ABSTRACT
Soil organic carbon tends to respond more sensitively to climate change and land use intensification in ecologically fragile and economically marginal regions of mountainous areas. This study aims to evaluate the soil organic carbon stock dynamic across various land uses at different altitudes in the Bagrot valley, Northern Karakoram, Gilgit-Baltistan, Pakistan. Soil samples from 0–20, 20–40 and 40–60 cm depth were collected from three land uses: pasture, forest, and adjacently located arable land at different altitude (ranging from 2100–4163 m). The variables investigated were soil bulk density (BD), soil organic carbon concentration (SOC), soil organic carbon stock (SOCS) and pH. A significant variation in all tested variables were found across the land uses and altitudes. Likewise, soil under forest had significantly higher values of SOCS (59.35 Mg ha−1) than pasture (42.48 Mg ha−1) and arable land (23.63 Mg ha−1). Similarly, SOCS increased with increasing altitude and decreased with soil depth in all land uses. In addition, SOCS had a negative relationship with BD and pH. Overall results indicated that the land use intensification and climate change (increase in temperature and decrease in precipitation) were associated with declining SOCS. These results suggest restoration of degraded agricultural land to the forest, especially at higher altitude, and decrease in intensity of land use could increase SOCS in the study area as well as other similar mountainous regions.
Introduction
Soil organic carbon (SOC) plays an important role in food security and climate change mitigation (Lal Citation2004). The soil is the largest and a significant pool of carbon in the terrestrial ecosystem (Schlesinger Citation1997) and a significant constituent of global C cycle. Two-thirds of SOC is stored in soil (Schlesinger Citation1997), which is three times greater than that of plants and two times that in the atmosphere (Wang et al. Citation2011). SOC improve soil properties (Mulongoy & Merckx Citation1993) by holding a great amount of important soil nutrients for plant growth (Leu Citation2007) and providing energy to the microbial community (Mulongoy & Merckx Citation1993).
SOC can be influenced by various factors such as altitude and climate (Djukic et al. Citation2010), soil parent material and texture (Hoffmann et al. Citation2009), slope- aspect (Schwanghart & Jarmer Citation2011; Lozano-García et al. Citation2016), soil pH (Falloon & Smith Citation2009), vegetation (Luyssaert et al. Citation2008), and land uses (Morgan et al. Citation2010). Several studies have shown significant variation in SOC with relation to land use (Lemenih & Itanna Citation2004; Shufang et al. Citation2012; Iqbal et al. Citation2014; Mauryaa et al. Citation2014; Meng et al. Citation2014) and it decreases with the increase in soil depth (Yang et al. Citation2010). The type of land use is a vital element controlling SOC stock in soil; the land use affects the amount of input and the rate of decomposition of litter in soil (Lehmann et al. Citation2000). Likewise, SOC stock was found to be decreased by cultivation (Chen et al. Citation2010; Vikas et al. Citation2014).
Knowledge of vertical patterns of SOC can improve our understanding of the dynamics of SOC along with a profile and the potential response of SOC to climate change; a small variation in climate at higher altitude will drastically change the ecology and function of the mountain ecosystem (Jodha Citation1992). Similarly, altitude has a strong effect on the micro-climate and landscape (Bendix et al. Citation2008; Gerold et al. Citation2008). With increasing altitude, normally temperature becomes cooler and precipitation increases, resulting in vegetation distribution change, which impacts SOC stock (Tanner et al. Citation1998; Proctor et al. Citation2007). Various researchers have reported a positive relationship between SOC and altitude (Woldeamlak & Stroosnijder Citation2003; Genxu et al. Citation2004; Emiru & Gebrekidan Citation2013) although not always (e.g. Sheikh et al. Citation2009; Jahangeer et al. Citation2013). Similarly, SOC stock has been observed to increase with an increase in precipitation and decrease with an increase in temperature (Schlesinger Citation1997; Kidanemariam et al. Citation2012). However, SOC stock depends on C inputs from plant biomass production and outputs through decomposition (Schlesinger Citation1997).
Considerable studies were undertaken on effects of land use and altitude (climate change) on SOC stock in stable core areas. However, the researchers have given less attention to ecologically highly vulnerable and economically extremely marginal regions: these regions tend to respond more sensitively to changes in their framework conditions such as a change in land cover, land uses and climate change as compared to the stable core areas of human activities (Ehlers Citation2005). The natural environment of Karakoram in Gilgit-Baltistan is that of high-altitude limits of human land use and heavily glaciated milieu with an extremely steep and diversified topography; it is also a distinct vegetation zone, with cold and snow-rich winters (Ehlers Citation1995, Citation2005). Human occupation confined to the narrow river terraces and valley bottom allowing irrigated agriculture and mountain slopes with humid forest and high altitude pastures permitting seasonal grazing. Heavy rains, earthquakes, avalanches and other natural phenomena are continuous, which causes natural disasters such as landslides, mudflow, rock fall etc., sometimes causing severe damage to land and life (Hewitt Citation1988; Kreutzmann Citation1994). In general, due to the topographical setting and harsh climatic situation of the mountain areas, there are limited natural resources in this region (Ehlers Citation1995).
However, increasing population growth and construction of the Karakoram Highway (KKH) in 1970 has drastically increased the interference of man in these ecologically highly vulnerable and economically extremely marginal areas of the Karakoram; it involves over-exploitation of natural resources such as expansion and intensification of agriculture activities, overgrazing and severe deforestation (Ehlers Citation1995, Citation2005). These activities enhance the processes of degradation, soil erosion, slope destabilization, rockfalls, and rocks slides, which already common in the Karakoram mountains (Kreutzmann Citation1994; Ehlers Citation1995). Furthermore, these processes causing environmental degradation and local deterioration of the natural environment and soil health (Sitaula et al. Citation2004; Ehlers Citation2005). The possible changes that may occur increase the emission of greenhouse gases (Post & Kwon Citation2000), decrease SOC stock (Fenton et al. Citation1999) and other soil parameters (Kay Citation1995; Sahani & Behera Citation2001; Abbasi et al. Citation2010; Abbasi et al. Citation2010).
The objective of this study is to determine the effects of different land uses and altitudes (in terms of climate change) on SOC dynamics in the ecological and economically marginal land in the Bagrot Valley of Karakoram. Understanding the effect of land use and altitude on SOC stock is essential for effective land use planning and mitigation of climate change. This study may also provide information on what might happen to SOC pool under a warming global climate scenario in ecologically highly vulnerable and economically extremely marginal mountain areas.
Materials and methods
Study area
The Bagrot Valley is situated in the southern part of the Karakoram Range, about 17 km to the northeast of Gilgit, Pakistan. The Karakoram Range lies in the center of the high mountain node: Hindukush in the west, Pamir and Tien Shah to the northwest, Kulun Shah in the northeast, and Himalaya in the southeast. The total watershed area is 440 km2: 16.14% comprised of pasture, 16.43% forest, 3.35% arable land, 64.08% ice, glacier, and rock (WWF Pakistan Citation2009). With rising altitude, summer becomes shorter and cooler whereas winters are longer. The relationship between altitude and climate (temperature and precipitation) variation in the study area (Bagrot Valley) has been calculated by Cramer (Citation2000): There is an average temperature decline of 0.63°C /100 m and a statistical precipitate increases from + 17.6 mm per 100 m. The annual mean air temperature decreases from 11.6°C at 2210 m (Sinker) to – 2.2°C in 4030 m (Diran). Similarly, the annual precipitation increases from 142 mm (2210 m) to 721 mm (4030 m). Geologically, the area consists of the Chalt Volcanic Group, which has a high content of SiO2, MgO, Fe2O3 and CaO (Petterson et al. Citation1991). In the Bagrot Valley, people have practiced ‘Mixed Mountain Agriculture’ for at least 1000 years. This includes agriculture, pastoralism, and forestry. The forest site in this study represents an undisturbed ecosystem extending from 2700 m to 3600 m altitude, where Juniperus, Pinus wallichiana (pine/chi), Picea smithiana (spruce/kachul) and Betula utilis are the dominant plants. Similarly, the native grassland sites in the study started from 3000 m altitude to 4200 m, and mostly consist of alpine grass including Bistorta affinis, Leontopodium, Gentiana tianschanica, Pedicularis cheilanthifolia, Poa alpina, Carex stenocarpa, Festuca alatavica and Swertia petiolate. The agriculture land are located from 2000 m to 3010 m altitude, where wheat (Triticum aestivum), maize (Zea mays), and potatoes (Solamum tuberosum) are grown ().
Experimental design and sampling
In the present study, variation in SOCS was determined across the different land uses and along the altitudinal gradients in the Bagrot Valley, Northern Karakoram, Pakistan. To determine the variation in SOCS across the land use, soil samples were collected from adjacent land uses such as pastures, forests and arable land located at the similar altitudes. Five sample plots of 20 × 20 m2 were randomly selected in each land use. Similarly, for the investigation of variation in SOCS along the altitude gradient, 10 sampling sites at different altitudes were selected in each of the land uses including pasture (3317–4163 m), forest (2787–3600 m), and arable land (2100–2930 m). Furthermore, five sample plots of 20 × 20 m2 were located in the sites.
In each plot, five randomly points were chosen for sampling. After removing the vegetation, litter, roots, stones, and debris from the surface of the sampling point, then soil samples were taken from three depths (0–20, 20–40 and 40–60 cm) using soil agar in a ‘X’ type pattern and making a composite sample for each layer. In this way, five composite replicates samples were taken from each plot. One undisturbed core sample was also collected from each plot for bulk density by using a 100 cm3 core ring and which was packed in the sealed bag. A total of 720 soil samples were collected for the current investigation. This includes 270 samples for land use study (three land uses × five plots × three depths × five replicates), and 420 samples (three land use × ten sites × three depths × five replicates) for altitudinal investigation. The exact location of sampling points and altitude were recorded by a GPS (Garmin nuvi 3490 LMT 4.3-Inch). The vegetation and morphological characteristics were also recorded. The samples were packed in separate polyethylene bags and labeled. These samples were brought to the laboratory and then air dried. The dried samples were lightly ground, sieved through a (< 2 mm), and used for analysis SOC and pH.
Laboratory analysis
Bulk density was determined by the core method described by Blake and Hartge (Citation1986). The samples were weighed in their sealed bags, clumps broken by hand and then oven dried at 40°C to constant weight. Thereafter, each sample was oven dried at 105°C for 4 h, which allowed for the calculation of soil bulk density. The samples were then be dry sieved (< 2 mm) and the rock fragment > 2 mm was weighed. The method described by Rowel (Citation1994) was used for determination of pH in 1: 2.5 (soil: water). The soil organic carbon was determined by Walkley and Black (Nelson & Sommers Citation1982). The conversion of oxidizable organic C to total C was done by multiplying with the factor 1.30. The total soil organic carbon stock (Mg ha−1) was calculated by multiplying the C concentration (g 100 g) with the calculated bulk density (g cm−3) and depth of soil layer (cm) by using the following formula described by Batjes (Citation1996):
Where, F is the volume of the fraction of fragments > 2 mm.
Data analysis
An analysis of variance (ANOVA) was used to determine the effect of land use and altitude on BD, SOC, and SOCS at the different depths. A Least Significant Difference (LSD) comparison test was carried out to separate statistically different means (p < 0.05). Linear regression analysis was carried out to determine the relationship of SOC with altitude and other variables. Statistical Package for Social Sciences (SPSS) was used for all analyses (Einstein & Abernethy Citation2000).
Results and discussion
Effect of land uses on soil organic carbon stock
Analysis of variance () showed that land use and soil depth had significant effects on bulk density (g cm−3), SOC concentration (%) and SOC stock (Mg ha−1). The overall mean SOC and SOCS in the forest (3.74% and 59.35 Mg ha−1) was significantly higher than pasture (2.51% and 42.48 Mg ha−1) and was the lowest in arable land (0.93% and 23.63 Mg ha−1) (). Similar trends were observed for each depth. The highest SOC and SOCS were observed more often in the upper layer (0–20 cm) than in the middle layer (20–40 cm) and was lowest in the bottom layer (40–60 cm). SOC plays a significant role in food security and climate change mitigation (Lal Citation2004). It also helps to control soil erosion, increase infiltration and porosity of clay soil, improve soil structure and stability; which contributes to the cycling of plant nutrients and provides energy to microbes and fauna, increasing their activity (Schlesinger Citation1997). In this study, SOC was found to be very sensitive to land use intensification especially in ecologically highly vulnerable and economically extremely marginal land. Due to intensive agricultural activities, about 60.18% less SOC was found in arable land as compared to the forest and 44.37% less than pasture. The intensive land uses such as continuous plowing and cultivation accelerated the rate of organic matter decomposition and mineralization. Further, the removal of biomass during harvesting and periodic tillage breaks up macro aggregates, resulting in increased soil erosion and runoff. Other researchers also reported that cultivation can reduce SOC (Billings Citation2006; Wang et al. Citation2008), and can change the balance between humification and mineralization processes (Saviozzi et al. Citation2001). Likewise, tillage changes the soil moisture, aeration, and nutrient concentrations, resulting in increased oxidation of soil organic matter (Lal Citation1995; Pandey et al. Citation2010) and the loss via soil microorganisms (Reicosky & Forcella Citation1998); this can in turn reduce the inputs of organic matter from above- and below-ground vegetation (Zhao et al. Citation2005; Yimer et al. Citation2006). In contrast, the higher SOC in the forest could be due to the greater biomass input by vegetative material, which is the main source of soil organic carbon. Organic matter generally has a positive relationship with plant density (Rees et al. Citation2005; Thomas et al. Citation2007). Forests store more C than any other terrestrial ecosystems and represent a significant carbon pool for the global carbon budget (Houghton Citation2007). It is estimated that almost 60% of the world’s terrestrial carbon is stored in forest vegetation and soil (Winjum et al. Citation1992); it comprised of 80% of aboveground terrestrial C and 40% of soil C (Dixon et al. Citation1994; Batjes Citation1996; Waring Citation1998; Goodale et al. Citation2002).
Figure 2. Variation Soil bulk density, SOC (%) and SOCS (Mg/Ha) across different land uses in various depths. Values having the same letter do not differ significantly from each other with respect to land uses and depth at p ≤ 0.05. Letters x, y, z indicate the variation within land uses in different depths, while, letters a, b, c indicate the variation among the land uses in each depth. Abbreviation BD (bulk density); SOC (soil organic carbon) and SOCS (soil organic carbon stock).
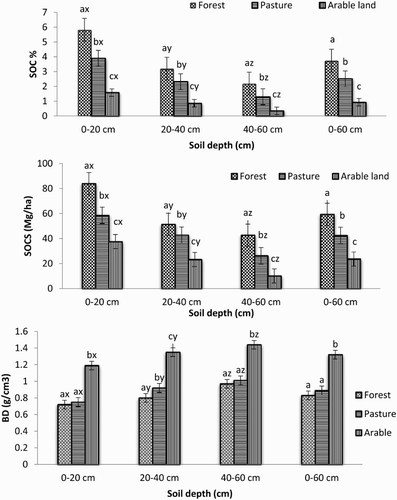
Table 1. Effect of land uses on soil BD (g cm−3) and SOC (%) and stock in different depth.
In this study, about 50–60% of SOC was found in the top layer, while 25–30% was in the middle and 10–15% in the bottom layer. The highest SOC and SOCS in the upper layer could be due to continuous addition of undecayed and partially decomposed plant and animal remains in the top soils. Other researchers have also reported that surface layers have accumulated more organic materials than deeper layers (Wang et al. Citation2008; Emiru & Gebrekidan Citation2013).
Effect of altitude on soil organic carbon stock
Analysis of variance () showed that both altitude and soil depth had significant effect on BD (g cm−3), SOC (%) and SOCS (Mg ha−1) in all land uses (p < 0.01). In arable land the overall mean SOC was significantly increased from 0.52–1.0% and SOCS from 15.48 to 23.87 Mg ha−1, with increasing altitude from 2100–2930 m. A similar trend was observed for each depth. Furthermore, SOC and SOCS decreased significantly with increasing depth (). In contrast, in the forest the SOC increased (from 2.43 to 12.49%), as well as SOCS (from 48.42–165.26 Mg ha−1), with an increase in altitude from 2797 to 3460 m (). Above this altitude, a slight decrease in SOC was found up to 3600 m. The same pattern was also observed in the 0–20, 20–40 and 40–60 cm soil layers (). Similarly, in pasture, the SOC concentration significantly increased from 1.21–8.57% as did SOCS (from 33.82–102.0 Mg ha−1), with increasing altitude from 3017–3767 m, and then slightly decreased to 4163 m (). Similar results were observed for the top, middle and bottom layers. Furthermore, SOC (%) and SOCS (Mg ha−1) had a significantly negative relationship with bulk density, soil depth and soil pH ().
Figure 3. Relationship of soil organic carbon stock with different variable in arable land.
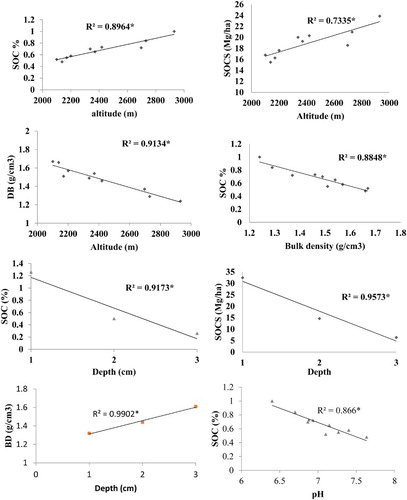
Figure 4. Relationship of soil organic carbon stock with different variable in forest.
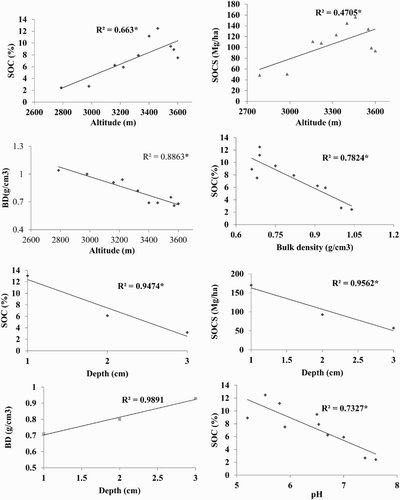
Figure 5. Relationship of soil organic carbon stock with different variable in Pasture.
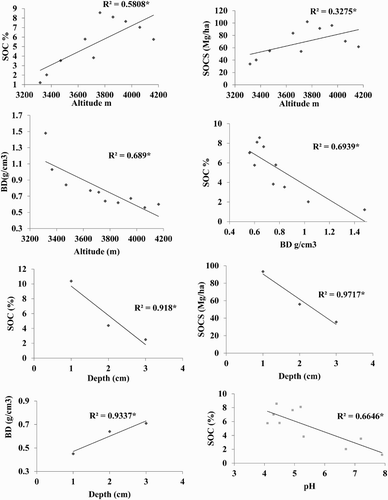
Table 2. Effect of altitude on soil organic carbon stock in each land uses in various depth.
Knowledge of vertical patterns of soil organic C can improve our understanding of the dynamics of SOC along a profile and the potential response of SOC to climate change; a small variation in climate at higher altitudes will drastically change the ecology and function of the mountain ecosystem (Jodha Citation1992). Similarly, altitude has a strong effect on the micro-climate and landscape (Bendix et al. Citation2008; Gerold et al. Citation2008). With increasing altitude, temperature normally becomes cooler and precipitation increases, resulting in vegetation distribution change, which impacts SOC stock (Tanner et al. Citation1998; Proctor et al. Citation2007). In addition, climate shifts in temperature and precipitation have a major influence on the decomposition and amount of SOC stored within an ecosystem and released into the atmosphere.
In the study area have the same parent material and vegetation along with the altitudes, however increased in SOC and SOCS with increasing altitude could be due to variation in climatic parameter along with an elevation gradient. In the area, the average temperature decreased by 0.63°C per 100 m and precipitation increased + 17.6 mm per 100 m Cramer (Citation2000). Cooler temperatures and higher precipitation with altitude could retard the decomposition of litter. In turn, the accumulation of organic matter may have increased with this altitude. Other researchers have reported a positive relation between SOC with altitude (Sims & Nielsen Citation1986; Ineson et al. Citation1998; Charan et al. Citation2012). Furthermore, the lightly decreased amount of SOCS at higher altitudes in forest and pasture could be due to harsh climatic conditions, which provided less time for soil formation, restrict decomposition of the organic matter and produced low net primary production. At the upper timberline (Dame 3780 m), there were 102 frosting and 135 ice days, and vegetation period were short (112 days). Likewise, in the alpine meadow (4150 m), there were seventy-three frost and 188 ice days indicating the rough climate conditions. The thermal vegetation period lasted 95 days. As a result, vegetation density decreased in forest and pasture at higher elevations due to short growing period. The vegetative material, which is the main source of soil organic carbon and SOC, generally has a positive relationship with plant density (Rees et al. Citation2005; Thomas et al. Citation2007). Other researchers have also found a decrease in SOC at higher altitudes (Panthi Citation2010; Saeed et al. Citation2014). The low SOC at lower altitudes could be due to higher temperatures and less precipitation. In addition, mineralization of soil organic matter increased with rising temperature (Garcia-Pausas et al. Citation2007; Djukic et al. Citation2010) and the rate of reaction doubled with each 10°C increase in mean temperature (Bot & Benites Citation2005).
The highest SOC (21.61%) in this analysis was observed in the top layer in Betula utilis forest at an altitude of 3460 m. Similar SOC (21.9%) have been reported by Schickhoff (Citation2005) in Batura II (3500 m). Bhat (Citation2010) reported the highest SOC in Betula utilis forest (3550 m) in high mountain temperate Himalayan forests of India and further suggested that Betula utilis was a good source of biomass production and carbon balance. In this study, about 50 -60% SOCS was observed in the top layer, while 25–30% was in the middle and 10–15% in the bottom layer. This could be due to the continuous accumulation of undecayed and partially decomposed plant and animal residue in the surface soils. Other researchers have also reported the highest SOCS in the top soil layer (Woldeamlak & Stroosnijder Citation2003; Emiru & Gebrekidan Citation2013).
SOC had a negative relationship with bulk density and pH (–5) in this current study. Bulk density value is dependent on the amount of organic matter. The soil with high organic matter has a low bulk density (White Citation1997; Gupta Citation2004). Furthermore, high organic matter, by oxidation, produced organic acids in the soil solution causing a decrease in soil pH. Fey (Citation2001) also has reported that organic matter can have a negative relationship with pH.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Shamsher Ali, Ph.D., is a Lecturer and Environmental Chemist at the Karakoram International University, Pakistan, working on the response of soil organic carbon to land use intensification and environmental change.
Farida Begum, Ph.D., is an Assistant professor and Environmental Scientist at the Karakoram International University, Pakistan, working on Carbon Dynamics and Sequestration potential under different land use systems for sustainable management of agro ecosystem and mitigation of climate change.
Rifat Hayat, Ph.D., is an Associate Professor and Soil Scientist at the Pir Maher Ali Shah Arid Agriculture University, Pakistan, working on Soil microbiology/Systematic/Soil Metagenomics under different cropping systems.
Brendan J. M. Bohannan, Ph.D., is a Professor of Biology and Environmental Studies at the University of Oregon, Eugene, USA, working on causes and consequences of microbial biodiversity and the response of bacterial and archaeal communities to environmental change. Prof. Bohannan is especially interested in promoting the integration of microbial ecology into the general science of ecology.
References
- Abbasi MK, Zafar M, Sultan T. 2010. Changes in soil properties and microbial indices under different land cover types in the mountain region of Rawalakot Azad Jammu and Kashmir. Commun Soil Sci Plant Anal. 41:768–782. doi: 10.1080/00103620903565985
- Batjes NH. 1996. Total carbon and nitrogen in the soils of the world. Eur J Soil Sci. 47:151–163. doi: 10.1111/j.1365-2389.1996.tb01386.x
- Bendix J, Rollenbeck R, Richter M, Fabian P, Emck P. 2008. Climate. In: Beck E, Bendix J, Kottke I, Makeschin F, Mosandl R, editors. Gradients in a tropical mountain ecosystem of ecuador. Berlin, Heidelberg: Springer-Verlag; p. 63–73.
- Bhat MI. 2010. Characterization, classification and nutrient indexing of saffron growing soils of district Pulwama [Ph.D. Thesis]. SKUAST-Kashmir.
- Billings SA. 2006. Soil organic matter dynamics and land use change at a grassland/forest ecotone. Soil Biol Biochem. 38:2934–2943. doi: 10.1016/j.soilbio.2006.05.004
- Blake GR, Hartge KH. 1986. Bulk density. In: Klute A, editor. Methods of soil analysis. Part 1: physical and mineralogical methods. Soil science society of America. Book Series No. 5, Part 1. Madison, WI: ASA, SSSA; p. 363–375.
- Bot A, Benites J. 2005. The importance of soil organic matter. Rome: Soils Bulletin 80, Food and Agriculture Organization of the United Nations (FAO).
- Charan G, Bharti VK, Jadhav SE, Kumar S, Angchok D, Acharya S, Kumar P, Srivastava RB. 2012. Altitudinal variations in carbon storage and distribution patterns in cold desert high altitude region of India. Afr J Agric Res. 7:6313–6319. doi: 10.5897/AJAR12.1168
- Chen DD, Zhang SH, Dong SK, Wang XT, Du GZ. 2010. Effect of land-use on soil nutrients and microbial biomass of an Alpine region on the Northeastern Tibetan Plateau, China. Land Degrad Dev. 22:446–452.
- Cramer T. 2000. Topo-climatic studies of Bagrot Valley-Karakorum, Pakistan. GEO currently, Vol. 3. (terrimago-Verl.), Göttingen. 231 S.
- Dixon RK, Brown S, Houghton REA, Solomon AM, Trexler MC, et al. 1994. Carbon pools and flux of global forest ecosystems. Science. 263:185–190. doi: 10.1126/science.263.5144.185
- Djukic I, Zehetner F, Tatzber M, Gerzabek MH. 2010. Soil organic-matter stocks and characteristics along an Alpine elevation gradient. Global Change Biol. 4:143–152.
- Ehlers E. 1995. The organization of space and time - population growth, Resource management and appropriate land use in Bagrot/Karakoram. Pet Geogr Mitt. 139:105–120.
- Ehlers E. 2005. Interaction of society and environment in economically and ecologically marginal lands. In: Elena M, Yukio H, Ivan B, editors. Understanding land uses and land cover change in global and regional context. Enfield (NH): Science publishers, Inc; p. 23–29.
- Einstein G, Abernethy K. 2000. Statistical package for the social sciences (SPSS) Version 12.0. Greenville: Furman University.
- Emiru N, Gebrekidan H. 2013. Effect of land use changes and soil depth on soil organic matter, total nitrogen and available phosphorus contents of soils in Senbat watershed, Western Ethiopia. ARPN J Agric Biol Sci. 8:206–212.
- Falloon P, Smith P. 2009. Modeling soil carbon dynamics. Soil carbon dynamics. An integrated methodology. Berlin: Cambridge University Press.
- Fenton TE, Brown JR, Maubach MJ. 1999. Effects of long-term cropping on organic matter content of soils: implication for soil quality. In: Lal R, editor. Soil quality and soil erosion. Ankeny, IA: CRC Press; p. 95–124.
- Fey MV. 2001. Consequences of a land scape turned sour: the effect of excessive soil acidity on the natural resources, agriculture and forestry. Proc. 5th international symposium on soil plant interactions at low pH; Alpine Health, South Africa; p. 52–62.
- Garcia-Pausas J, Casals P, Camarero LS, Huguet C, Sebastia MT, Thompson JA, Romanya J. 2007. Soil organic carbon storage in mountain grasslands of the Pyrenees: effects of climate and topography. Biogeochemistry. 82:279–289. doi: 10.1007/s10533-007-9071-9
- Genxu W, Haiyan M, Ju Q, Juan C. 2004. Impact of land use changes on soil carbon, nitrogen and phosphorus and water pollution in an arid region of northwest China. Soil Use Manage. 20:32–39. doi: 10.1079/SUM2003220
- Gerold G, Schawe M, Bach K. 2008. Hydrometeorology, pedologic and vegetation patterns along an elevational transect in the montane forest of the Bolivian Yungas. Erde. 139:141–168.
- Goodale CL, Apps MJ, Birdsey RA, Field CB, Heath LS, Houghton RA, Jenkins JC, Kohlmaier GH, Kurz W, Liu S, et al. 2002. Forest carbon sinks in the Northern Hemisphere. Ecol Appl. 12:891–899. doi: 10.1890/1051-0761(2002)012[0891:FCSITN]2.0.CO;2
- Gupta PK. 2004. Plant analysis. In: Gupta PK, editor. Soil, plant, water and fertilizer analysis. Jodhpur, India: Agrobios; p. 258–292.
- Hewitt K. 1988. Catastrophic landslides deposits in the Karakoram Himalaya. Science. 242:64–67. doi: 10.1126/science.242.4875.64
- Hoffmann T, Glatzel S, Dikau R. 2009. A carbon storage perspective on alluvial sediment storage in the Rhine catchment. Geomorphology. 108:127–137. doi: 10.1016/j.geomorph.2007.11.015
- Houghton RA. 2007. Balancing the global carbon budget. Annu Rev Earth Planet Sci. 35:313–347. doi: 10.1146/annurev.earth.35.031306.140057
- Ineson P, Taylory T, Harrison AF, Poskitt J, Benham DG, Tipping E, Woof C. 1998. Effects of climate change on nitrogen dynamics in upland soils. A transplant approach. Global Change Biol. 4:143–152. doi: 10.1046/j.1365-2486.1998.00118.x
- Iqbal MA, Hossen MS, Islam MN. 2014. Soil organic carbon dynamics for different land uses and soil management practices in Mymensingh. Proceedings of 5th International Conference on Environmental Aspects of Bangladesh; Bangladesh; p. 16–17.
- Jahangeer BA, Kaiser I, Munesh K, Negi AK, Todaria N. 2013. Carbon stock of trees along an elevational gradient in temperate forests of Kedarnath Wildlife Sanctuary. Forest Sci Pract. 15:137–143. doi: 10.1007/s11632-013-0210-1
- Jodha NS. 1992. Mountain perspective and sustainability, a framework for development. In: Jodha NS, Banskota M, Partap T, editors. Sustainable mountain agriculture. New Delhi, India: Oxford & IBM Publishing Co Pvt. Ltd.; p. 41–82.
- Kay, B. 1995. Rates of change of soil structure under different cropping systems. Adv Soil Sci. 12:1–52.
- Kidanemariam A, Gebrekidan H, Mamo T, Kibret K. 2012. Impact of altitude and land use type on some physical and chemical properties of acidic soils in Tsegede Highlands, Northern Ethiopia. Open J Soil Sci. 2:223–233. doi: 10.4236/ojss.2012.23027
- Kreutzmann H. 1994. Habitate conditions and settlement processes in Hindukush-Karakoram. Petermanns Geographische Mitteilungen. 138:337–357.
- Lal R. 1995. Global soil erosion by water and carbon dynamics. In: Lal R, Kimble JM, Levine E, Stewart BA, editors. Soil and global change. Boca Raton (FL): CRC/Lewis Publishers; p. 131–142.
- Lal R. 2004. Soil carbon sequestration impacts on global climate change and food security. Science. 304:1623–1627. doi: 10.1126/science.1097396
- Lehmann J, Da Silva Cravo M, Zech W. 2000. Organic matter stabilization in a Xanthic Ferralsol of the central Amazon as affected by single trees: chemical characterization of density, aggregate, and particle size fractions. Geoderma. 99:147–168. doi: 10.1016/S0016-7061(00)00070-7
- Lemenih M, Itanna F. 2004. Soil carbon stocks and turnovers in various vegetation type and arable lands along an elevation gradient in southern Ethiopia. Geoderma. 123:177–188. doi: 10.1016/j.geoderma.2004.02.004
- Leu A. 2007. Organics and soil carbon: increasing soil carbon, crop productivity, and farm profitability. Managing the Carbon Cycle’ Katanning Workshop, 21-22 March 2007. Available from: www.amazingcarbon.com.
- Lozano-García B, Parras-Alcántara L, Brevik EC. 2016. Impact of topographic aspect and vegetation (native and reforested areas) on soil organic carbon and nitrogen budgets in Mediterranean natural areas. Sci Total Environ. 544:963–970. doi: 10.1016/j.scitotenv.2015.12.022
- Luyssaert S, Schulze ED, Börne A, Knohl A, Hessenmöller D, Law BE, Ciais P, Grace J. 2008. Old-growth forests as global carbon sinks. Nature. 455:213–215. doi: 10.1038/nature07276
- Mauryaa B, Singh V, Dhyanib P, Kashyap S. 2014. Impact of altitudes on soil characteristics and enzymatic activities in the forest and Fallow lands of Almora District of Central Himalaya. Octa J Environ Res. 2:1–9.
- Meng X, Xiaoliang L, Xiaobu C, Jingping G, Xiaolin L. 2014. Soil microbial community structure and activity along a montane elevational gradient on the Tibetan Plateau. Eur J Soil Biol. 64:6–14. doi: 10.1016/j.ejsobi.2014.06.002
- Morgan JA, Follett RF, Allen LHJ, Del Grosso S, Derner JD, Dijkstra F, Franzluebbers A, Fry R, Paustian K, Schoeneberger MM. 2010. Carbon sequestration in agricultural lands of the United States. J Soil and Water Conservation. 65:6A–13A. doi: 10.2489/jswc.65.1.6A
- Mulongoy K, Merckx R. 1993. Soil organic matter dynamics and sustainability of tropical agriculture. Leuven: John Wiley and Sons; p. 3–18.
- Nelson DW, Sommers LE. 1982. Total carbon, organic carbon, and organic matter. In: Page AL, editor. Methods of soil analysis. Part 2. 2nd ed. Agronomy monograph 9. Madison, WI: Amer. Soc. Agronomy and Soil Sci. Soc. Amer; p. 539–594.
- Pandey CB, Singh GB, Singh SK, Singh RK. 2010. Soil nitrogen and microbial biomass carbon dynamics in native forests and derived agricultural land use in a humid tropical climate of India. Plant Soil. 333:453–467. doi: 10.1007/s11104-010-0362-x
- Panthi J. 2010. Altitudinal variation of soil fertility: a case study from langtang national park [M.Sc thesis]. Nepal: Central Department of Environmental Science Tribhuvan University Kathmandu.
- Petterson MG, Windley BF, Luff IW 1991. The Chalt volcanics, Kohistan, Pakistan: High-Mg tholeiitic and low-Mg Calc-Alkaline volcanism in a Cretaceous Iceland Arc. In: Sharma KK, editor. Geology and geodynamic evolution of the Himalayan collision zone, Part 1. New York: Pergamon Press; p. 19–30.
- Post WM, Kwon KC. 2000. Soil carbon sequestration and land-use change: processes and potential. Global Change Biol. 6:317–327. doi: 10.1046/j.1365-2486.2000.00308.x
- Proctor J, Edwards ID, Payton RW, Nagy L. 2007. Zonation of forest vegetation and soils of mount Cameroon, West Africa. Plant Ecol. 192:251–269. doi: 10.1007/s11258-007-9326-5
- Rees RM, Bingham IJ, Baddeley JA, Watson CA. 2005. The role of plants and land management in sequestering soil carbon in temperate arable and grassland ecosystems. Geoderma. 128:130–154. doi: 10.1016/j.geoderma.2004.12.020
- Reicosky DC, Forcella F. 1998. Cover crop and soil quality interactions in agro-ecosystem. J Soil Water Conserv. 53:224–229.
- Rowel DL. 1994. The preparation of saturation extracts and the analysis of soil salinity and sodicity. In: Rowell DL, editor. Soil science methods and applications. England (UK): Wesley Longman Singapore Publishers (Pte) Ltd; p. 350.
- Saeed S, Muhammad BY, Alia A, Syed SH. 2014. Impact of altitude on soil physical and chemical properties in Sra Ghurgai (Takatu mountain range) Quetta, Balochistan. Int JSci Eng Res. 5:730–735.
- Sahani U, Behera N. 2001. Impact of deforestation on soil physicochemical characteristics, microbial biomass and microbial activity of tropical soil. Land Degrad Dev. 12:93–105. doi: 10.1002/ldr.429
- Saviozzi A, Levi-Minzi R, Cardelli R, Riffaldi R. 2001. A comparison on soil quality in adjacent cultivated, forest and native grassland soils. Plant Soil. 233:251–259. doi: 10.1023/A:1010526209076
- Schickhoff U. 2005. The Upper Timberline in the Himalayas, Hindu Kush and the Karakorum: a review of geographical and ecological aspects. In: Keplin GB, editor. Mountain ecosystems; studies in treeline ecology. Berlin, Heidelberg: Springer-Verlag; p. 327–328.
- Schwanghart W, Jarmer T. 2011. Linking spatial patterns of soil organic carbon to topography - a case study from south-eastern Spain. Geomorphology. 126:252–263. doi: 10.1016/j.geomorph.2010.11.008
- Schlesinger WH. 1997. Biogeochemistry. An analysis of global change. San Diego: Academic Press; p. 588.
- Sheikh MA, Kumar M, Bussmann RW. 2009. Altitudinal variation in soil organic carbon stock in coniferous subtropical and broadleaf temperate forests in Garhwal Himalaya. Carbon Balance Manage. 4:1–6. doi: 10.1186/1750-0680-4-6
- Shufang W, Xiaoke W, Zhiyun O. 2012. Effects of land use, climate, topography and soil properties on regional soil organic carbon and total nitrogen in the UpstreamWatershed of Miyun Reservoir, North China. J Environ Sci. 24:387–395. doi: 10.1016/S1001-0742(11)60789-4
- Sims ZR, Nielsen GA. 1986. Organic carbon in Montana soils related to clay content and climate. Soil Sci Soci Am J. 50:1261–1271.
- Sitaula BK, Bajracharya RM, Singh BR, Solberg B. 2004. Factors affecting organic carbon dynamics in soils of Nepal/Himalayan Region - a review and analysis. Nutr Cycl Agroecosys. 70:215–229. doi: 10.1023/B:FRES.0000048474.85331.7d
- Tanner EVJ, Vitousek PM, Cuevas E. 1998. Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology. 79:10–22. doi: 10.1890/0012-9658(1998)079[0010:EIONLO]2.0.CO;2
- Thomas GA, Dalal RC, Standley J. 2007. No-till effects on organic matter, pH, cation exchange capacity and nutrient distribution in a Luvisol in the semi-arid subtropics. Soil Till Res. 94:295–304. doi: 10.1016/j.still.2006.08.005
- Vikas S, Shabeer H, Sharma RK, Arya VM. 2014. Labile carbon pools and soil organic carbon stocks in the foothill Himalayas under different land use systems. Geoderma. 232–234:81–87. doi: 10.1016/j.geoderma.2014.04.039
- Wang J, Soininen J, Zhang Y, Wang B, Yang X, Shen J. 2011. Contrasting patterns in elevational diversity between microorganisms and macroorganisms. J Biogeogr. 38:595–603. doi: 10.1111/j.1365-2699.2010.02423.x
- Wang ZP, Han XG, Li LH. 2008. Effects of grassland conversion to croplands on soil organic carbon in the temperate Inner Mongolia. J Environ Manage. 86:529–534. doi: 10.1016/j.jenvman.2006.12.004
- Waring RH, Running SW. 1998. Forest ecosystems: analysis at multiple scales Academic Press. San Diego, CA: Academic Press.
- White RE. 1997. Principles and practices of soils science: the soil is the natural resource. 3rd ed. Oxford (UK): Blackwell Scientific; p. 348.
- Winjum JK, Dixon RK, Schoroeder PE. 1992. Estimating the global potential of forest and agro forestry management practices to sequester carbon. Water Air Soil Pollut. 64:213–227. doi: 10.1007/BF00477103
- Woldeamlak B, Stroosnijder L. 2003. Effects of agro-ecological land use succession on soil properties in the Chemoga watershed, Blue Nile basin, Ethiopia. Geoderma. 11:85–98.
- WWF. 2009. Land cover mapping of the central Karakoram national Park, Version 2.0. Lahore: WWF – Pakistan.
- Yang K, Zhu J, Zhang M, Qiaoling Y, Sun OJ. 2010. Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: a comparison between natural secondary forest and larch plantation. J Plant Ecol. 3:175–182. doi: 10.1093/jpe/rtq022
- Yimer F, Stig L, Abdelkadir A. 2006. Soil property variations in relation to topographic aspect and vegetation community in the south-eastern highlands of Ethiopia. For Eco Manage. 232:90–99. doi: 10.1016/j.foreco.2006.05.055
- Zhao WZ, Xiao HL, Liu ZM, Li J. 2005. Soil degradation and restoration as affected by land use change in the semiarid Bashang area, Northern China. Catena. 59:173–186. doi: 10.1016/j.catena.2004.06.004