ABSTRACT
Ambient UV radiation is recognised as an important environmental factor in the regulation of plant growth and development, and selenium (Se) as a beneficial nutrient that can increase plant tolerance to different environmental constraints. The effects on hybrid buckwheat plants of full (+UV) and reduced (−UV) ambient UV radiation without (−Se) and with (+Se) foliar Se treatment (10 mg L−1 sodium selenate) provided the four conditions of Se−UV–, Se–+UV, +Se−UV and +Se+UV. Plant morphological, biochemical and physiological properties were examined, along with leaf elemental composition and content, efficiency of Se enrichment, and production parameters. Leaf anatomical parameters under all conditions were mainly affected by UV radiation, and less so by Se. +Se+UV plants showed a trade-off between primary and secondary metabolism, which resulted in high levels of protective substances (e.g. anthocyanins, UV absorbing compounds), and low levels of photosynthetic pigments. All +UV plants were significantly shorter in comparison to those under the reduced −UV, while biomass production was highest for −Se+UV plants and lowest for +Se+UV plants. +Se plants accumulated ∼20-fold greater Se content compared to −Se plants, and full ambient UV radiation partly reduced this effect. +UV plants showed increased phosphorus content in leaves, independent of Se status, while +Se plants showed increased K content. Si content was increased by Se treatment and decreased by the full UV exposure. Se treatment and the ambient UV radiation, separately have positive effects on growth and production of this hybrid buckwheat, while the combination of the Se treatment and ambient UV resulted in lower yields. However, under these conditions (+Se+UV), the buckwheat plants established good protection against the different environmental constraints that are becoming more and more frequent due to changes to our climate.
Introduction
The planet Earth is facing rapid changes in the environmental conditions for plant growth, which include changes in the quality of the solar radiation that reaches the Earth surface, and changes in regional climates and soil quality (The United Nations Environment Programme Citation2015). The growth environment, and especially the light conditions, can also be altered for plants if they are grown in chambers under artificial light or in greenhouses, and also with plant protection against hail and herbivores when growing in the field. All of these changes demand well-planned management of crop production. Only detailed knowledge of the responses of important crops to different environmental factors will assure successful crop production in the future.
Although a number of studies have shown that elevated UV radiation can cause stress to plants (Rozema et al. Citation2002; Kataria et al. Citation2014), the ambient UV radiation is also an evolutionarily important environmental factor in the regulation of plant growth and development (Jansen et al. Citation2012; Björn Citation2015). Therefore, the exclusion or reduction of UV radiation during plant growth and development can significantly affect plant traits (Golob et al. Citation2017).
Indeed, Wargent and Jordan (Citation2013) reported that many desirable crop traits are regulated by the presence of UV radiation. Plants have evolved a variety of repair and protection mechanisms, among which the biosynthesis and accumulation of UV-absorbing compounds is one of the most consistent (Rozema et al. Citation2002). These are mostly phenolic compounds (flavonoids, hydroxicynnamic acids, anthocyanins, etc) and are involved in the protection of plants against the stress induced by UV-B radiation, as well as other environmental stresses, such as herbivores and pathogens (Wargent and Jordan Citation2013; Ballaré Citation2014).
In addition, these UV-absorbing compounds have relevance for food nutrition, because of their high antioxidative potential (Germ and Gaberščik Citation2016). These mainly accumulate in the epidermal cells of leaves, and along with differences in leaf structure, they affect the penetration of UV-B into leaf tissue, which further affects UV-induced morphogenetic changes to leaf tissue (Wargent and Jordan Citation2013; Barnes et al. Citation2015).
UV radiation also affects the different mineral element contents of plants, as has been shown in studies on soybean (Peng and Zhou Citation2010; Shen et al. Citation2014).
The selenium (Se) levels in plants are directly related to the phyto-availability of Se in the soil, which depends on the soil properties, including pH, salinity, and calcium carbonate content (Kabata Pendidas Citation2001). A balanced elemental composition of soil is crucial for crop production. However, many soils can become Se deficient or contain excess Se (Foster and Sumar Citation1995). Se is considered to be a beneficial nutrient for plants, as it can increase the tolerance of plants to environmental stress and provide resistance against pathogens and herbivores (White Citation2016). The treatment of plants with low concentrations of Se also improves their tolerance to drought (Tadina et al. Citation2007; Hajiboland et al. Citation2014; Hajiboland et al. Citation2015; Nawaz et al. Citation2015), high and low temperatures (Djanaguiraman et al. Citation2010; Abbas Citation2012), and heavy-metal toxicity (Cartes et al. Citation2010; Hawrylak-Nowak et al. Citation2014). Furthermore, Se can stimulate the growth of plants exposed to UV-B radiation, due to mitigation of oxidative stress (Hartikainen et al. Citation2000; Xue et al. Citation2001; Seppänen et al. Citation2003; Germ et al. Citation2005). Although numerous studies have suggested such protective roles of Se in plants exposed to stress, data on the physiological role of Se under stress conditions are scarce. Yao et al. (Citation2010) observed that in wheat seedlings exposed to UV-B radiation, Se application increased the content of anthocyanins, flavonoids and phenolic compounds, increased the activities of peroxidase and superoxide dismutase, and reduced superoxide radical production and malondaldehyde content.
Buckwheat is attracting greater research interest because of its suitability for ecological cultivation and its health benefits for consumers. Buckwheat has strong ecological adaptability, and therefore it can grow successfully in environments with relatively unfavourable conditions (Li and Zhang Citation2001; Kreft et al. Citation2002). It can also thrive at high altitudes with elevated UV-B radiation levels (Bonafaccia et al. Citation2003), and it has high potential for synthesis of UV-absorbing substances (Kreft et al. Citation2002; Fabjan et al. Citation2003). The most commonly used types of buckwheat around the world are common and Tartary buckwheat (Bonafaccia et al. Citation2003). Hybrid buckwheat is a new buckwheat taxon that was recently obtained by the interspecific crossing of Fagopyrum tataricum (4x = 32) × Fagopyrum giganteum (Fesenko and Fesenko Citation2010), although little is known about its properties (Golob et al. Citation2015).
The present study was designed to analyse the combined effects of Se foliar treatment and full and reduced ambient UV radiation on selected chemical parameters and production parameters in hybrid buckwheat. We hypothesised that UV radiation affects the biochemical properties and elemental composition and content of buckwheat leaves, and that these effects are altered in plants enriched with Se. We also hypothesised that Se treatment and ambient UV radiation will compromise the investment of plants in primary metabolism, due to the need for the plants to provide protection against possible negative effects of such treatments and to assure undisturbed plant function.
Materials and methods
Experimental design
Hybrid buckwheat was grown outdoors in 16 plastic pots (each, 0.8 m × 0.36 m × 0.3 m; as four pots per treatment), in an experimental field in Ljubljana (geographic coordinates: Lat: 46,051,023°, Lon: 14,470,608°, altitude 297 m). Sixty buckwheat plants were grown (from 5/6/2014 to 9/8/2014) in each pot, under two types of panels. The first panels were transparent to UV and visible radiation, thus transmitting wavelengths from 290 nm and above (+UV), and the second panels were transparent to only the visible region of the spectrum, with only low levels (about 15%) of the UV transmission (−UV), with transmission of wavelengths >380 nm.
At the beginning of flowering, half of the experimental plants under each UV exposure had the foliage treated with a solution of 10 mg L−1 sodium selenate (total about 30 µg Se per plant) (+Se), with the other half of the plants treated with only water (−Se). This resulted in the four different plant growth conditions of −Se−UV, −Se+UV, +Se−UV and +Se+UV. Two weeks after the Se treatments, five plants from each pot (subsamples) out of the four pots for each treatment were used for morphological, anatomical, biochemical and physiological analyses. At the end of the experiment, the plants were harvested and the biomasses of their roots, leaves, stems and grain were determined. These plant parts were air dried and lyophilised (Christ Alpha freeze dryer), then homogenised in an agate planar micromill, and used for analysis of the elemental contents.
Morphological and anatomical parameters
The anatomical analysis of the leaves was performed on transverse sections of each leaf. The thicknesses of the leaf, the upper and lower cuticle and epidermis, and the mesophyll, were determined at 100× magnification, using a digital camera (XC30; Olympus, Japan) and the CellSens software (Olympus, Japan). The density and length of the leaf stomata and prickle hairs on the upper and lower leaf surfaces were also measured. The specific leaf area was determined as the leaf area per dry mass.
Biochemical analysis
The contents of photosynthetic pigments were determined according to Lichtentaler and Buschman (Citation2001a, Citation2001b). The absorbances of samples were measured using a UV/Vis spectrometer. The chlorophyll contents (Chl a, b) and carotenoid contents are expressed per unit area (mg dm−2). The anthocyanin contents were determined according to Drumm and Mohr (Citation1978), and are expressed in relative units per leaf area. The contents of UV-A and UV-B absorbing compounds were measured according to Caldwell (Citation1968), and are expressed as relative units per leaf area.
Physiological parameters
Chlorophyll fluorescence was measured in situ in five plants from each pot, using a portable fluorometer (PAM 2500 Portable Chlorophyll Fluorometer; Heinz Walz GmbH, Germany). The potential photochemical efficiency of photosystem (PS)II was evaluated as the ratio of the variable fluorescence to the maximal fluorescence (Fv/Fm). After 15 min in the dark (provided by dark-adaptation clips), measurements of the minimal (F0) and maximal Fm chlorophyll fluorescences were taken. The fluorescence was excited using a saturating beam of ‘white light’ (photosynthetic photon flux density, 8000 µmol m−2 s−1; 0.8 s). Fv was calculated as the difference between Fm and F0. The effective photochemical efficiency was determined after saturation with a pulse of white light, with the calculation of , where
is the maximum fluorescence signal of an illuminated leaf after the pulse of saturating light, and F is the steady-state fluorescence (Schreiber et al. Citation1996).
The transpiration rate was measured using a steady-state leaf porometer (Decagon Devices, Inc. Pullman, WA, USA), which measured the rate of water vapour diffusion via the leaf surfaces. The respiratory potential of the mitochondria was determined in the plants via the terminal electron transport system (ETS) activity, as described by Packard (Citation1971) and modified by Kenner and Ahmed (Citation1975). Fresh leaves from the plants were homogenised in 4 mL ice-cold homogenisation buffer, followed by ultrasonic homogenisation (4710; Cole-Parmer, Vernon Hills, IL, USA) for 20 s at 40 W. The homogenates were centrifuged at 8496 × g for 6 min at 0°C (2–16 PK; Sigma, Germany). Triplicate 0.5 mL supernatant samples were added to a mixture of 1.5 mL substrate solution containing NADH, NADPH, Triton X-100, and 0.5 mL 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyl tetrazolium chloride (INT) reagent solution, and incubated for 40 min at 20°C. Formazan production was determined spectrophotometrically (Lambda 12; Perkin-Elmer, Norwalk, CT, USA) from the absorbance of the samples at 490 nm (against a blank), within 10 min of stopping the reaction with a mixture of formaldehyde and phosphoric acid (1:1; v/v). The respiratory potential of the mitochondria was determined in the plants via the terminal ETS activity, as described by Packard (Citation1971) and modified by Kenner and Ahmed (Citation1975).
Selenium content
The plants were analysed for Se content of the roots, stems, leaves and grain. The plant samples were collected at the mature stage, and the roots were washed with water and cut into pieces. All of the samples were freeze dried (Alpha 1-4; Osterode am Herz, Germany), weighed, and milled (Pulverisette 7; Fritsch, Idar-Oberstein, Germany). The total Se content was determined using hydride generation atomic fluorescence spectrometry. Details of the method of digestion of the plant material and the optimal measurement conditions were described by Smrkolj and Stibilj (Citation2004). The accuracy of the method was validated with the use of the certified reference material ‘Spinach Leaves’ (NIST 1570a).
Contents of other elements
The contents of the other elements, namely Si, Ca, P, K and S, in the leaves were determined using energy dispersive X-ray fluorescence spectrometry. The lyophilised leaves were pulverised and homogenised in an agate planar micromill. From 200 to 400 mg of powdered material was pressed into pellets using a pellet die and hydraulic press. 55Fe was used as the primary excitation source for the analysis. The fluorescence radiation emitted was collected using a Si drift diode (Amptek, USA). The energy resolution of the spectrometer at count rates below 1000 counts s−1 was 145 eV at 5.9 keV. The X-ray fluorescence analysis was performed under vacuum, and the samples were irradiated for 2000 s, to obtained spectra with sufficient statistics (Nečemer et al. Citation2008). The analysis of the X-ray spectra was performed using an iterative least squares programme (Vekemans et al. Citation1994), as included in the quantitative X-ray analysis system software package (Vekemans et al. Citation1994). Element quantification from the measured spectra was performed using the quantitative analysis of environmental samples based on fundamental parameters (Kump et al. Citation2011). Quality assurance for the element analyses was performed using standard reference materials: NIST SRM 1573a (tomato leaves as a homogenised powder), and GeoPT24, certified through an international proficiency test of the International Association of Geoanalysts, also analysed in the form of pressed pellets.
Statistical analysis
The normal distribution of the data was tested using Shapiro-Wilk tests. Differences between the conditions were tested using one-way analysis of variance followed by Tukey or Tamhane post-hoc tests. To test for statistically significant effects of the factors (i.e. Se, UV) and their interaction (i.e. Se×UV), factorial ANOVA was used. The level of significance was accepted at p < 0.05. The SPSS Statistics software, version 20.0 (IBM) was used for these calculations.
Results
Buckwheat morphological, biochemical and functional responses to treatments
The ambient UV radiation and Se treatment affected the morphological parameters of the buckwheat leaves in different ways. Testing the data with factorial ANOVA showed that the leaves from plants exposed to the full UV radiation (+UV) were thinner and had thinner palisade mesophyll and adaxial cuticle, with narrower stomata on both epidermis, and more dense trichomes on the adaxial epidermis, as compared to the leaves from the plants grown under reduced UV radiation (−UV). The Se addition (+Se) significantly increased the thickness of the palisade mesophyll, the length of the trichomes on both leaf surfaces, and the length of the stomata on the abaxial leaf surface of the treated plants. Se treatment of the plants grown under reduced UV radiation (−UV) increased the specific leaf area, the stomata density on the adaxial leaf surface, and the width of the stomata on the abaxial surface, while in plants exposed to ambient UV radiation (+UV), Se addition decreased these parameters. Leaves from the −Se−UV plants had significantly more stomata per area on both leaf surfaces, although the stomata were shorter than those for leaves from the +Se−UV plants ().
Table 1. Anatomical parameters of the buckwheat leaves under the four treatments, and impact on the measured parameters of Se and UV, and their interaction (Se×UV).
The reduction of UV radiation (−UV) resulted in statistically significant decrease in the contents of anthocyanins and UV-absorbing compounds per leaf area, while the Se treatment increased the production of these compounds. The highest contents of anthocyanins, UV-A absorbing compounds, and UV-B absorbing compounds were in the leaves from the +Se+UV plants, and the lowest were in the leaves from the Se−UV– plants ().
Table 2. Biochemical and physiological parameters of the buckwheat leaves under the four treatments, and impact on the measured parameters of Se and UV, and their interaction (Se×UV).
The influence of the Se treatment on the photosynthetic pigments was not clear. According to the factorial ANOVA, for plants grown under reduced UV radiation (−UV), Se treatment significantly increased chlorophyll a and b contents, while in plants grown under ambient UV radiation (+UV), Se treatment decreased the chlorophyll a and b contents. For the +UV plants, there was decreased content of chlorophylls and carotenoids in the leaves. For the leaves here, those from the +Se−UV plants had the highest chlorophyll a and b contents per leaf area, those from the −Se−UV pants had higher chlorophyll a and carotenoid contents per leaf area than those from the +Se+UV plants, and those from the +Se−UV plants had significantly higher carotenoid contents than the +Se+UV plants ().
Factorial ANOVA showed that in these buckwheat leaves, Se treatment (+Se) increased transpiration rate and decreased the potential photochemical efficiency of PSII. Ambient UV radiation (+UV) decreased the potential photochemical efficiency of PSII and increased the ETS activity. Addition of Se (+Se) increased the ETS activity in the plants grown under reduced UV radiation (−UV), and decreased the ETS activity in the plants grown under ambient UV radiation (+UV). Testing the data with the factorial ANOVA showed that in buckwheat leaves, Se treatment (+Se) and ambient UV radiation (+UV) decreased the water content ().
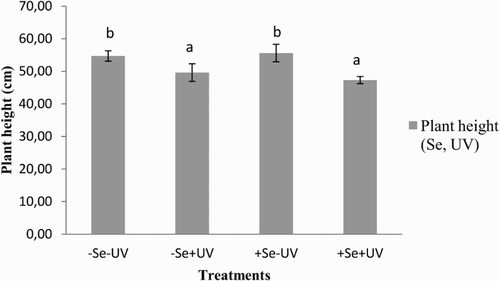
Irrespective of the Se treatment, the plants exposed to ambient UV radiation (+UV) were significantly shorter than those grown under the reduced UV radiation (−UV) (). Testing the data with the factorial ANOVA showed that for the buckwheat plants, Se treatment (+Se) and ambient UV radiation (+UV) decreased their heights.
Figure 1. Heights of the buckwheat plants grown under the different treatment conditions. Different letters indicate statistically significant differences between conditions (p < 0.05; Tukey tests). Se and UV in brackets indicate the results of factorial ANOVA, showing the factors that significantly influence plant height (p < 0.05).
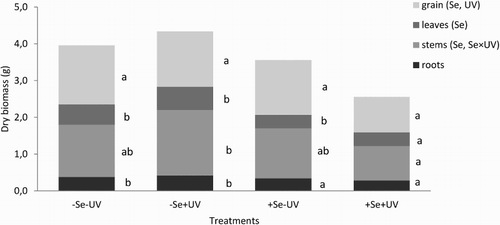
Biomass of buckwheat plant parts
Factorial ANOVA showed that the treatment of the buckwheat plants with Se (+Se) significantly lowered the dry biomass of the leaves, stems and grain. These effects of Se on the dry mass were more pronounced in the plants exposed to ambient UV radiation (+UV), as the +Se+UV plants had significantly lower of leaf, stem and grain biomasses than the Se–+UV plants. The most evident differences here were between the stems and the grain. For the +Se−UV plants, significant decrease was seen only for the biomass of the leaves, and not of the stems, grain and roots (). The +Se+UV plants had the highest root/above-ground biomass ratio.
Figure 2. Biomass of the buckwheat plant parts (as indicated) under the four conditions. Different letters indicate significant differences of plant parts between the different treatments (p < 0.05; Tukey tests). Se and/or UV and Se×UV in brackets indicate the results of factorial ANOVA, showing the factors and their interaction that significantly influence biomass of different plant parts (p < 0.05).
Elemental analysis of buckwheat leaves
Exposure of the buckwheat to the ambient UV radiation (+UV) increased the P content in the leaves, and decreased the Si content. On the other hand, the Se treatment (+Se) increased the Si and K contents in the buckwheat leaves. The increased K content in the leaves was higher for the plants under ambient UV radiation (+UV) in comparison to those under reduced UV radiation (−UV). Exposure to either ambient UV radiation (+UV) or Se treatment (+Se) did not affect the S and Ca contents in the buckwheat leaves ().
Table 3. Elemental analysis of the buckwheat leaves under the four conditions, and impact of Se and UV and their interaction (Se×UV) on the parameters measured.
The plants treated with Se (+Se) accumulated approximately 20-fold higher Se content compared to the untreated ones (−Se) in the leaves, stems and grain, and 3-fold higher in the roots (). In the plants treated with Se, the ambient UV radiation (+Se+UV) decreased the accumulation of Se in the grain and roots, in comparison to the reduced UV radiation (+Se−UV). However, in the plants not treated with Se, the ambient UV radiation (−Se+UV) increased the Se content in the stems and grain, in comparison to the plants grown under reduced UV radiation (−Se−UV) ().
Table 4. Se levels in the plant parts under the different conditions of the buckwheat plants, and the impact of Se and UV and their interaction (Se×UV) on the parameters measured.
Discussion
Effects of treatments on structural and production parameters
In the present study, these buckwheat plants grown under ambient UV (+UV) without and with foliar Se treatment were significantly shorter in comparison to the plants under reduced ambient UV (−UV) (). This is due to the UV-B sensitivity of auxin, which promotes apical dominance (Teramura and Ziska Citation1996). The −Se+UV and +Se+UV plants also differed significantly in their total biomass production, which was the highest for all of the conditions in the −Se+UV plants (i.e. those grown under ambient UV radiation without Se) (). These −Se+UV plants therefore had the highest biomass/plant height ratio (0.088), while the +Se+UV plants had the lowest (0.054). This pronounced difference in biomass between the −Se and +Se treated plants grown under ambient UV radiation that were similar in height is a consequence of branching in the −Se+UV plants, due to their loss of apical dominance. In both of the Se treatments, as −Se and +Se, growth under reduced ambient UV radiation (−UV) showed no differences in the above-mentioned parameters, with the exception of root biomass. The biomass/plant height ratios were, on average, 0.072 and 0.064, respectively. Previous studies using the same Se treatment revealed little or no effects of Se on plant biomass; however, this has been shown to be species dependent (Germ et al. Citation2005; Mechora et al. Citation2011; Kreft et al. Citation2013).
The leaf anatomical parameters under all of these conditions were mainly affected by UV radiation, and less so by Se treatment. In contrast to the data from the literature (Jansen Citation2002; Kakani et al. Citation2003), where UV exposed plants had low specific leaf areas and thicker leaves with thicker cuticle, in the present study with hybrid buckwheat, the −Se+UV plants had the highest specific leaf area, and thinner leaves with thinner upper cuticle (). This was possibly the consequence of the densely branched shoots that might result in self-shading, which prevents the penetration of the light (Beckwith et al. Citation2004). Here also, ambient UV radiation (+UV) significantly increased trichome density at the upper leaf surface (), which is in agreement with previous studies (Golob et al. Citation2017), while Se treatment (+Se) significantly increased trichome length at the upper and lower leaf surfaces and stomata length at lower leaf surfaces, as shown by the factorial analysis. The former appears to be related to accumulation of Se in the trichomes, as has been shown for Mn and Zn (McNear and Kupper Citation2014). The increased size of stomata may be related to their lower density, as shown in some other species (Larcher, Citation2003).
The +Se−UV and +Se+UV plants differed across all of the biochemical parameters measured, while the differences between the −Se−UV and −Se+UV plants indicated changes only in the contents of protective substances; i.e. anthocyanins and UV-absorbing compounds (). This is in agreement with the literature data (Kakani et al. Citation2003). Additional positive effects on the production of protective substances was seen for Se treatment (+Se), which was not the case for the contents of the photosynthetic pigments (). However, factorial analysis of the influence of ambient UV radiation (+UV) showed pronounced reduction in photosynthetic pigments, and strong promotion of protective substances (), which was also shown in a study with wheat (Golob et al. Citation2017). This appears to be the consequence of a trade-off between promoting plant protection or primary metabolism, which consequently resulted in biomass production. Biomass production was lowest in the +Se+UV plants (), where the contents of protective substances were the highest under all of these conditions (). Caretto et al. (Citation2015) supported the hypotheses that there is a trade-off between plant growth and plant defence for the plant cells, and that this trade-off is mediated by resource availability.
The physiological parameters measured showed only slight effects under these four conditions. The potential photochemical efficiency of PSII was decreased by both ambient UV radiation (+UV) and Se treatment (+Se) (). The plant respiratory potential was increased by the UV radiation in plants with no Se treatment (−Se+UV) (). The leaf respiratory potential is a measure of the available energy, and it is affected by the severity of environmental constraints (Germ and Gaberščik Citation2003; Germ et al. Citation2006). The relatively small differences in respiratory potential and also in potential photochemical efficiency of PSII across the four conditions revealed the absence of stress in the studied plants.
Effect of treatments on plant elemental composition
Foliar treatment with Se resulted Se accumulation in all plant parts of hybrid buckwheat, which is in agreement with the results of other studies on buckwheat (Vogrinčič et al. Citation2009; Golob et al. Citation2015). Here, the Se-treated plants (+Se) had up to 20-fold higher Se content in stems, leaves and grain, compared to the untreated plants (−Se). Results showed increased uptake of Se from soil and its accumulation in stems and grain in hybrid buckwheat exposed to ambient UV radiation (+UV) and without Se treatment (-Se) (). This is in agreement with findings of Ožbolt et al. Citation2008, that UV-B radiation increased content of Se in leaves, stems and seeds in common buckwheat grown from seeds previously soaked in solutions with different concentrations of Se. On the other hand in hybrid buckwheat, foliarly treated with Se (+Se), ambient UV radiation decreased Se accumulation in roots and grain (). Different impact of ambient UV radiation on Se accumulation between Se treated and untreated hybrid buckwheat, could be due to the impact of UV radiation on leaf morphology.
Regarding sulphur (S) content in leaf tissue we did not find any differences among treatments (). Due to the chemical similarities between Se and S, the uptake, transport and assimilation of selenate follow the sulphate pathway (White et al. Citation2004), therefore increased soil concentration of Se may negatively affect the uptake of sulphate. In our experiment the plants were foliary treated therefore negative interactions were not expected, however the interactions within plants are still possible. In fact, study of Lyons et al. (Citation2005) showed, that once a relatively high threshold level of soil/medium selenate is reached, high Se seems to cause upregulation of the main sulphate transporter, resulting in higher S accumulation in leaves.
In the present study, there were no effects of ambient UV radiation (+UV) on the potasium (K) content, although there was increased K content in plants treated with Se (+Se) and ambient UV (+UV) (). This was not expected since in a study on maize, Se treatment at 10 mmol (SeO2) L−1 decreased uptake and accumulation of K in plant leaves (Pazurkiewicz-Kocot et al. Citation2003), which was possibly the consequence of lower concentration of Se used for treatment.
Se had no effect on phosphorus (P) content in hybrid buckwheat’s leaves. On the other hand, exposure to ambient UV radiation on increased P content in leaves (). The study of Shen et al. (Citation2014) is in agreement with these results. Increased P content might have negative effects on the plants, because P deficiency in plants reduces their UV-B sensitivity (Jordan Citation1993).
The Ca contents in hybrid buckwheat where relatively high, but similar among treatments, and neither ambient UV (+UV) nor Se treatment (+Se) affected these in the leaf tissue (). In some plants, including Polygonaceae, Ca oxalate is present in high amounts (Franceschi and Nakata Citation2005). Excess free Ca2+ ions in the cytoplasm is toxic to plants, and therefore deposition of this Ca into physiologically inactive forms, such as crystal Ca oxalate, can prevent potential toxic effects (He et al. Citation2012), which was possibly also the case in hybrid buckwheat.
Si is found in plants at concentrations ranging from 0.1% to 10%, and this is species specific (Epstein Citation1999). The Si contents determined here for F. hybridum were low () and were reduced by the ambient UV radiation (+UV), which is opposite to that seen in a study with wheat, where Si contents >1% represented an important structural element (Golob et al. Citation2017).
Acknowledgements
Authors are grateful to Christopher Berrie for revising the English writing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributor
Dr. Aleksandra Golob works at the University of Ljubljana, Biotechnical Faculty, Slovenia. Research topics are: ecology of plants, mineral nutrition, effects of UV radiation on plants and biofortification.
Dr. Vekoslava Stibilj is retired. She was employed as Senior Research Scientist at Jožef Stefan Institute, Ljubljana with expertise in Analytical Chemistry, Food Science, Agricultural Plant Science. Her research topics are: development of the analytical procedures for speciation of selenium and iodine in the environmental and biological samples, especially in food, bioavailability of trace elements from soils and sediments, investigations of oxidation–reduction processes of iodine and selenium, investigations related to safe disposal of waste materials (sewage sludge, tannery waste), investigations of the uptake, kinetics and transformation of various selenium species in plants. She is the main coordinator of the basic projects.
Prof. Ivan Kreft is employed at the Nutrition Institute, Ljubljana, Slovenia. He is a member of Slovenian Academy of Sciences and Arts. He was a professor of Genetics at the Biotechnical Faculty University of Ljubljana. Ivan Kreft is involved in training and education of young scientists and professionals as a mentor to many doctoral students, master candidates, graduate students and authors of student research papers. Ivan Kreft has served on numerous organizational boards and committees of international scientific symposia on a range of international scientific meetings with invited keynote lectures and has lectured in many universities abroad including in Sweden and Japan. He has received several national and international awards.
Associate Prof. Dr. Katarina Vogel-Mikuš works at the University of Ljubljana, Biotechnical Faculty, Slovenia. Her research topics are: metal stress in plants, metal accumulation and tolerance in plants, metal hyperaccumulator plants, mycorrhiza, interactions of engineered nanoparticles and plants, phytoremediation, biofortification, environmental monitoring, radionuclides, food chains. She was a proposer or experiment leader for more than 20 synchrotron experiments (beamtime allocated on the basis of peer-reviewed proposals) at ESRF, Grenoble, Elettra, Trieste and DESY, Hamburg. She was a visiting scientist at TwinMic, Elettra, Trieste and iThemba Labs, Somerset West, South Africa.
Associate Prof. Dr. Alenka Gaberščik works at the University of Ljubljana, Biotechnical Faculty, Slovenia. She is involved in undergraduate and postgraduate programs teaching subjects such as Ecology, Plant Ecology, Ecosystems and Environmental Changes and Nature Conservation. Her current research interests are (1) the influence of UV-B on plants and possibly interactions with other stresses, (2) ecology of aquatic plants and (3) processes in the intermittent Lake Cerknica. She is a coordinator of the research program Plant Biology, a head of the Chair of ecology and environment protection, an editor of scientific journal Acta Biologica Slovenica and a president of the Association of Biologists of Slovenia.
Associate Prof. Dr. Mateja Germ works at the University of Ljubljana, Biotechnical Faculty, Slovenia. Research topics are: ecology of plants, mineral nutrition, effects of different environmental parameters: UV-B radiation, Se, draught on plants, distribution of macrophytes in relation to environmental parameters. She has lectured in different subjects and is responsible for many master and doctoral theses. She is the main coordinator of the basic and applicative projects. She has co-organized many international symposiums.
Additional information
Funding
References
- Abbas SM. 2012. Effects of low temperature and selenium application on growth and the physiological changes in sorghum seedlings. J Stress Physiol Biochem. 8:268–286.
- Ballaré CL. 2014. Light regulation of plant defense. Annu Rev Plant Biol. 65:335–363. doi: 10.1146/annurev-arplant-050213-040145
- Barnes PW, Flint SD, Ryel RJ, Tobler MA, Barkley AE, Wargent JJ. 2015. Rediscovering leaf optical properties: new insights into plant acclimation to solar UV radiation. Plant Physiol Biochem. 93:94–100. doi: 10.1016/j.plaphy.2014.11.015
- Beckwith AG, Zhang Y, Seeram NP, Cameron AC, Nair MG. 2004. Relationship of light quantity and anthocyanin production in Pennisetum setaceum cvs. ‘Rubrum’ and ‘Red Riding Hood’. J Agric Food Chem. 52:456–461. doi: 10.1021/jf034821+
- Björn LO. 2015. On the history of phyto-photo UV science (not to be left in skoto toto and silence). Plant Physiol Biochem. 93:3–8. doi: 10.1016/j.plaphy.2014.09.015
- Bonafaccia G, Marocchini M, Kreft I. 2003. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 80:9–15. doi: 10.1016/S0308-8146(02)00228-5
- Caldwell MM. 1968. Solar UV radiation as an ecological factor for alpine plants. Ecol Monogr. 38:243–268. doi: 10.2307/1942430
- Caretto S, Linsalata V, Colella G, Mita G, Lattanzio V. 2015. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int J Mol Sci. 16:26378–26394. doi: 10.3390/ijms161125967
- Cartes P, Jara AA, Pinilla L, Rosas A, Mora ML. 2010. Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann Appl Biol. 156:297–307. doi: 10.1111/j.1744-7348.2010.00387.x
- Djanaguiraman M, Prasad PVV, Seppanen M. 2010. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem. 48:999–1007. doi: 10.1016/j.plaphy.2010.09.009
- Drumm H, Mohr H. 1978. The mode of interaction between blue (UV) light photoreceptor and phytochrome in anthocyanin formation of the sorghum seedling. Photochem Photobiol. 27:241–248. doi: 10.1111/j.1751-1097.1978.tb07595.x
- Epstein E. 1999. Silicon. Annu Rev Plant Physiol Plant Mol Biol. 50:641–664. doi: 10.1146/annurev.arplant.50.1.641
- Fabjan N, Rode J, Košir IJ, Wang Z, Zhang Z, Kreft I. 2003. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J Agricult Food Chem. 51:6452–6455. doi: 10.1021/jf034543e
- Fesenko NI, Fesenko NN. 2010. Fagopyrum hybridum: a process of the new buckwheat crop development. Proceedings of the 11th International Symposium on Buckwheat, Orel (Russia), 308–313.
- Foster LH, Sumar S. 1995. Selenium in the environment, food and health. Nutr Food Sci. 95:17–23. doi: 10.1108/00346659510093991
- Franceschi VR, Nakata PA. 2005. Calcium oxalate in plants: formation and function. Annu Rev Plant Biol. 56:41–71. doi: 10.1146/annurev.arplant.56.032604.144106
- Germ M, Gaberščik A. 2003. Dihalni potencial - kazalnik stresa pri rastlinah = Respiratory potential - indicator of stress in plants. Zbornik Biotehniške fakultete Univerze v Ljubljani, Kmetijstvo, Agricultural Issue. 81(2):335–339.
- Germ M, Gaberščik A. 2016. The effect of environmental parameters on buckwheat. In: Zhou M, Kreft I, Woo SH, Chrungoo N, Wieslander G, editors. Molecular breeding and nutritional aspects of buckwheat. Amsterdam: Elsevier; p. 273–281.
- Germ M, Kreft I, Osvald J. 2005. Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II; Yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant Physiol Biochem. 43:445–448. doi: 10.1016/j.plaphy.2005.03.004
- Germ M, Mazej Z, Gaberščik A, Trošt Sedej T. 2006. The response of Ceratophyllum demersum L. and Myriophyllum spicatum L. to reduced, ambient, and enhanced ultraviolet-B radiation. Hydrobiologia. 570:47–51. doi: 10.1007/s10750-006-0160-x
- Golob A, Germ M, Kreft I, Zelnik I, Kristan U, Stibilj V. 2015. Selenium uptake and Se compounds in Se-treated buckwheat. Acta Bot Croat. 75:17–24. doi:10.1515/botcro-2016-0016.
- Golob A, Kavčič J, Stibilj V, Gaberščik A, Vogel-Mikuš K, Germ M. 2017. The effect of selenium and UV radiation on leaf traits and biomass production in Triticum aestivum L. Ecotoxicol Environm Safety. 136:142–149. doi: 10.1016/j.ecoenv.2016.11.007
- Hajiboland R, Sadeghzadeh N, Ebrahimi N, Sadeghzadeh B, Mohammadi SA. 2015. Influence of selenium in drought-stressed wheat plants under greenhouse and field conditions. Acta Agric Slov. 105:175–191. doi: 10.14720/aas.2015.105.2.01
- Hajiboland R, Sadeghzadeh N, Sadeghzadeh B. 2014. Effect of Se application on photosynthesis, osmolytes and water relations in two durum wheat (Triticum durum L.) genotypes under drought stress. Acta Agric Slov. 103:167–179. doi: 10.14720/aas.2014.103.2.2
- Hartikainen H, Xue T, Piironen V. 2000. Selenium as an antioxidant and pro-oxidant in ryegrass. Plant Soil. 225:193–200. doi: 10.1023/A:1026512921026
- Hawrylak-Nowak B, Dresler S, Wójcik M. 2014. Selenium affects physiological parameters and phytochelatins accumulation in cucumber (Cucumis sativus L.) plants grown under cadmium exposure. Sci Hortic. 172:10–18. doi: 10.1016/j.scienta.2014.03.040
- He H, Bleby TM, Veneklaas EJ, Lambers H, Kuo J. 2012. Precipitation of calcium, magnesium, strontium and barium in tissues of four acacia species (Leguminosae: Mimosoideae). PLoS One. doi:10.1371/journal.pone.0041563.
- Jansen MAK. 2002. Ultraviolet-B radiation effects on plants: induction of morphogenic responses. Physiol Plant. 116:423–429. doi: 10.1034/j.1399-3054.2002.1160319.x
- Jansen MAK, Coffey AM, Prinsen S. 2012. UV-B induced morphogenesis. Four players or a quartet? Plant Signal Behav. 7:1185–1187. doi: 10.4161/psb.21260
- Jordan BR. 1993. The molecular biology of plants exposed to ultraviolet-B radiation and the interaction with other stresses. In: Jackson MB, Black CR, editors. Interacting stresses on plants in a changing climate, Vol. 16, NATO ASI Series. Berlin, Heidelberg: Springer-Verlag; p. 153–170.
- Kabata Pendidas A. 2001. Trace elements in soils and plants. 3th ed. Boca Raton (FL): CRC Press; p. 241–252.
- Kakani VG, Reddy KR, Zhao D, Sailaja K. 2003. Field crop responses to ultraviolet-B radiation: a review. Agr Forest Meteorol. 120:191–218. doi: 10.1016/j.agrformet.2003.08.015
- Kataria S, Baroniya SS, Bhaghel L, Kanungo M. 2014. Effect of exclusion of solar UV radiation on plants. Plant Sci Today. 1:224–232. doi: 10.14719/pst.2014.1.4.61
- Kenner AA, Ahmed SI. 1975. Measurements of electron transport activities in marine phytoplankton. Mar Biol. 33:19–127.
- Kreft I, Mechora Š, Germ M, Stibilj V. 2013. Impact of selenium on mitochondrial activity in young Tartary buckwheat plants. Plant Physiol Biochem. 63:196–199. doi: 10.1016/j.plaphy.2012.11.027
- Kreft S, Štrukelj B, Gaberščik A, Kreft I. 2002. Rutin in buckwheat herbs grown at different UV-B radiation levels: comparison of two UV spectrophotometric and an HPLC method. J Exp Bot. 53:1801–1804. doi: 10.1093/jxb/erf032
- Kump P, Nečemer M, Rupnik Z, Pelicon P, Ponikvar D, Vogel-Mikuš K, Regvar M, Pongrac P. 2011. Improvement of the XRF quantification and enhancement of the combined applications by EDXRF and micro-PIXE. In: Integration of nuclear spectrometry methods as a new approach to material research. Final report of a Coordinated Research Project 2006–2009; p. 101–110.
- Larcher W. 2003. Physiological plant ecology, ecophysiology and stress physiology of functional groups, 4th ed. Berlin: Springer.
- Li S, Zhang H. 2001. Advances in the development of functional foods from buckwheat. Crit Rev Food Sci Nutr. 41:451–464. doi: 10.1080/20014091091887
- Lichtenthaler HK, Buschmann C. 2001a. Extraction of photosynthetic tissues: chlorophylls and carotenoids. Current Protocols in Food Analytical Chemistry, F4.2.1-F4.2.6. John Wiley in Sons Inc., New York.
- Lichtenthaler HK, Buschmann C. 2001b. Chlorophylls and carotenoids: measurement and characterisation by UV-VIS. Current Protocols in Food Analytical Chemistry, F4.3.1-F4.3.8. John Wiley in Sons Inc., New York.
- Lyons GH, Stangoulis JCR, Graham RD. 2005. Tolerance of wheat (Triticum aestivum L.) to high soil and solution selenium levels. Plant Soil. 270:179–188. doi: 10.1007/s11104-004-1390-1
- McNear Jr, DH, Kupper JV. 2014. Mechanisms of trichome-specific Mn accumulation and toxicity in the Ni hyperaccumulator Alyssum murale. Plant Soil. 377:407–422. doi: 10.1007/s11104-013-2003-7
- Mechora Š, Stibilj V, Radešček T, Gaberščik A, Germ M. 2011. Impact of Se (VI) fertilization on Se concentration in different parts of red cabbage plants. J Food Agric Environ. 9:357–361.
- Nawaz F, Ashraf MY, Ahmad R, Waraich EA, Shabbir RN, Bukhari MA. 2015. Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem. 175:350–357. doi: 10.1016/j.foodchem.2014.11.147
- Nečemer M, Kump P, Sčančar J, Jaćimović R, Simčič J, Pelicon P, Budnar M, Jeran Z, Pongrac P, Regvar M, et al. 2008. Application of X-ray fluorescence analytical techniques in phytoremediation and plant biology studies. Spectrochim Acta B. 63:1240–1247. doi: 10.1016/j.sab.2008.07.006
- Ožbolt L, Kreft S, Kreft I, Germ M, Stibilj V. 2008. Distribution of selenium and phenolics in buckwheat plants grown from seeds soaked in Se solution and under different levels of UV-B radiation. Food Chem. 110:691–696. doi: 10.1016/j.foodchem.2008.02.073
- Packard TT. 1971. The measurement of respiratory electron transport activity in marine phytoplankton. J Mar Res. 29:215–244.
- Pazurkiewicz-Kocot K, Galas W, Kita A. 2003. The effect of selenium on the accumulation of some metals in Zea mays L. plants treated with indole-3-acetic acid. Cell Moll Biol Lett. 8:97–103.
- Peng Q, Zhou Q. 2010. Effects of enhanced UV-B radiation on the distribution of mineral elements in soybean (Glycine max) seedlings. Chemosphere. 78:859–863. doi: 10.1016/j.chemosphere.2009.11.050
- Rozema J, Björn LO, Bornman JF, Gaberščik A, Häder DP, Trošt T, Germ M, Klisch M, Gröniger A, Sinha RP, et al. 2002. The role of UV-B radiation in aquatic and terrestrial ecosystems—an experimental and functional analysis of the evolution of UV-absorbing compounds. J Photochem Photobiol B Biol. 66:2–12. doi: 10.1016/S1011-1344(01)00269-X
- Schreiber U, Kühl M, Klimant I, Reising H. 1996. Measurement of chlorophyll fluorescence within leaves using a modified PAM Fluorometer with a fiber-optic microprobe. Photosynth Res. 47:103–109. doi: 10.1007/BF00017758
- Seppänen M, Turakainen M, Hartikainen H. 2003. Selenium effects on oxidative stress in potato. Plant Sci. 165:311–319. doi: 10.1016/S0168-9452(03)00085-2
- Shen X, Li Z, Duan L, Eneji AE, Li J. 2014. Silicon effects on the partitioning of mineral elements in soybean seedlings under drought and ultraviolet-B radiation. J Plant Nutr. 37:828–836. doi: 10.1080/01904167.2013.873458
- Smrkolj P, Stibilj V. 2004. Determination of selenium in vegetables by hydride generation atomic fluorescence spectrometry. Anal Chim Acta. 512:11–17. doi: 10.1016/j.aca.2004.02.033
- Tadina N, Germ M, Kreft I, Breznik B, Gaberščik A. 2007. Effects of water deficit and selenium on common buckwheat (Fagopyrum esculentum Moench.) plants. Photosyntetica. 45:472–476. doi: 10.1007/s11099-007-0080-7
- Teramura AH, Ziska HL. 1996. Ultraviolet-B radiation and photosynthesis. In: Baker NR, editor. Photosynthesis and the environment. Amsterdam: Springer; p. 435–450. doi:10.1007/0-306-48135-9_18.
- The United Nations Environment Programme. 2015. Annual Report. 62 p.
- Vekemans B, Janssens K, Vincze L, Adams F, Van Espen P. 1994. Analysis of X-ray spectra by iterative least squares (AXIL): new developments. X-Ray Spectrom. 23:278–285. doi: 10.1002/xrs.1300230609
- Vogrinčič M, Cuderman P, Kreft I, Stibilj V. 2009. Selenium and its species distribution in above-ground plant parts of selenium enriched buckwheat (Fagopyrum esculentum Moench). Analyt Sci. 25:1357–1363. doi: 10.2116/analsci.25.1357
- Wargent JJ, Jordan BR. 2013. From ozone depletion to agriculture: understanding the role of UV radiation in sustainable crop production. New Phytol. 197:1058–1076. doi: 10.1111/nph.12132
- White PJ. 2016. Selenium accumulation by plants. Ann Bot. 117:217–235.
- White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, et al. 2004. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J Exp Bot. 55(404):1927–1937. doi: 10.1093/jxb/erh192
- Xue T, Hartikainen H, Piironen V. 2001. Antioxidative and growth-promoting effect of selenium in senescing lettuce. Plant Soil. 237:55–61. doi: 10.1023/A:1013369804867
- Yao X, Chu J, Ba C. 2010. Antioxidant responses of wheat seedlings to exogenous selenium supply under enhanced ultraviolet-B. Biol Trace Elem Res. 136:96–105. doi: 10.1007/s12011-009-8520-9
