ABSTRACT
Old potato cultivars from the Nordic Genetic Resource Center (NordGen) and advanced breeding clones from the Swedish University of Agricultural Sciences (SLU) were evaluated for susceptibility to Potato Virus Y (PVY) and Potato Leafroll Virus (PLRV), foliar and tuber resistance to late blight – caused by Phytophthora infestans – as well as for glycoalkaloid content and crossing ability. Enzyme-Linked Immunosorbant Assay (ELISA) tests were used for PVY and PLRV screening during two years that were characterized by intensive virus incidence and severity in the field, while foliar and tuber resistances to late blight were assessed under artificial inoculation with an aggressive Swedish isolate of P. infestans. Hybrid seeds were obtained by crossing cultivars such as ‘Kiva’, ‘Sarpo Mira’, ‘Rosamunda’ and ‘Superb’ with SLU advanced breeding clones and a selected clone of Solanum tuberosum Group Andigena. Some cultivars (‘Hårek’, ‘Sarpo Mira’) and breeding clones (04-2662, 04-2085) with late blight resistance did not show virus infection. The α-solanin and α-chaconin contents of some of the old Nordic potato cultivars and breeding clones were similar to the known Dutch table cultivar ‘Bintje’ after 3 years of testing. This research allows identification of promising Nordic potato cultivars and SLU breeding clones for further use in developing germplasm aiming at organic and conventional farming systems.
Introduction
Diet quality depends on agro-biodiversity worldwide. Diverse Solanum germplasm used for breeding potato cultivars with host plant resistance to pathogens and pests contributes to increasing productivity for this crop, thereby closing yield gaps. Potato breeders include germplasm of diverse genetic background in their crossing plans. Newly bred cultivars replace those previously grown, particularly among small farmers. Genebanks preserve plant germplasm, which include released cultivars (under use or obsolete), and make them available to both breeders and researchers. Denmark, Finland, Iceland, Norway and Sweden in the Nordic region are working together to preserve their potato cultivars and selected breeding clones at NordGen, the Nordic Genetic Resource Center situated in Alnarp, Sweden. The long-term storage of potato germplasm at NordGen includes around 70 pathogen-tested accessions that are kept in vitro. Old Nordic potato cultivars have previously been characterized with amplified fragment length polymorphism (AFLP) and 57 morphological descriptors. Most of them were distinguished based on the morphological characters (Veteläinen et al. Citation2005). To benefit from germplasm collections, the evaluation data of material for several desirable traits including host plant resistance to pathogens and pest are required.
Late blight, caused by the oomycete Phytophthora infestans (Mont.) de Bary, is one of the most economically important potato diseases that can affect stems, leaves and tubers of the potato plant. P. infestans ability for sexual recombination leads to more pathogenic types (Goodwin et al. Citation1998). In Europe and Asia, a new population displaced the previously existing ones, which led to new challenges on late blight control (Fry Citation2008). Oospores produced via hybridization of A1 and A2 mating types are being the sources of strain variation. Large spectrum of variation within the European populations of P. infestans was reported (Lebecka et al. Citation2007; Lehtinen et al. Citation2008; Runno-Paurson et al. Citation2009; Zoteyeva & Patrikeeva Citation2011). In the last 10 years, significant changes have occurred in P. infestans populations in Europe as new genotypes of the pathogen have been identified. Among them the most notably is strain ‘Blue 13’ or 13_A2 (Cooke et al. Citation2012). The genotyping of P. infestans isolates sampled from different potato fields in Denmark (2011 and 2012) showed that the Danish P. infestans population is highly diverse, thereby indicating oospore-borne inoculum in Danish fields. The share of 13_A2 strain in local P. infestans populations is increasing (Bent et al. Citation2014). Potato Leafroll Virus (PLRV) is probably the most damaging and widespread virus of potato (Solomon-Blackburn & Barker Citation2001). Potato Virus Y (PVY) is another severe potato pathogen throughout the world because it causes significant tuber yield loss in susceptible but popular potato cultivars (Valkonen et al. Citation1996). PVY can reduce yield by 80% (Hane & Hamm Citation1999). The low rate of certified seeds planted by farmers (about 8%) leads to high PVY infections in widely grown cultivars (Zimnoch-Guzowska et al. Citation2013). The amount of certified seed potato has been estimated at approximately one third of the total volume used (Swedish Board of Agriculture Citation2006). The search for new sources of resistance to viral infection remains to be important because the range of virus resistant cultivars still is limited and the costs of producing potato tuber propagules are considerably high. In this regard, field screening of breeding populations for viral infection along with late blight resistance evaluation is a must for developing new potato cultivars.
Glycoalkaloid amount in tubers is an important threshold trait in potato breeding. These amounts vary due to both genetics and environments where potato grows, and their interaction (Maga Citation1994; Friedman & McDonald Citation1997; Friedman Citation2006). The main potato glycoalkaloids are α-solanine and α-chaconine. Tuber glycoalkaloid content may not be responsible for host plant resistance to late blight (Andrivon et al. Citation2003), though a significant correlation was noted between tuber late blight resistance and ɑ-chaconine content (Carlson-Nilsson et al. Citation2012). High glycoalkaloid amounts in potato tubers are evidently harmful for consumer’s health. The upper threshold for total glycoalkaloid content is 200 mg per 1 kg of potato, above this it may be toxic (Cantwell Citation1996).
Crossing ability within and among related species is crucial for potato breeding. Since 1930s, Solanum species have been widely used in potato breeding due to their host plant resistance to pathogens and pests, such as these causing late blight or PVY. The South American potato species S. tuberosum Gr. Andigena (2n = 4x = 48) is frequently used in breeding for crossing with S. tuberosum Gr. Tuberosum which is the preferred cultigen worldwide. Along with other potato species resistance to PVY has been also introgressed from S. tuberosum Gr. Andigenum (Ortiz Citation2001). This species is also known as a source of resistance to PLRV (Marczewski et al. Citation2001; Velásquez et al. Citation2007). Hence, the aim of this research was to screen potato germplasm from the Nordic region for resistance to late blight in leaves and tubers, viral infection, glycoalkaloid content and crossing ability.
Materials and methods
Plant material
This research includes Swedish, Norwegian and Finnish cultivars and landraces conserved at NordGen as well as a number of advanced breeding clones (SW-clones) originating from a former breeding program at Svalöf Weibull (Sweden). These breeding clones were introduced to research and breeding program conducted at Swedish University of Agricultural Sciences (SLU-Alnarp) in 2006. The pedigrees of SW-clones are given in of Supplement. In addition some more modern cultivars developed in other countries were included in this evaluation.
Table 1. Potato plant material evaluated (+) for resistance to Phytophthora infestans in leaflet (PLT) and tuber (PTT) tests, viral infection in ELISA tests and glycoalkaloid content (GA).
In total 42 accessions were evaluated in leaflet tests (40) and tuber tests (29) for resistance to P. infestans and ELISA tests (20) for detecting PVY and PLRV infections. Sixteen clones were evaluated for glycolkaloid (α-chaconine and α-solanine) contents (). Crossing ability was assessed after hybridization of 4 cultivars and 4 breeding clones that showed leaf or tuber late blight resistance and a tuber late blight resistant clone of S. tuberosum Gr. Andigena.
P. infestans inoculation tests
Detached leaflet assays were performed according to Eucablight protocols (http://www.eucablight.org) and were used for assessment of P. infestans infection rate. For the preparation of the inoculum, the P. infestans isolate SE03058 (virulence 1.3.4. 7.10.11., A1 mating type) obtained from Dr. Björn Andersson (Department of Forest Mycology and Pathology, SLU) was used in both leaflet and tuber tests. The isolate was maintained on rye B agar media (Caten & Jinks Citation1968). For inoculation, mycelia was flooded from Petri dishes with sterile water. Sporangial suspension was filtered through a 100 µm mesh and the concentration was adjusted to 24,000 sporangia/ml.
Suspension was incubated at 15°C in the dark for 2 h. Three leaflets of four tested plants of each genotype were detached, placed on nets in plastic boxes containing moistened filter paper and drop inoculated with P. infestans suspension. The incubation proceeded in a climate chamber at 17°C with a light regime: 16/8 h day/night, respectively. On the 7th day after inoculation the diseased leaf area was scored as the percentage of lesion area out of the whole leaflet area. Inoculation of tubers was performed one month after harvest. The tuber resistance assay was developed based on the method described by Zoteyeva and Zimnoch-Guzowska (Citation2004). The number of tubers of each clone varied from 7 to 10. Cut tuber surfaces of 7 mm diameter were inoculated with a droplet of inoculum comprising 24,000 sporangia/ml. The tubers of each clone were inoculated in two replications and incubated in the dark at 18°C. The mycelium growth was estimated 6 days after inoculation using an original scale from 0 to 3 (grade 0 representing lack of mycelia and grade 3 representing intensive growth). On the 14th day after inoculation tubers were longitudinally cut and the lesions’ area was scored using a 1–9 scale, where 9 is resistant.
In leaf and tuber inoculation tests evaluated clones were compared with the foliar and tuber late blight susceptibility of the widely grown Dutch cultivar ‘Bintje’. Leaflet and tuber resistance evaluations were done using two replications in laboratory tests during one year. Host plant resistance rating in both leaf and tuber tests led to rating clones as resistant (score grades ranging from 6.1 to 9), moderately resistant (5–6) and susceptible (1–4.9).
Viral infection assessment
ELISA readings led to characterization of breeding clones and cultivars according to their host plant resistance to both PVY and PLRV. ELISA tests were performed for two years and twice during the vegetative season; i.e. in the end of June and in the beginning of August. ELISA kits were acquired from BIOREBA AG (Switzerland). The absorbance values of healthy plants ranged from 0 to 0.001, and any values above 0.1 were regarded as positive for PVY and PLRV virus infection.
Glycoalkaloid (GA) content quantification
For GA analysis, five tubers from each genotype were selected, washed, dried and cut into small cubes and homogenized (including skin and cortex). Tubers were analyzed using high performance liquid chromatography (HPLC) for total GA content; i.e. ɑ-solanine and ɑ-chaconine. The tubers were rinsed in tap water. Each sample was finely diced and mixed, and 20 g was homogenized with an Ultra Turrax homogenizer TP 18/10 with shaft 18-N (Janke & Kunkel KG, IKA-Werk, D-7813, Staufen, Germany) for 2 min with 100 ml water: acetic acid: ascorbic acid, 100:5:1 (vol/vol/wt). The volume was adjusted to 200 ml with the same solvent, clarified by centrifugation at 4°C at 10,000 rpm (Sorvall Evolution RC) for 10 min and filtered through 1F (Munktells). Ten ml of the supernatant was put onto a Sep-Pak C18 cartridge previously activated by acetonitrile in accordance with the method of Hellenäs and Branzell (Citation1997), which was also used for the subsequent analytical procedure. ɑ-solanine and ɑ-chaconine (Sigma Chemical Co.) were used as standards for the HPLC determination. The concentrations were given in mg kg−1 fresh weight.
Crossing ability
The cultivars of S. tuberosum Gr. Andigena and S. tuberosum Gr. Tuberosum, and potato breeding clones were grown in the field for further use to determine their crossing ability. The crossing was made using detached branches with flower buds of female parents. Flowers buds were emasculated 1 or 2 days before pollination. The anthers from the flowers of the male parents were collected and then dried. Pollination was performed by contacting emasculated flowers (female) with the pollen (male) obtained. Cut branches were maintained in glass bottles with water until natural fruit size formation. Fruits were collected and kept in room temperature. Seed extraction was done after fruit maturation (as determined by berry softening) between November and December.
Statistical analyses
Statistical analyses of the data were performed using the Minitab Program, version 17.01. Differences in (percentage of infected area on the leaflets and lesion size on infected tubers) were analyzed with one - way ANOVA. Mean comparisons were performed using Tukey’s test.
Results
Resistance to Phytophthora infestans
Leaflet assay
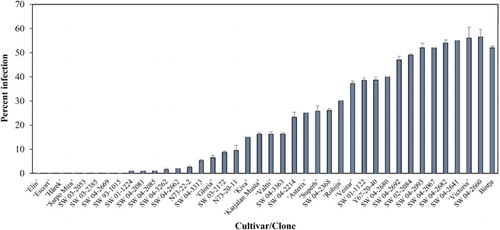
There were highly significant differences (P < 0.001) in host plant resistance according to this trial. The breeding clones SW03-2385, SW03-2053, SW93-1015 and SW04-2669, as well as the cultivars ‘Elin’, ‘Escort’, ‘Hårek’ and ‘Sarpo Mira’ did not show any late blight symptoms in the leaflet assay. Hence, they were rated as having the highest resistance; i.e. 0% of the infected leaflet area ().
There were other breeding clones: SW04-2081, SW04-2085, SW01-1224, SW04-2662, SW04-3262, N73-22-2, SW04-3313, SW03-2172, N73-20-11, as well as one cultivar ‘Gloria’ showing high partial resistance to P. infestans, as noted by their low percentage of infection (average ratings from 1% to 9.4% of the infected area).
The breeding clones SW04-3363, SW-2214 and SW04-2368 as well as the cultivars ‘Kiva’, ‘Valtti’, ‘Karjalan Musta’, ‘Robjin’, ‘Superb’ and ‘Asterix’ were rated as moderately resistant (infected leaflet areas from 15% to 30%), while the breeding clones Y-67-20-40, SW01-1122, SW02-2084, SW04-2680, SW04-2692, SW04-2640, and cultivar ‘Vestar’ were moderately susceptible (from 37.2% to 49%). The ratings for the susceptible breeding clones SW04-2067, SW04-2682, SW04-2641, SW04-2660, SW04-2093 and cultivar ‘Victoria’ exceeded 50% of the infected area. Statistical analyses of the data obtained in the leaflet tests showed significant differences (P = 0.0001) among the potato accessions regarding infected leaflet area rates.
Tuber assay
The host plant resistance to late blight among breeding clones and cultivars was significantly different (P < 0.001) regarding infection lesions (), necrotic reaction and intensity of the mycelium growth. The susceptible control ‘Bintje’ had a score of 3.2. Sixteen out of 29 clones tested were rated as partial or moderately resistant, eight as moderately susceptible and five as susceptible. The resistant breeding clones were SW04-2081, SW04-2662, SW04-2669, SW04-3262 and N73-20-11, which had same host plant resistance like the cultivar ‘Kiva’. Their average scores ranged from 7.6 to 8.8, without showing mycelia growth. Hypersensitive response (HR) was observed 5 days after inoculation on tubers of the breeding clone N73-20-11 and cultivar ‘Kiva’. HR was also noted on the cut tuber surface of cultivar ‘Matilda’ (7.2) and of breeding clone SW04-2085 (7.3) (). The breeding clones Y67-20-40, SW03-2053 and cultivars ‘Asterix’, ‘Elin’, ‘Escort’, ‘Hårek’, ‘Rosamunda’, ‘Sarpo Mira’ and ‘Vestar’ were rated as moderately resistant (6–6.9), while cultivars ‘Robjin’ (5.7), ‘Valtti’, ‘Victoria’ (5.3), ‘Karjalan Musta’ (5) and ‘Superb’ (5), plus breeding clones SW03-2385 (5.9) and N73-22-2 (5.3) were rated as moderately susceptible. No mycelium growth was observed on cut tuber surfaces of breeding clone SW03-2053 and of cultivar ‘Escort’ (data not shown). Only five out of 29 breeding clones and cultivars were rated as susceptible due to their low score that ranged from 3.6 to 4.7 ().
Table 2. Late blight screening using tuber assays (grade scale from 1 to 9, where 9 is resistant).
Virus host plant resistance
The absorbance values of the PVY susceptible cultivar ‘Magnum Bonum’ ranged from 2.19 to 2.37. The breeding clones SW04-2640, SW04-2662, SW04-3262, SW93-1015, SW04-2085 and SW04-2662, plus the cultivars ‘Hårek’, ‘Sarpo Mira’ and ‘Superb’ were found to be free of PVY infection. The breeding clones SW04-3262 and SW03-2053 had low PVY absorbance values; i.e. 0.12 and 0.15, respectively.
Four breeding clones (SW93-1015, SW04-2081, SW04-2084, SW04-3262) and three cultivars (‘Asterix’, ‘Matilda’, ‘Superb’) showed a positive reaction to PLRV in both years as per the ELISA tests. The highest PLRV absorbance value was noted in breeding clone SW93-1015 (1.43), which did not show any PVY infection. Low amount of PLRV infection was detected in plants of cultivars ‘Matilda’ (0.11) and ‘Asterix’ (0.15), although they did not show any visible symptoms.
Glycoalkaloid content
The breeding clones expressing leaf or tuber resistance to late blight were assessed for their contents of ɑ-solanine and ɑ-chaconine during three years. They show significant differences (P < 0.001) for glycoalkaloid (GA) content. The table cultivar ‘Bintje’ –showing 31.4 and 54.3 mg kg−1 f.w. of ɑ-solanine and ɑ-chaconine, respectively– was used as control. The average contents (mg kg−1 f.w.) of ɑ-solanine and ɑ-chaconine, of breeding clones SW01-1224 (26.8 and 55.4), SW03-2385 (45.2 and 51.2), SW04-2214 (35.3 and 59.5), SW04-2669 (21.5 and 56) and SW03-2385 (45.2 and 51.2), and of cultivar ‘Superb’ (20.9 and 46.5) were not significantly different than those of ‘Bintje’. The breeding clones SW04-2640 (30.1 and 74.6), SW04-2662 (36.0 and 85.4), SW04-2081(46.5 and 98.0), SW04-2085 (42.3 and 102,6) and SW04-3262 (43.8 and 83.8), plus that of cultivar ‘Asterix’ (45 and 111.5) had ɑ-solanine content similar to ‘Bintje’ and ɑ-chaconine content exceeding that found in ‘Bintje’. The ɑ-solanine content of ‘Matilda’ was slightly above that of other cultivars and ‘Bintje’ (53.3), while its ɑ-chaconine content was almost twice (102.4) than that noted in ‘Bintje’. High GA content was also found in breeding clone SW93-1015 (74 and 241.7 for ɑ-solanine and ɑ-chaconine, respectively) and in cultivar ‘Magnum Bonum’ (367 and 378.7).
Crossing ability
Reciprocal crossing was performed using four cultivars, four breeding clones as well as a tuber resistant clone of S. tuberosum Gr. Andigena. There were 14 (out of 28) matings that led to hybrid seed formation. A high seed set was noticed in the reciprocal cross between cultivar ‘Sarpo Mira’ and breeding clone SW04-3262 as determined by the relatively high number of seeds per fruit (). A slightly higher number of seeds were noted when using ‘Sarpo Mira’ as a female parent in this cross, while in the other matings the cultivar ‘Sarpo Mira’ was most efficient as a pollinator. Seeds were obtained after crossing ‘Sarpo Mira’ with four female parents (clones SW04-2081, SW04-2662, SW04-3262 and SW93-1015) and with two males (SW04-3262 and ‘Superb’). Despite the high seed number after using ‘Sarpo Mira’ as the pollinator, the average seed set was lower than those obtained when ‘Sarpo Mira’ was female parent ().
Table 3. Average seed set (seed fruit -1) after crossing potato cultivars and SLU breeding clones.
The cultivars ‘Superb’ and ‘Kiva’ were also successful pollinators. The seed set after using ‘Superb’ varied significantly; i.e. between 92 and 225 seed per fruit. Large seed sets were obtained by crossing ‘Superb’ with either breeding clone SW04-3262 or cultivar ‘Rosamunda’. Four matings had high seed set when cultivars ‘Kiva’ was used as pollinator. All reciprocal matings with ‘Superb’ as a female parent were unsuccessful. Breeding clone SW04-2662 had low pollen production and seeds were not obtained when it was used as female parent. The breeding clone SW93-1015 set seeds as female parent with cultivars ‘Kiva’ and ‘Sarpo Mira’. Seed set obtained in both crosses was relatively low: 71.3 and 88 seeds per fruit, respectively (). This breeding clone failed as a pollinator in matings with cultivars ‘Kiva’, ‘Superb’ and ‘Asterix’. The cultivars ‘Kiva’, ‘Asterix’, ‘Superb’ and breeding clones SW04-2081, SW04-2662, SW04-3262 and SW93-1015 did not set any seed after crossing them as females with the S. tuberosum Gr. Andigena accession, which had plentiful pollen production (data not shown). SW04-3262 was the only successful male parent. Crossing this clone with cultivars ‘Sarpo Mira’ and ‘Rosamunda’ led to a high seed set. Hybrid seeds were obtained after using SW04-3262 as male for crossing with other breeding clones such as SW04-2081, SW04-2662, and SW93-1015 used as female parents.
Discussion
Many of previously developed potato cultivars have been lost but some are still preserved in genebanks. Collections of plant genetic resources are available and can be distributed to breeders upon request. A very important genebank activity is to evaluate potato clones preserved for various traits and making this information publicly available. One of the main goals of the majority of potato breeding programs is to develop new market-quality cultivars with durable resistance to late blight. Economic losses caused by late blight result from both foliage and tuber susceptibility of grown cultivars. The number of late blight resistant cultivars used for potato production is still insufficient.
Potato cultivars with resistance to late blight reduce tuber yield loss and decrease the costs of fungicide applications (Glover et al. Citation2010). The search for host plant resistance sources and development of breeding clones highly resistant to late blight is sought elsewhere (Douches et al. Citation1998, Citation2001; Platt & Tai Citation1998; Corsini et al. Citation1999; Stewart et al. Citation2003; Kirk et al. Citation2005; Abou-Taleb et al. Citation2010; Tahtjarv et al. Citation2013; Haynes et al. Citation2014; Jo et al. Citation2014; Plich et al. Citation2015). In potato, foliar and tuber susceptibility to late blight does not often correlate. Both positive (Stewart et al. Citation1994) and negative (Douches et al. Citation2002) correlations on resistance to P. infestans in foliage and tubers have been reported. Differentially expressed genes, gene groups and ontology bins were identified to observe similarities and differences in foliage and tuber defense mechanisms against P. infestans. Results show that R gene dosage and shared biochemical pathways contribute to potato-P. infestans interactions in both foliage and tubers (Gao & Bradeen Citation2016). For many decades, the potato breeders focused on foliar resistance. In the first part of the last century many foliar resistant cultivars were bred using S. demissum Lindl. Major R-genes from S. demissum failed, however, to provide durable resistance due to fast adaptation of the pathogen overcoming such type of resistance (Bradshaw et al. Citation1995). Research shows that material from northern Europe (e.g. ‘Elin’, ‘Hårek’, ‘Kiva’ and ‘Valtti’) adapted to the climatic conditions favorable for P. infestans (i.e. high humidity, difference of night/day temperatures), were foliar resistant (). Nonetheless, foliar resistance could not fully protect tubers against P. infestans due to infection accumulation in seed tubers (Platt et al. Citation1999). Even slightly blighted foliage during the growing season leads to a high proportion of infected tubers (Schwinn & Margot Citation1991). Study on interaction between the tubers of the host-plants and the strains of the pathogen provide strong indication that the stability of tuber resistance is a must (Flier et al. Citation2001). Hence, the search for new genetic sources of resistance not only in leaves but also in tubers is important (Kirk et al. Citation2001). Our study found tuber resistance in both breeding clones and cultivars (). A few of them reacted to inoculation with a necrotic reaction (HR). The nature of the observed resistance response suggests the presence of R genes or quantitative trait loci providing resistance to tuber blight.
Highly resistant clones are often offspring of wild germplasm and may consequently possess relatively high glycoalkaloid content. ‘Backcrossing’ to the cultigen may be used to breed S. tuberosum clones with low glycoalkaloid content. This time-consuming approach may, however, lead to decrease of the host plant resistance levels to late blight. Our research led to identifying about one-third of breeding clones and cultivars whose GA content was similar to that found in tubers of table cultivar ‘Bintje’.
Success of potato breeding is highly dependent on the crossing ability of the parental clones involved in hybridization. The cultigen S. tuberosum is a tetrasomic polyploid (2n = 4x = 48 chromosomes) with an endosperm balance number (EBN) of 4 (Johnston et al. Citation1980). However some crossing barriers are observed even when both parents ‘belong’ to S. tuberosum. The explanation might be that many breeding clones and cultivars are not ‘true’ S. tuberosum and have in their background wild or cultivated species germplasm (The European Cultivated Potato Database, https://www.europotato.org). Likewise, tetraploid 2 EBN wild species S. stoloniferum has been incorporated to S. tuberosum germplasm due to its resistance to PVY. However it also caused male sterility in cultivars like German ‘Assia Petra’ and ‘Ute’ (Song & Schwarzfischer Citation2008), which limits their further use as male parents. The results of crosses performed in our study showed that crossing success was highly dependent on if the different clones were used as male or female parents. In our research some cultivars and breeding clones were able to cross with a tuber late blight resistant clone of S. tuberosum Gr. Andigena (adg) (). We noted success and failures after crossing adg × ‘Kiva’, adg × ‘Superb’, ‘Sarpo Mira’ × SW04-3262, and ‘Superb’ × SW04-2662. Our results confirm the previous finding by Hermsen and Sawicka (Citation1979), who noted differences in the crossing ability among different plants within the same accessions.
Nowadays, potato breeding requires diverse sources for several useful traits. Breeders search for plants combining resistance to two or more pathogens. We were able to identify within the potato germplasm preserved at NordGen and material used by the SLU breeding program, various Nordic cultivars and breeding clones combining leaf and tuber resistance to late blight, lack of viral infection and glycoalkaloid content at the same level as the table cultivar ‘Bintje’. Our study also led to the development of several promising hybrids after crossing cultivars and breeding clones bearing desired traits. This material was incorporated into the SLU potato breeding program. Since 2011 when tubers obtained from the hybrid seedlings were planted in the experimental field for the first time, the hybrid populations have been evaluated yearly for late blight. The selection of the best clones is still in process. Evaluation results of field late blight resistance of some SW–derived hybrid progenies were recently reported (Carlson-Nilsson & Zoteyeva Citation2014).
Acknowledgements
We thank Ingegerd Nilsson (SLU) for helping with the ELISA tests, Firuz Odilbekov (SLU) for assisting with statistical analyses, and Lena Ansebo (NordGen) for verifying names of old potato varieties and landraces used in the evaluation.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Nadezhda Zoteyeva http://orcid.org/0000-0003-2266-0467
Ulrika Carlson-Nilsson http://orcid.org/0000-0002-6467-2215
Therese Bengtsson http://orcid.org/0000-0003-4784-1723
Rodomiro Ortiz http://orcid.org/0000-0002-1739-7206
Additional information
Funding
Notes
* Supplemental data for this article can be accessed doi:10.1080/09064710.2017.1324042
References
- Abou-Taleb EM, Aboshosha SM, El-Sherif EM, El-Komy MH. 2010. Genetic diversity among late blight resistant and susceptible potato genotypes. Saudi J Biol Sci. 17:133–138. doi: 10.1016/j.sjbs.2010.02.006
- Andrivon D, Corbiere R, Lucas JM, Pasco C, Gravoueille JM, Pelle R, Dantec JP, Ellisseche D. 2003. Resistance to late blight and soft rot in six potato progenies and glycoalkaloid contents in the tubers. Am J Pot Res. 80:125–134. doi: 10.1007/BF02870211
- Bent JN, Cooke DEL, Hansen JG. 2014. Monitoring the Danish populations of potato late blight pathogen, Phytophthora infestans in 2011–2012 and occurrence of 13_A2. In: Shepers HTAM, editor. Proceeding of the Fourteen EuroBlight workshop; 2013 May 12–15; Limassol (CY).
- Bradshaw JE, Wastie RL, Stewart HE, Mackay GR. 1995. Breeding for resistance to late blight in Scotland. In: Dowley LJ, Bannon E, Cooke LR, Keane T, O’Sullivan E, editors. Phytophthora infestans 150, European Association for Potato Research (EAPR) Pathology Section Conference. Boole Press and Teagasc (IE). pp. 246–254.
- Cantwell M. 1996. A review of important facts about potato glycoalkaloids. Perishables Handl Newsl. 87:26–27.
- Carlson-Nilsson BU, Zoteyeva NM. 2014. Development of late blight resistant breeding material. Poster session presented at: 19th Triennial EAPR Conference; Brussels (BE).
- Carlson-Nilsson U, Zoteyeva N, Reslow F. 2012. Glycoalkaloid content in potato tubers with different levels of resistance to Phytophthora infestans. In: Schepers HTAM, editor. Proceedings of the Thirteenth EuroBlight Workshop; 2011 Oct 9–12; St. Petersburg (RU).
- Caten CE, Jinks JL. 1968. Spontaneous variability of single isolates of Phytophthora infestans. I. Cultural variation. Can J Bot. 46:329–348. doi: 10.1139/b68-055
- Cooke DEL, Cano LM, Raffaele S, Bain RA, Cooke LR, Etherington GJ, Deahl KL, Farrer RA, Gilroy EM, Goss EM, et al. 2012. Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLOS Pathogens. 8:e1002940. doi: 10.1371/journal.ppat.1002940
- Corsini D, Pavek J, Brown C, Inglis D, Martin M, Powelson M, Dorrance A, Lozoya Saldana H. 1999. Late blight resistant potato germplasm release AWN86514-2. Am J Pot Res. 76:45–49. doi: 10.1007/BF02853557
- Douches D, Jastrzebski K, Coombs J, Kirk W, Felcher K, Hammerschmidt R, Chase R. 2001. Jacqueline Lee: A late-blight-resistant tablestock variety. Am J Pot Res. 78:413–419. doi: 10.1007/BF02896372
- Douches DS, Jastrzebski K, Long C, Walters K, Coombs J, Bertram A, Bisognin D. 1998. Michigan State University potato breeding program 1998 status report. In: 1998 Michigan potato research report 130. Lansing, MI (US): The Michigan Potato Industry Commission.
- Douches DS, Kirk WW, Bertram MA, Coombs JJ, Niemira BA. 2002 Foliar and tuber assessment of late blight (Phytophthora infestans (Mont.) de Bary) reaction in cultivated potato Solanum tuberosum L.). Potato Res. 45:215–224. doi: 10.1007/BF02736116
- Flier WG, Turkensteen LJ, van den Bosch GBM, Vereijken PFG, Mulder A. 2001. Differential interaction of Phytophthora infestans on tubers of potato cultivars with different levels of blight resistance. Plant Pathol. 50:292–301. doi: 10.1046/j.1365-3059.2001.00574.x
- Friedman M. 2006. Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J Agric Food Chem. 54:8655–8681. doi: 10.1021/jf061471t
- Friedman M, McDonald GM. 1997. Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Crit Rev Plant Sci. 16:55–132. doi: 10.1080/07352689709701946
- Fry W. 2008. Phytophthora infestans: the plant (and R gene) destroyer. Mol Plant Pathol. 9:385–402. doi: 10.1111/j.1364-3703.2007.00465.x
- Gao L, Bradeen JM. 2016. Contrasting potato foliage and tuber defense mechanisms against the late blight Pathogen Phytophthora infestans. Plos One. 11:e0159969. doi: 10.1371/journal.pone.0159969
- Glover B, Syrovy L, Prasad R. 2010. Late blight control alternatives: resistant varieties and organic fungicides. Final report. Organic Sector Development Program Lower Mainland Horticultural Improvement Association Fraserland Organics (CA).
- Goodwin SB, Smart CD, Sandrock RW, Deahl KL, Punja ZK, Fry WE. 1998. Genetic change within populations of Phytophthora infestans in the United States and Canada during 1994 to 1996: role of migration and recombination. Phytopathol. 88:939–949. doi: 10.1094/PHYTO.1998.88.9.939
- Hane DC, Hamm PB. 1999. Effects of seedborne potato virus Y infection in two potato cultivars expressing mild disease symptoms. Plant Dis. 83:43–45. doi: 10.1094/PDIS.1999.83.1.43
- Haynes KG, Qu X, Christ BJ. 2014. Two cycles of recurrent maternal half-sib selection reduce foliar late blight in a diploid hybrid Solanum phureja-S. stenotomum population by two-thirds. Am J Pot Res. 91:254–259. doi: 10.1007/s12230-013-9345-9
- Hellenäs K-E, Branzell C. 1997. Liquid chromatographic determination of the glycoalkaloids alpha-solanine and alpha-chaconine in potato tubers: NMKL interlaboratory study. Nordic committee on food analysis. J AOAC Int. 80:549–554.
- Hermsen JG, Sawicka E. 1979. Incompatibility and incongruity in tuber-bearing Solanum species. In: Jellis JC, Richardson DE, editors. The production of new potato varieties. Technological advances. Cambridge (UK): Cambridge University Press; p. 172–185.
- Jo K-R, Kim C-J, Kim S-J, Kim T-Y, Bergervoet M, Maarten JA, Visser RGF, Jacobsen E, Vossen JH. 2014. Development of late blight resistant potatoes by cisgene stacking. BMC Biotechnol. 14:50. doi: 10.1186/1472-6750-14-50
- Johnston SA., den Nijs TPM, Peloquin SJ, Hanneman Jr RE. 1980. The significance of genic balance to endosperm development in interspecific crosses. Theor Appl Genet. 57:5–9. doi: 10.1007/BF00276002
- Kirk W, Felcher KJ, Duchs D, Niemira B, Hammerschmidt R. 2001. Susceptibility of potato (Solanum tuberosum L.) foliage and tuber to the US8 genotype of Phytophthora infestans. Am J Pot Res. 78:319–322. doi: 10.1007/BF02875697
- Kirk WW, Abu-El Samen FM, Muhinyuza JB, Hammerschmidt R, Douches DS, Thill CA, Groza H, Thompson AL. 2005. Evaluation of potato late blight management utilizing host plant resistance and reduced rates and frequencies of fungicide applications. Crop Prot. 24:961–970. doi: 10.1016/j.cropro.2004.12.016
- Lebecka R, Sliwka J, Sobkowiak S, Zimnoch-Guzowska E, 2007. Phytophthora infestans population in Poland. In: Shepers HTAM, editor. Proceedings of the Tenth Workshop of an European Network for Development of an Integrated Control Strategy of Potato Late Blight; 2007 May 2-5; Bologna (IT).
- Lehtinen A, Hannukkala A, Andersson B, Hermansen A, Le VH, Nærstad R, Brurberg MB, Nielsen BJ, Hansen JG, Yuen J. 2008. Phenotypic variation in Nordic populations of Phytophthora infestans in 2003. Plant Pathol. 57:227–234. doi: 10.1111/j.1365-3059.2007.01739.x
- Maga AJ. 1994. Glycoalkaloids in Solanaceae. Food Rev Int. 10:385–418. doi: 10.1080/87559129409541010
- Marczewski W, Flis B, Syller J, Schäfer-Preg R, Gebhardt C. 2001. A major quantitative trait locus for resistance to Potato leafroll virus is located in a resistance hotspot on potato chromosome XI and is tightly linked to N-gene-like markers. Mol Plant Microbe Interact. 14:1420–1425. doi: 10.1094/MPMI.2001.14.12.1420
- Ortiz R. 2001. The state of the use of potato genetic diversity. In: Cooper HD, Spillane C, Hodgkin T, editors. Broadening the genetic base of crop production. Wallingford, Oxfordshire (UK): CABI; p. 181–200.
- Platt HW, Peters RD, Mendina M, Arsenault W. 1999. Impact of seed potatoes infected with Phytophthora infestans (US-1 or US-8 genotypes) on crop growth and disease risk. Am J Pot Res. 76:67–73. doi: 10.1007/BF02855202
- Platt HW, Tai G. 1998. Relationship between resistance to late blight in potato foliage and tubers of cultivars and breeding selections with different resistance levels. Am J Pot Res. 75:173–178. doi: 10.1007/BF02853569
- Plich J, Tatarowska B, Lebecka R, Śliwka J, Zimnoch-Guzowska E, Flis B. 2015. R2-like gene contributes to resistance to Phytophthora infestans in Polish potato cultivar Bzura. Am J Pot Res. 92:350–358. doi: 10.1007/s12230-015-9437-9
- Runno-Paurson E, Fry WE, Myers KL, Koppel M, Mand M. 2009. Characterization of Phytophthora infestans isolates collected from potato in Estonia during 2002–2003. Eur J Plant Pathol. 124:565–575. doi: 10.1007/s10658-009-9442-2
- Schwinn FJ, Margot P. 1991. Control with chemicals. In: Ingram DS, Williams PH, editors. Advances in plant pathology. Phytophtora infestans, the cause of potato late blight, Vol. 7. London: Academic Press Limited, San Diego, CA (US); p. 225–265.
- Solomon-Blackburn RM, Barker H. 2001. Breeding virus resistant potatoes (Solanum tuberosum): a review of traditional and molecular approaches. Heredity. 86:17–35. doi: 10.1046/j.1365-2540.2001.00799.x
- Song YS, Schwarzfischer A. 2008. Development of STS markers for selection of extreme resistance (Rysto) to PVY and maternal pedigree analysis of extremely resistant cultivars. Am J Pot Res. 85:159–170. doi: 10.1007/s12230-008-9012-8
- Stewart HE, Bradshaw JE, Pande B. 2003. The effect of the presence of R-genes for resistance to late blight (Phytophthora infestans) of potato (Solanum tuberosum) on the underlying level of field resistance. Plant Pathol. 52:193–198. doi: 10.1046/j.1365-3059.2003.00811.x
- Stewart HE, Bradshaw JE, Wastie RL. 1994. Correlation between resistance to late blight in foliage and tubers in potato clones from parents of contrasting resistance. Potato Res. 37:429–434. doi: 10.1007/BF02358357
- Swedish Board of Agriculture. 2006. Utredning om utsädespotatis, Rapport.
- Tahtjarv T, Tsahkna A, Tamm S. 2013. Comparison of late blight resistance and yield of potato varieties. Proc Latvian Acad Sci., Section B. 67:254–258.
- Valkonen JPT, Jones RAC, Slack SA, Watanabe KN. 1996. Resistance specificities to viruses in potato: standardization of nomenclature. Plant Breed. 115:433–438. doi: 10.1111/j.1439-0523.1996.tb00952.x
- Velásquez AC, Mihovilovich E, Bonierbale M. 2007. Genetic characterization and mapping of major gene resistance to potato leafroll virus in Solanum tuberosum ssp. andigena. Theor Appl Genet. 114:1051–1058. doi: 10.1007/s00122-006-0498-5
- Veteläinen M, Gammelga E, Valkonen JPT. 2005. Diversity of Nordic landrace potatoes (Solanum tuberosum L.) revealed by AFLPs and morphological characters. Genet Res Crop Evol. 52:999–1010. doi: 10.1007/s10722-003-6129-y
- Zimnoch-Guzowska E, Yin Z, Chrzanowska M, Flis B. 2013. Sources and effectiveness of potato PVY resistance in IHAR’s breeding research. Am J Pot Res. 90:21–27. doi: 10.1007/s12230-012-9289-5
- Zoteyeva NM, Patrikeeva MV. 2011. Phenotypic characteristics of North-West Russian populations of Phytophthora infestans (2003–2008). In: Shepers HTAM, editor. Proceeding of the Twelfth EuroBlight workshop; 2010 May 3–6; Arras (FR).
- Zoteyeva NM, Zimnoch–Guzowska EM. 2004. Nowy metod ocenki ustojchyvosty klubnej kartofelia k fitoftorozu [New method of evaluation for resistance to Phytophthora infestans in potato]. Micologia i fitopathologia. 38:89–93. Russian.