ABSTRACT
Nemarioc-AL and Nemafric-BL phytonematicides consistently reduced populations of plant-parasitic nematodes. The contribution of juvenile hatch inhibition to the overall reduction of the nematode numbers by the two phytonematicides, with cucurbitacin A and B as active ingredients, respectively, remains undocumented. The objectives of this study were to examine (i) the response of Meloidogyne incognita second-stage juveniles (J2) hatch to increasing concentration of cucurbitacins A and B, (ii) the potential saturation of J2 hatch when exposed to cucurbitacins for extended incubation periods, (iii) the minimum inhibition concentration for J2 hatch and (iv) the reversibility of J2 hatch inhibition.. Eggs of M. incognita were exposed to a series of purified cucurbitacin A and B concentrations over five incubation periods of 24, 48, 72 h and extended incubation periods of 7 and 10 days. Methanol-dissolved cucurbitacin A and B were each diluted and pipetted into well-plates making 11 concentrations, ranging from 0.0 to 2.5 µg.ml−1 water solvent. Juvenile counts were made after 24, 48 and 72 h, with those for saturation assessed at 7 and 10 days. Thereafter, treatments were diluted five times, incubated again for 5 days and counted to establish reversibility of J2 hatch inhibition. In all incubation periods, treatment effects were highly significant (P ≤ 0.01), with J2 hatch and cucurbitacin concentrations exhibiting quadratic relations. Minimum inhibition concentrations of the two cucurbitacins were between 1.13 and 1.40 µg.ml−1. Treatment effects for reversibility to J2 hatch inhibition were not significant (P > 0.05). In conclusion, J2 hatch inhibition could be one of the waysthrough which the two phytonematicides reduced population densities of Meloidogyne species.
Introduction
Cucurbitacin A (C32H46O8) and cucurbitacin B (C32H46O9) are tetracyclic triterpenoids (Chen et al. Citation2005), synthesised by the mevalonic acid pathway (Chen et al. Citation2014). The water-soluble cucurbitacin A is unstable and oxidises to cucumin (C27H40O9) and leptodermin (C27H38O8), which had been shown to have both insect repellent and insecticidal activities (Damalas Citation2011; Gobinda et al. Citation2014). The two cucurbitacins are active ingredients in Nemarioc-AL (A = cucurbitacin A, L = liquid formulation) and Nemafric-BL (B = cucurbitacin B) phytonematicides, respectively. The two phytonematicides consistently suppressed population densities of root-knot (Meloidogyne species) nematodes and citrus nematode (Tylenchulus semipenetrans) under various conditions (Mashela et al. Citation2011; Maile et al. Citation2013). The efficacy of Nemarioc-AG phytonematicide (G = granular formulation) was comparable to that of the synthetic chemicals aldicarb and fenamiphos in suppressing the second-stage (J2) population densities of M. incognita in tomato (Mashela et al. Citation2008), whereas that of Nemarioc-AL phytonematicide was comparable to that of Velum (a.i. Fluopyram) (Seshweni Citation2016).
Active ingredients in botanical pesticides occur in multiple forms, with multiple modes of action, which are relatively well-documented for phytoinsecticides (Nzanza & Mashela Citation2012), but to a limited extent for ingredients of phytonematicides (Wuyts et al. Citation2006). Phytonematicides contain a large number of active ingredients which could affect nematodes (Okwute Citation2012). Juvenile hatch inhibition, inhibition of second-stage juveniles (J2) motility and J2 paralysis are commonly listed as some of the modes of action involved in nematode suppression by chemical compounds (Wuyts et al. Citation2006).
In order to identify the precise mode of action of phytonematicides, bioassays should be carried out with purified active ingredients, preferably dose-response effects. The objectives of this study were to examine (i) the response of Meloidogyne incognita J2 hatch to increasing concentration of cucurbitacins A and B, (ii) the potential saturation of J2 hatch when exposed to cucurbitacins for extended incubation periods, (iii) the minimum inhibition concentration (MIC) for J2 hatch and (iv) the reversibility of J2 hatch inhibition.
Materials and methods
Preparation of active ingredients and nematode eggs
Purified (≈ 98%) cucurbitacin A and B (1000 µg each), obtained from ChemFaces (Wuhan, China), were first dissolved in 5 µl methanol (98% purity) to enhance solubility before adding 1 ml distilled water to make stock solutions. Egg masses of almost similar colour were obtained from greenhouse raised 2-month-old tomato plants (Solanum lycopersicum) cv. ‘Floradade’ seedlings. Egg masses were dislodged from tomato roots using a tooth pick and placed in a petri dish containing 5 ml distilled water prior to transfer into cucurbitacin concentrations.
Bio-assay
In separate trials, cucurbitacin A and B were pipetted into 96 well-plates at 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25 and 2.5 µg.ml−1 water solvent. Methanol concentration of 0.005%, equivalent to the percentage in the highest cucurbitacin concentration, was included as a control. In all trials, treatments were arranged in a completely randomised design in an incubator at 25 ± 3°C. Each treatment was replicated 3 times. After 24, 48 and 72 h, after 7 and 10 days incubation periods, the J2 were counted under a stereomicroscope. Ten days after the initial incubation, all the treatments were diluted 5 times and the eggs and J2 incubated for another 5 days for establishing the eventual reversibility of egress inhibition. For each product three independent experiments were conducted.
Statistical analysis
Cumulative J2 counts were made per well after each incubation period, but statistical analysis was performed on noncumulative counts. Data were transformed using log10(x + 1) prior to analysis of variance (ANOVA) using SAS software (SAS Institute Citation2008). Treatment means were separated using Waller-Duncan multiple range test at the probability level of 5% and subjected to lines of the best fit.
Results
There were no significant differences between methanol and distilled water controls therefore distilled water control only was used in data analysis. There were also no significant differences between the three independent experiments hence data were pooled (n = 99) and subjected to ANOVA.
Inhibition of juvenile hatch
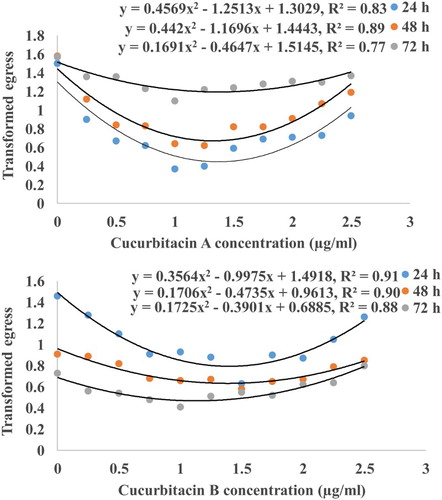
In the 24, 48 and 72 h incubation periods, treatment effects were highly significant (P ≤ 0.01), with juvenile hatch and cucurbitacin A and B concentrations exhibiting quadratic relations. For cucurbitacin A, the quadratic model explained the relations between egress and concentrations at 24, 48 and 72 h of incubation by 83, 89 and 77%, respectively (). Similarly, for cucurbitacin B, the quadratic model explained the relations between the two at the incubation periods mentioned above by 91, 90 and 88%, respectively.
Reversibility of juvenile hatch inhibition
Treatment effects for extended incubation periods of 7 and 10 days, were not significant, regardless of the cucurbitacin.
Minimum inhibition concentration
The minimum inhibition concentrations for J2 hatch were relatively similar for both cucurbitacins at all incubation periods (). Average MIC for cucurbitacin A and B were 1.37 and 1.31 µg.ml−1, respectively.
Table 1. Minimum inhibition concentration (MIC) of pure cucurbitacin A and B on second-stage juvenile hatch of Meloidogyne incognita.
Discussion
Inhibition of juvenile hatch
Relative to the 24 h incubation time, more J2 hatched at all concentrations compared to the two other incubation periods for cucurbitacin A, whereas the opposite was observed for cucurbitacin B (). Overall, the quadratic relations between juvenile hatch and increasing concentrations of cucurbitacins A and B constituted the main feature of density-dependent growth (DDG) patterns (Salisbury & Ross Citation2005). Opposite trends as shown by the positions of the quadratic models between cucurbitacins A and B could probably be due to the instability of cucurbitacin A (). Cucurbitacin A oxidises to cucumin and leptodermin, our findings suggests that these two chemical compounds are more potent than their precursor cucurbitacin A. It has been demonstrated that cucumin and leptodermin have each a very high bioactivity on insects (Damalas Citation2011; Gobinda et al. Citation2014), with limited information on nematode bioactivities.
The major observation in DDG patterns generated by our study was that at low concentration ranges the products gradually reduced juvenile hatch (inhibition) (), with others depicting this as negative linear models (Giannakou Citation2011; Odeyemi & Adewale Citation2011). Inhibition phases were followed by concentration ranges where juvenile hatch levelled off (neutral) (), depicted in other studies as no effect on juvenile hatch (Payan et al. Citation1987; Oka et al. Citation2000). The neutral effect was possible due to the ability of J2 inside the eggs to enter survival stages (McSorley Citation2003). Finally, high concentration ranges, where juvenile hatch was stimulated (), were depicted by others as positive linear models (Skantar et al. Citation2005; Meyer et al. Citation2008). In agreement with our observations, Wuyts et al. (Citation2006) showed that at least 14 purified active ingredients had no effect on M. incognita juvenile hatch, whereas salicyclic acid and caffeic acid inhibited the activity.
The unique feature of the sedentary endoparasitic root-knot nematode is that females lay their eggs in specialised structures if possible outside the roots in an egg mass and gelatinous matrix. Upon development, first-stage juveniles (J1) moult prior to juvenile hatch, resulting in J2 which use their stylets to break egg shells in response to exogenous factors, which may include chemicals from root exudates. Nematodes use chemoreceptors to discern these exogenous chemical stimuli (Perry Citation1996). Nematode bodies are covered with at least 14 types of chemoreceptors (Troemel et al. Citation1995), which are able to respond to multiple chemicals at very low concentrations for water-soluble chemoattractant chemicals (Troemel et al. Citation1995). At low concentration ranges in the current study, J2 might have interpreted the detected condition as being evidence of waning root exudates with the onset of plant senescence, thereby entering the dauer stage, which is a form of survival strategy (McSorley Citation2003). In contrast, as cucurbitacin concentrations increased, J2 in our study were tricked into perceiving the situation as being analogous to increased root exudates in trap crops (Wuyts et al. Citation2006) and increasingly hatched, thereby exposing their bodies to unfavourable conditions induced by cucurbitacins in solution.
Reversibility of juvenile hatch inhibition
Incubation for 7- and 10-days, regardless of the cucurbitacins, resulted in saturation of juvenile hatch. Observations at the two incubation periods across all the concentrations in the two cucurbitacins were important since they added an empirically-based observation of the neutral phase in DDG patterns. The Curve-fitting Allelochemical Response Data (CARD) computer based-model clarified the three phases of DDG patterns, with the neutral phases being at the top of the convex quadratic curves, between the stimulation and inhibition phases (Liu et al. Citation2003). Our findings during 24, 48 and 72 h incubation periods suggested that neutral phases can also start from inhibition to stimulation phases, which is biological sound due to the existence of survival strategies in nematodes. Post-extended incubation periods, juvenile hatch inhibition was irreversible at any level of cucurbitacins. The observation agreed with empirically-based extended incubation period observations where treatment effects were not significant since eggs were saturated with cucurbitacins. Others observed that juvenile hatch inhibition was reversed for certain active ingredients and nematode species (Wuyts et al. Citation2006).
Minimum inhibition concentration
Average MICs of cucurbitacin A and B for J2 hatch were fairly low, at 1.37 and 1.31 µg.ml−1, respectively (). Low MIC indicates high level of toxicity of the cucurbitacins to J2 hatch. When compared with synthetic chemical aldicarb, cucurbitacin MIC were observed to be in the same range (Hough & Thomason Citation1975). This provides evidence for observed comparable efficacy between Nemarioc-AG phytonematicide and aldicarb in the suppression of population densities of M. incognita in tomato (Mashela et al. Citation2008). The observed MIC for J2 hatch were higher than those of J2 immobility which averaged 0.6 µg.ml−1 (Dube Citation2016). Previous studies on plant phytotoxicity of Nemarioc-AL and Nemafric-BL phytonematicides had observed that different plant organs had different sensitivity, with those that come in direct contact with the phytonematicides, such as the roots being more sensitive than the shoots (Pelinganga et al. Citation2013). This could explain the observed lower MIC concentrations for J2 immobility when compared to J2 hatch, with egg shell probably causing the difference in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Z.P. Dube is a researcher and P.W. Mashela is senior professor of nematology at the University of Limpopo.
Additional information
Funding
References
- Chen C, Kuo TC, Yang M, Chien T, Chu M, Huang L, Chen C, Lo H, Jeng S, Chen LO. 2014. Identification of cucurbitacins and assembly of a draft genome for Aquilaria agallocha. BMC Genomics. 15:578–00. doi: 10.1186/1471-2164-15-578
- Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu GA. 2005. Cucurbitacins and cucurbitane glycosides: structure and biological activities. Nat Prod Rep. 22:386–399. doi: 10.1039/b418841c
- Damalas CA. 2011. Potential uses of turmeric (Curcuma longa) products as alternative means of pest management in crop production. Plant Omics. 4:136–141.
- Dube ZP. 2016. Nemarioc-AL and Nemafric-BL Phytonematicides: Bioactivities in Nematodes, Crops and Soil [PhD Thesis]. Sovenga: University of Limpopo.
- Giannakou IO. 2011. Efficacy of a formulated product containing Quillaja saponaria plant extracts for the control of root-knot nematodes. Eur J Plant Pathol. 130:587–596. doi: 10.1007/s10658-011-9780-8
- Gobinda CR, Kaushik C, Parthasarathi N, Moitra MN. 2014. Pros and cons of curcumin as bioactive phyto-compound for effective management of insect pests. Am Sci Res J Eng Technol Sci. 7:31–43.
- Hough A, Thomason IJ. 1975. Effects of aldicarb on the behavior of Heterodera schachtii and Meloidogyne javanica. J Nematol. 7:221–229.
- Liu DL, An M, Johnson IR, Lovett JV. 2003. Mathematical modeling of allellopathy. III. A model for curve-fitting allellochemical dose responses. Nonlinear Biol Toxicol Med. 1:37–50.
- Maile KD, Mashela PW, Tseke P. 2013. Responses of the citrus nematode to a phytonematicide Nemarioc-AG with and without micro-organisms in citrus production. Afr Crop Sci Conf Proc. 11:333–337.
- Mashela PW, De Waele D, Pofu KM. 2011. Use of Indigenous Cucumis Technologies as alternative to synthetic nematicides in management of root-knot nematodes in low-input agricultural farming systems: A review. Sci Res Essays. 6:6762–6768.
- Mashela PW, Shimelis HA, Mudau FN. 2008. Comparison of the efficacy of ground wild cucumber fruits, aldicarb and fenamiphos on suppression of the root-knot nematode in tomato. J Phytopathol. 156:264–267. doi: 10.1111/j.1439-0434.2007.01353.x
- McSorley R. 2003. Adaptations of nematodes to environmental extremes. Florida Entomol. 86:138–142. doi: 10.1653/0015-4040(2003)086[0138:AONTEE]2.0.CO;2
- Meyer SLF, Lakshman DK, Zasada IA, Vinyard BT, Chitwood DJ. 2008. Dose-response effects of clove from Syzygium aromaticum on the root-knot nematode Meloidogyne incognita. Pest Manag Sci. 64:223–229. doi: 10.1002/ps.1502
- Nzanza B, Mashela PW. 2012. Control of whiteflies and aphids in tomato (Solanum lycopersicum L.) by fermented plant extracts of neem leaf and wild garlic. Afr J Biotechnol. 11:16077–16082. doi: 10.5897/AJB12.775
- Odeyemi IS, Adewale KA. 2011. Phythonematoxic properties and nematicidal potential of Tithonia diversifolia extract and residue on Meloidogyne incognita infecting yam (Discoria rotundata). Arch Phytopathol Plant Prot. 44:1745–1753. doi: 10.1080/03235400903461039
- Oka Y, Nacar S, Putievsky E, Ravid U, Yaniv Z, Spiegel Y. 2000. Nematicidal activity of essential oils and their compounds. J Phytopathol. 90:710–715. doi: 10.1094/PHYTO.2000.90.7.710
- Okwute SK. 2012. Plants as potential sources of pesticidal agents: A review. In Soundararajan RP, editor. Pesticides: Advances in chemical and botanical pesticides. Detroit: Academic Press; p. 207–232.
- Payan LA, Johnson AW, Littrell RH. 1987. Effects of nematicides and herbicides alone or combined on Meloidogyne incognita juvenile hatch and development. Ann Appl Nematol. 1:67–70.
- Pelinganga OM, Mashela PW, Mphosi MS, Mafeo TP, Dube ZP. 2013. Using computer-based model to determine phytotoxicity concentration of Nemarioc-A phytonematicide in tomato production. Afr Crop Sci Conf Proc. 11:349–353.
- Perry RN. 1996. Chemoreception in plant parasitic nematodes. Annu Rev Phytopathol. 34:181–199. doi: 10.1146/annurev.phyto.34.1.181
- Salisbury FB, Ross C. 2005. Plant physiology. New York (NY): Willey; p. 392.
- SAS Institute. 2008. SAS/STAT® 9.2 Qualification tools user’s guide. Cary (NC): SAS Institute Inc.
- Seshweni MD. 2016. Integrated system for the management of population densities of Meloidogyne javanica in potato production [Master Dissertation]. Sovenga: University of Limpopo.
- Skantar AM, Agama K, Meyer SLF, Carta LK, Vinyard BT. 2005. Effects of Geldanamycin on hatching and juvenile motility in Caenorhabditis elegens and Heterodera gylcines. J Chem Ecol. 31:2481–2490. doi: 10.1007/s10886-005-7114-z
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in Caenorhabditis elegans. Cell. 83:207–218. doi: 10.1016/0092-8674(95)90162-0
- Wuyts N, Swennen R, De Waele D. 2006. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. J Nematol. 8:89–101. doi: 10.1163/156854106776179953