. 1997. How a corn plants develops. Spec. Publ. 48. Ames, IA: Iowa State University Cooperative Extension Service.
- Salem M, Kohler J, Wurst S, Rillig MC. 2013. Earthworms can modify effects of hydrochar on growth of Plantago lanceolata and performance of arbuscular mycorrhizal fungi. Pedobiologia. 56:219–224. doi: 10.1016/j.pedobi.2013.08.003
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. San Diego: Academic.
- Smith SE, Smith FA. 2011. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Ann Rev Plant Physio. 62:227–250.
- Sorensen JN, Larsen J, Jakobsen I. 2008. Pre-inoculation with arbuscular mycorrhizal fungi increases early nutrient concentration and growth of field-grown leeks under high productivity conditions. Plant Soil. 307:135–147. doi:10.1007/s11104-008-9591-7.
- Tabatabai MA. 1982. Soil enzymes. In: AL Page, RH Miller, DR Keeney, editor. Methods of soil analysis, part 2. Madison, WI: American Society of Agronomy; p. 903–947.
- Tao J, Griffiths B, Zhang S, Chen X, Liu M, Hu F, Li HX. 2009. Effects of earthworms on soil enzyme activity in an organic residue amended rice–wheat rotation agro-ecosystem. Appl Soil Ecol. 42:221–226. doi:10.1016/j.apsoil.2009.04.003.
- Tian H, Drijber RA, Niu XS, Zhang JL, Li XL. 2011. Spatio-temporal dynamics of an indigenous arbuscular mycorrhizal fungal community in an intensively managed maize agroecosystem in North China. Appl Soil Ecol. 47:141–152. doi:10.1016/j.apsoil.2011.01.002.
- Tisserant E, Kohler A, Dozolme-Seddas P, Balestrini R, Benabdellah K. 2012. Thetranscriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol. 193:755–769. doi:10.1111/j.1469-8137.2011.03948.x.
- Tuffen F, Eason WR, Scullion J. 2002. The effect of earthworms and arbuscular mycorrhizal fungi on growth of and 32P transfer between Allium porrum plants. Soil Biol Biochem. 34:1027–1036. doi:10.1016/S0038-0717(02)00036-6.
- van der Heijden MGA, Bardgett RD, van Straalen NM. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett. 11:296–310. doi:10.1111/j.1461-0248.2007.01139.x.
- Wardle DA. 2006. The influence of biotic interactions on soil biodiversity. Ecol Lett. 9:870–886. doi:10.1111/j.1461-0248.2006.00931.x.
- Wurst S, Gebhardt K, Rillig MC. 2011. Independent effects of arbuscular mycorrhiza and earthworms on plant diversity and newcomer plant establishment. J Veg Sci. 22:1021–1030. doi:10.1111/j.1654-1103.2011.01321.x.
- Xiang D, Li H. 2014. Nutrient uptake in mycorrhizal plants – role of earthworms. Acta Agr Scand B-S P. 64:434–441. doi:10.1080/09064710.2014.920412.
- Yang N, Schuetzenmeister K, Grubert D, Jungkunst HF, Gansert D, Scheu S, Polle A, Pena R. 2015. Impacts of earthworms on nitrogen acquisition from leaf litter by arbuscular mycorrhizal ash and ectomycorrhizal beech trees. Environ Exp Bot. 120:1–7. doi:10.1016/j.envexpbot.2015.06.013.
- Zaller JG, Heigl F, Grabmaier A, Lichtenegger C, Piller K, Allabashi R, Frank T, Drapela T. 2011. Earthworm–mycorrhiza interactions can affect the diversity, structure and functioning of establishing model grassland communities. PLoS One. 6:e29293. doi:10.1371/journal.pone.0029293.
- Zhang H, Wu X, Li G, Qin P. 2011. Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol Fert Soils. 47:543–554. doi:10.1007/s00374-011-0563-3.
- Zhang L, Xu M, Liu Y, Zhang F, Hodge A, Feng G. 2016. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 210:1022–1032. doi:10.1111/nph.13838.