ABSTRACT
Purpose: The purposes of this study were to determine the effect of matrine on the migration and the colonization dynamics of the two fluorescent-tagged rhizobia in Gannong No.5 alfalfa (Medicago sativa L. Gannong No. 5) tissues, and also to determine the effect of the combination treatments on alfalfa seedlings’ growth.
Materials and methods: 0, 100, 200, 300 and 400 mg L−1 matrine levels were added into two cyan fluorescent protein (CFP)-tagged rhizobia; Ensifer meliloti LZgn5f (gn5f) and Ensifer meliloti 12531f (12531f), respectively; and drenched the alfalfa root with the inoculants. Then the migration and colonization of the two rhizobia in alfalfa on D7, D14, D21 and D28, and subsequently seedling growth were investigated.
Results: The results showed that the optimum matrine level enhanced the colonization of both fluorescent-tagged rhizobia in alfalfa roots and the highest colonization densities of log 6.31 cfu g−1 and log 5.87 cfu g−1 were achieved by adding 300 mg L−1 matrine into 12531f and adding 100 mg L−1 matrine into gn5f, respectively. They could migrate to the aerial tissues and most colonize stems through the application of adding 300 mg L−1 matrine into 12531f and 100 mg L−1 matrine into gn5f, respectively. No fluorescent-tagged rhizobia were detected in the control treatment. Alfalfa seedling growth parameters like leaf chlorophyll content, seedling growth rate, root length, seedling biomass and total N percentage also increased the most when 300 mg L−1 matrine was added into 12531f and 100 mg L−1 matrine added into gn5f treatments.
Conclusions: Our results suggest that 300 mg L−1 matrine added into 12531f and 100 mg L−1 matrine added into gn5f might be exploited to promote the colonization of rhizobia in alfalfa tissue and positively impact growth and yield, indicating possible benefits for plant cultivation.
Introduction
Alfalfa (Medicago sativa L.) is a widely planted forage specie in arid and semi-arid areas (Mohamed et al. Citation2009; Xie et al. Citation2013) due to its high productivity, better nutritive value and capable of fixing nitrogen. The use of chemical fertilizers can temporarily improve plants yield and quality, while for the long-term use not only increased the cost of farming but also damaged to natural ecosystem (Dang Citation2003; Ortiz-Santaliestra et al. Citation2006). The excess usages of nitrogen fertilizers significantly decreased the diversities of rhizobia and soil nitrogen-fixing bacteria, also inhibited the growth of soil nitrogen-fixing bacteria (Zhang Citation2008; Liu and Wang Citation2012). Rhizobia can develop symbiotic relationships with legume plants, often resulting in the fixation of atmospheric nitrogen, which has considerable economic and ecological benefits because of improving soil fertility, plant health, crop yield, and farm profitability, also can develop endophytic relationship with plants apart from legume (Peoples et al. Citation2009; Ji et al. Citation2010). The application of rhizobia could reduce the use of nitrogen fertilizer and the pollution of the environment; also increase the yield and quality of plants (Guo et al. Citation2012). Thus, inoculating alfalfa with rhizobia is a widely used method.
The main pre-inoculation methods are seed dressing and seed coating, but these methods are always unstable. The quantity of rhizobia in pre-inoculated legumes seeds (including vetch, clover, and alfalfa) are very low compared with that on freshly inoculated seeds (Hartley et al. Citation2012). Various factors, such as plants varieties, rhizobia species, external environment, inoculation methods, and the limitation of biology or non-biology can affect the nitrogen fixation ability and reduce the plants yield (Afzal et al. Citation2013). Therefore, selecting convenient methods to improve the alfalfa inoculation abilities are very necessary.
The isolation of endophytic rhizobia from plants capable of high sustainable yields without input of chemical fertilizers has suggested that endophytic rhizobia is an efficient means for enhancing alfalfa growth. Endophytic rhizobia can be found not only in seeds but also almost inside all plants tissues, meanwhile can migrate and colonize the internal plant tissues with no negative effect on their hosts (Li et al. Citation2015; Peralta et al. Citation2016). Compared with resident rhiziobia, the endophytic rhizobia enable the elongating hypocotyl to interact with them prior to interact with resident rhizobia, which makes the endophytic rhizobia in seeds can efficiently fix nitrogen. Despite a widespread occurrence of this natural endophytic rhizobia-legume association, much remains unknown about its migration and colonization dynamics. Endophytic rhizobia mainly exist in roots of alfalfa, especially in fibrous roots, while fewer exist in the stems, leaves or seeds (Li et al. Citation2009). Understanding of endophytic rhizobia colonization and identification of target species inhabiting alfalfa throughout the seedling growth period can be helpful in the formulation of suitable inoculation methods for alfalfa.
Plants are normally associated with diverse microorganisms, such as bacteria and fungi (Liu et al. Citation2016); they may compete with the efficient endophytic rhizobia for the limited space and nutrients provided by the host plants. These competitions will decrease the colonization densities and the proliferation ability of the effective endophytic rhizobia. Thus, selecting exterior material to inhibit the competitions with other microbes is necessary.
Matrine, a low toxic alkaloid purified from the dry root of Sophora flavescens Ait, has extensive application in medicine for the treatment of cancer, cardiac diseases, viral hepatitis and skin diseases (Zhang et al. Citation2007). It has been regarded as natural plant antibacterial agent, which has strong specificity effect on the pathogen and insect. With the use of matrine, it is not easy to induce antibiotic resistance in microbes and rarely kills beneficial organisms (Huo Citation2014). Besides, matrine can enhance crop production, but does not harm humans and not pollute the environment (Gao et al. Citation2013). Previous studies by our group have found that matrine has the ability to inhibit soil-oriented and air-oriented micro-organisms, also improves the alfalfa seedlings growth when R.LH3436f rhizobia combination with matrine compares with single inoculation matrine treatment and control (Huo Citation2014). Matrine has also been shown to significantly increase of the germination and seedling growth of tomato and wheat (Xiong Citation2015; Zhou et al. Citation2016). Despite all the benefits of matrine, no research has yet been conducted on the effects of matrine and fluorescent tagged rhizobia (FTR) combination on colonization dynamics and migration characteristics in alfalfa seedlings. The potential benefits of this combination treatment on alfalfa growth are still blank.
Thus, the aims of this study were (I) to determine the effect of different matrine levels on the migration and the colonization dynamics of the two different FTR in various alfalfa tissues; and also (II) to determine the effect of the combination treatments on alfalfa seedlings growth. The results will not only enhance the colonization ability and improve the inoculation effect, but also help in determining the best inoculation practices and the precise matching between rhizobia strains and alfalfa varieties.
Materials and Methods
Rhizobia strains
Two rhizobia strains, exogenetic Ensifer melilot 12531f (12531f) and endophytic Ensifer meliloti LZgn5 (gn5). The original exogenetic rhizobium strain was 12531, it was a standard alfalfa rhizobium, which was obtained from the China General Microbiological Culture Collection Center (CGMCC) and isolated from Melilotus (Melilotus suaveolens L.). The original endophytic rhizobium strain was Ensifer meliloti LZgn5 (gn5), which was isolated from the M. sativa L. Gannong No. 5 seed. To generate CFP fluorescent tagged rhizobia strains, the donor Escherichia coli (E. coli) strain used in this study carried the pMP4517 plasmid containing a gentamicin resistance gene (40 μg mL−1) and a CFP encoding gene; the helper E. coli strain carried the pRK2073 plasmid containing the spectinomycin resistance gene (20 μg mL−1). Both strains were maintained on Luria Bertani solid medium (Hu et al. Citation2007). pMP4517 was inserted into the rhizobia strains with the help of pRK2073 according to a triparental conjugation method (Zhang et al. Citation2015). The resulting fluorescent tagged E. meliloti and E. meliloti strains were named 12531f and gn5f, respectively. Both strains were maintained on TY (Yeast Tryptone Agar medium) (Zhang et al. Citation1991), all strains were kept at 4°C at the Key Laboratory of Grassland Ecosystem of the Ministry of Education, College of Grassland Science, Gansu Agricultural University (GSAU), Lanzhou, China.
Matrine source
The matrine was purchased from Baoji F.S. Biological Development Co., LTD with the concentration of 10% and stored at room temperature (23–26°C) in a brown glass bottle.
Alfalfa seed materials
Seeds of M. sativa Gannong No. 5 were provided by the Key Laboratory of Grassland Ecosystem of the Ministry of Education, College of Grassland Science, GSAU, Lanzhou, China. At the time of the experiment, the seeds had been stored for 4 years at room temperature (23–26°C) with the germination percentage of 84% and the purity of 97%.
Preparation of FTR suspension with matrine
The two FTR strains were preserved on TY agar medium at 4°C for activation and then inoculated in TY liquid medium and cultured at 28°C on a rotary shaker (180 r min−1) until the optical density at 600 nm (OD600nm) reached 0.5 to 1. Each culture was centrifuged at 4000 rpm, 25°C for 10 min (Centrifuge Xiangyi, H1650, Changsha, China) and re-suspended in sterile distilled water to reach an OD600nm of 0.5.
One gram of matrine was dissolved in 1 mL of ethanol, diluted to 20 mg mL−1 solution with sterile distilled water, and sterilized by filtration (filter diameter 0.22 µm). The sterilized matrine was added into the FTR suspension to a final level of 100 mg L−1, 200 mg L−1, 300 mg L−1, and 400 mg L−1.
Experimental design and treatments
The experiment was established at the College of Grassland Science, GSAU, on 23 November 2014. The experimental design used in the experiment was a completely randomized design with six replications. Alfalfa seeds were placed in a sterilized flask and surface sterilized in iodophor disinfectant (concentration of 2500 mg mL−1 in available iodine) for 2 min, then thoroughly rinsed with sterile distilled water five times and dried thoroughly. The sand was screened with 2 mm sieve and soaked in 1 mol L−1 HCl in order to reach pH 7, rinsed eight times with distilled water, dried in an oven at 110°C, and sterilized in autoclaves at 121°C for 6 h.
Thirty sterilized seeds were sown in each polyvinyl plastic pot (diameter 6 cm, height 7.5 cm) at 2 cm depth, which had already loaded with 470 g of sterilized sand. Pots were placed in a basin with 500 mL Hoagland nitrogen solution before inoculation (Hoagland and Arnon Citation1950). When the first true leaves of alfalfa seedlings emerged, remaining 20 seedlings each pot, then 20 mL of matrine-rhizobia suspension was evenly irrigated into each pot. Pots were irrigated with 500 mL N-free hoagland nutrient solution every 10 days after inoculation. The daily consumption of water in the basin was supplemented with sterile distilled water. Single inoculated rhizobia and single inoculated sterile distilled water (un-inoculated control) also used.
Detection of FTR in different plant tissues over time
Alfalfa plants were collected from each pot of each treatment, excised, washed with sterile distilled water, dry and divided into five parts: roots, stems (upper and lower stems) and leaves (upper and lower leaves) every 7 days (D7) after inoculation.
The collected samples were first sterilized by iodophor disinfectant (concentration of 2500 mg L−1 in available iodine) and shaken for 3 min. Subsequently, the samples were rinsed with sterile distilled water three to five times under aseptic conditions. All sterile samples were then placed on the surface of TY agar medium for 30 min and incubated for 48 h at 25°C. The plates showed no colonies, verifying that the excised roots were successfully surface sterilized (Chi et al. Citation2005).
To enumerate the endophytic rhizobia, surface-disinfected tissues (1 g) were triturated with 2 mL of sterile distilled water using a mortar and pestle, and 0.2 mL of each triturate (5 min, 4000 rpm centrifuged) was inoculated on TY agar medium in three replicates (roots triturate were with concentration of 10−1, 10−2, 10−3, 10−4 and 10−5, which had three replications with each concentration). After incubation for 48 h at 25°C in the incubator, the FTR colonies were recorded using a UV lamp (336 nm) in a dark room (FTR show cyan color under UV light). Finally, the above FTR colonies were converted to per gram fresh weight tissues samples.
Stereo Fluorescence Microscope (V20 Stereo Fluorescence Microscope with FS47 filter) was also used to detect the migration channel and colonization position of FTR at different alfalfa tissues. With microscope (V20 Stereo Fluorescence Microscope with FS47 filter) technique, the green light was used to detect the FTR since it became white for FTR and black for non FTR. The surface sterile tissues of roots, stems (upper and lower stems) and leaves (upper and lower leaves) were dried and placed on the objective table examined using the microscope with green filters to capture the green fluorescence from FTR from host tissue. The same detection methods were used for control.
Responses of alfalfa seedling growth to inoculation treatments
Plants were harvested 30 days after inoculation treatments. Five seedlings of each treatment pot were randomly selected to measure the following items: root length, root nodules, relative growth rate, compound leaf number, fresh and dry weight, total N percentage and leaf chlorophyll content. Root length was recorded in cm with a ruler. Nodules were separated from each seedling roots, their number and weighed were then counted, their diameters were also measured with a ruler, nodule classification was conducted using a five–point method (Li et al. Citation2010): 1. Death of nodule (one point); 2. Ineffective nodule with gray transverse section (two points); 3. Pink nodule with a diameter less than 0.5 mm (three points); 4. Pink nodule with a diameter between 0.5 and 1 mm (four points); 5. Pink nodule with a diameter greater than 1 mm (five points). Relative growth rate was measured every 10 days. Compound leaf number of each seedling was counted. Fresh weight of root and aerial tissues were measured, these samples were oven dried at 70°C for 48 h for dry weight (Bertrand et al. Citation2007). Total N percentage was determined according to the method of Shetta and Alshahrani (Citation2016). The leaf chlorophyll content of each treatment was extracted by homogenizing 0.1 g of fresh leaves (main vein removed) with 25 mL of 99% acetone and 95% ethanol (1:1). The mixture was placed in the dark and shaken until the leaves turned white. Absorption measurements were recorded at wavelengths of 645 and 663 nm using a spectrophotometer (SP752, Spectrum, Shanghai, China). Concentrations of pigments [mg g−1 fresh weight] were obtained by calculation (Wang and Bai Citation2005).
Statistical analysis
An completely randomized design model was used to analyze all parameters. Statistical analysis was conducted using one-way ANOVA by Duncan test at 5% probability with SPSS 16.0 (SPSS Inc.). A separate analysis of variance was done on data from the two FTR with the addition of different matrine levels. All the results were expressed as mean ± standard error (SE).
Results
Effect of matrine on migration and colonization of FTR in alfalfa seedling roots
The two FTR colonized alfalfa roots were stable and improved the colonization ability with the addition of matrine treatments ( and ). At D7, the highest densities of 12531f showed in roots with the addition of 300 mg L−1 matrine treatment, were log 6.12 cfu g−1, then the densities increased gradually and reached highest at D14 with the addition of 300 mg L−1 matrine treatment, to be log 6.31 cfu g−1, following the densities decreased but to the balance point without significance (P > 0.05) at D28 ((a)). No FTR were found in the control ((a)).
Figure 1. Fluorescent tagged rhizobia (12531f and gn5f) population number in alfalfa seedling roots with the addition of different matrine levels after roots inoculation. (a) population number of 12531f; (b) population number of gn5f. control: inoculated with sterile distilled water, 12531f + 0 to 12531f + 400: 0 mg L−1 to 400 mg L−1 matrine added into 12531f, gn5f + 0 to gn5f + 400: 0 mg L−1 to 400 mg L−1 matrine added into gn5f, respectively. Each value is the mean of three tissue sample replicates plated on TY media and vertical bars give standard errors (SE) of the means. Different lowercase letters indicating that the mean are statistically different according to the Duncan test (P < 0.05).
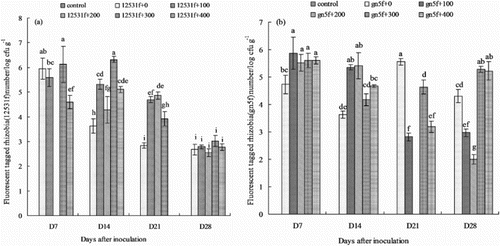
Figure 2. Stereo Fluorescence Microscope and UV lamp detection of endophytic fluorescent tagged rhizobia colonizing alfalfa roots. (a) and (b) show the results using the Stereo Fluorescence Microscope and UV lamp detection method for gn5f; (c) and (d) show the results using the Stereo Fluorescence Microscope and UV lamp detection method for control.
For gn5f, the densities reached highest at D7 with the addition of 100 mg L−1 matrine treatment (log 5.87 cfu g−1), then the densities decreased slightly during D14 to D28, and at D28, the densities still could maintain log 5.28 cfu g−1 ((b), (a) and (b). No FTR were found in the control ((b), (c) and (d)).
Effect of matrine on migration and colonization of FTR in alfalfa seedling aerial tissues
Most 12531f were detected in stems than in leaves. No 12531f was found in the control (). Matrine had a positive effect on 12531f migrating to and colonizing lower stems, lower leaves and upper stems with the addition of 300 mg L−1 matrine. The densities of 12531f reached higher than other treatments at D7 in lower stems, to be log 3.25 cfu g−1; then the densities increased gradually and reached highest at D14 (log 4.06 cfu g−1), but was not significantly (P > 0.05) from that of D7, and then it decreased. In lower leaves, the densities of 12531f reached highest at D28, to be log 1.01 cfu g−1, but it was not significantly different (P > 0.05) from other treatments. In upper stems, the densities of 12531f increased gradually and reached highest at D14 with the addition of 300 mg L−1 matrine treatment, to be log 1.75 cfu g−1, then decreased and no 12531f could be found at D28. Fewer 12531f could be found in upper leaves, the densities were only log 0.46 cfu g−1 at D7 with the addition of 100 mg L−1 matrine treatment.
Table 1. Fluorescent tagged rhizobia (12531f and gn5f) population number in alfalfa seedling aerial tissues with the addition of different matrine levels after roots inoculation.
The densities of gn5f in stems were higher than that in leaves. No gn5f was found in the control (). In lower stems, the densities of gn5f increased gradually and reached highest with the addition of 100 mg L−1 matrine treatment, to be log 3.94 cfu g−1, but were not significantly different (P > 0.05) from that at D7 with the addition of 100 mg L−1 and 300 mg L−1 matrine treatment. In lower leaves, the densities of gn5f increased highest at D14 with the addition of 200 mg L−1 matrine treatment (log 1.59 cfu g−1), but were not significantly different (P > 0.05) from the other inoculation treatments. In upper stems, the densities of gn5f at D7 was highest (log 2.03 cfu g−1) with the addition of 100 mg L−1 matrine treatment, but were not significantly different (P > 0.05) from the same treatment at D28. In upper leaves, gn5f could be found only in three treatments, and the highest densities were only log 0.83 cfu g−1 with the addition of 100 mg L−1 matrine treatment.
Effect of matrine added into FTR on alfalfa seedling nodules
Inoculation of the two FTR with appropriate matrine level could increase the number, weight, diameter, and classification of nodules (). The nodule number, individual nodule weight, nodule diameter and nodule classification all increased gradually and reached highest when added 300 mg L−1 matrine into 12531f, but for gn5f, the nodules index reached highest when added with 100 mg L−1 matrine.
Table 2. Effect of matrine added into fluorescent tagged rhizobia (12531f and gn5f) on alfalfa seedling nodule number, weight, diameter and classification.
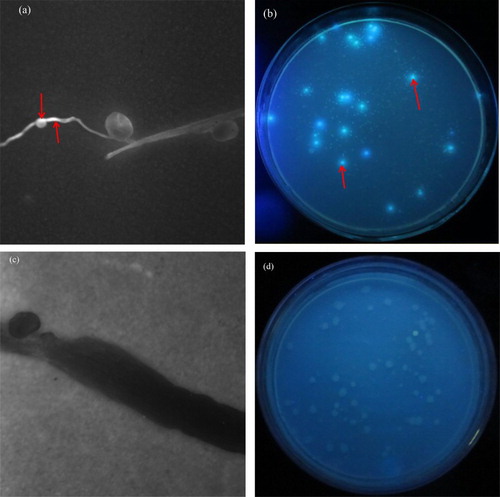
Effect of matrine added into FTR on alfalfa seedling growth characteristics
Whether matrine added into FTR or not tended to increase the relative growth rate of alfalfa seedlings compared with control (). The relative growth rate increased and reached highest when added 300 mg L−1 matrine into 12531f, which was significantly higher (P < 0.05) than control 80.95% ((a)); when inoculated gn5f, the relative growth rate reached highest when added with 100 mg L−1 matrine, which was significantly higher (P < 0.05) than control 57.14%, then it decreased gradually ((b)).
Figure 3. Effect of matrine added into fluorescent tagged rhizobia (12531f and gn5f) on alfalfa seedling relative growth rate. (a) matrine added into 12531f; (b) matrine added into gn5f. control: inoculated with sterile distilled water, 12531f + 0 to 12531f + 400: 0 mg L−1 to 400 mg L−1 matrine added into 12531f, gn5f + 0 to gn5f + 400: 0 mg L−1 to 400 mg L−1 matrine added into gn5f, respectively. Each value is the mean of five replicates and vertical bars give standard errors (SE) of the means. Different lowercase letters indicating that the mean are statistically different according to the Duncan test (P < 0.05).
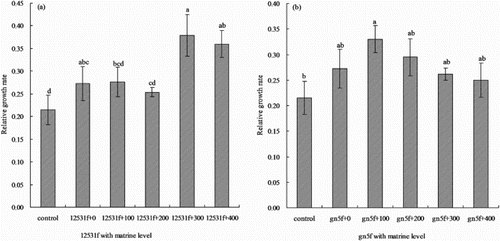
When single inoculated the two FTR or added with appropriate matrine level improved the root length of alfalfa seedlings (). It increased gradually and reached highest when 300 mg L−1 matrine added into 12531f, which was significantly higher (P < 0.05) than control 47.97% ((a)); when inoculated gn5f, the root length reached highest with the addition of 100 mg L−1 matrine treatment, which was significantly higher (P < 0.05) than control 40.19%, then it decreased gradually ((b)).
Figure 4. Effect of matrine added into fluorescent tagged rhizobia (12531f and gn5f) on alfalfa seedling root length. (a) matrine added into 12531f; (b) matrine added into gn5f. control: inoculated with sterile distilled water, 12531f + 0 to 12531f + 400: 0 mg L−1 to 400 mg L−1 matrine added into 12531f, gn5f + 0 to gn5f + 400: 0 mg L−1 to 400 mg L−1 matrine added into gn5f, respectively. Each value is the mean of five replicates and vertical bars give standard errors (SE) of the means. Different lowercase letters indicating that the mean are statistically different according to the Duncan test (P < 0.05).
Alfalfa seedling biomass was improved when single inoculated the two FTR or added with appropriate matrine level (). The highest biomass showed when 300 mg L−1 matrine added into 12531f. The aerial fresh weight was higher than control 89.63%, the aerial dry weight was higher than control 177.93%, but both were no significant difference (P > 0.05); the root fresh weight was significantly higher (P < 0.05) than control 584.66%; the root dry weight was significantly higher (P < 0.05) than control 461.22%. When added 100 mg L−1 matrine into gn5f, the aerial fresh weight was higher than control 130.49%, the aerial dry weight was higher than control 206.81%, but both were no significant difference (P > 0.05); the root fresh weight was higher than control 141.06%, but there was no significant difference (P > 0.05); the root dry weight was significantly higher (P < 0.05) than control 286.73%.
Table 3. Effect of matrine added into fluorescent tagged rhizobia (12531f and gn5f) on alfalfa seedling biomass.
Effect of matrine added into FTR on alfalfa seedling leaf chlorophyll content
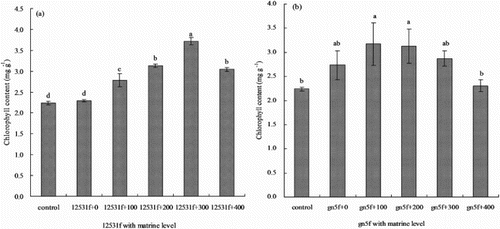
Leaf chlorophyll content tended to increase when single inoculated the two FTR or added with matrine (). The chlorophyll content increased gradually and reached highest when added 300 mg L−1 matrine into 12531f, it was significantly higher (P < 0.05) than control and single inoculated 66.07% and 62.45%, respectively ((a)); but the chlorophyll content reached highest when added 100 mg L−1 matrine into gn5f, it was significantly higher (P < 0.05) than control 41.52%, and single inoculated 16.12% without significant difference (P > 0.05), then gradually decreased with the matrine level increasing ((b)).
Figure 5. Effect of matrine added into fluorescent tagged rhizobia (12531f and gn5f) on alfalfa seedling leaf chlorophyll content. (a) matrine added into 12531f; (b) matrine added into gn5f. control: inoculated with sterile distilled water, 12531f + 0 to 12531f + 400: 0 mg L−1 to 400 mg L−1 matrine added into 12531f, gn5f + 0 to gn5f + 400: 0 mg L−1 to 400 mg L−1 matrine added into gn5f, respectively. Each value is the mean of five replicates and vertical bars give standard errors (SE) of the means. Different lowercase letters indicating that the mean are statistically different according to the Duncan test (P < 0.05).
Effect of matrine added into FTR on alfalfa seedling total N percentage
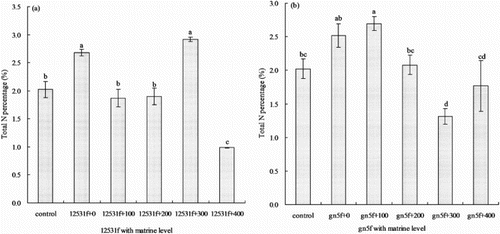
The total N percentage increased when single inoculated the two FTR or added with appropriate matrine (). When added matrine into 12531f, the total N percentage was only improved at 300 mg L−1 matrine level, which was higher than single inoculated treatment 8.96%, but there was no significant difference (P > 0.05) ((a)). Only added 100 mg L−1 matrine into gn5f, enhanced the total N percentage compared with single inoculated treatment. The percentage was higher than single inoculated 7.14% without significant difference (P > 0.05) ((b)).
Figure 6. Effect of matrine added into fluorescent tagged rhizobia (12531f and gn5f) on alfalfa seedling total N percentage. (a) matrine added into 12531f; (b) matrine added into gn5f. control: inoculated with sterile distilled water, 12531f + 0 to 12531f + 400: 0 mg L−1 to 400 mg L−1 matrine added into 12531f, gn5f + 0 to gn5f + 400: 0 mg L−1 to 400 mg L−1 matrine added into gn5f, respectively. Each value is the mean of five replicates and vertical bars give standard errors (SE) of the means. Different lowercase letters indicating that the mean are statistically different according to the Duncan test (P < 0.05).
Discussion
The concept of biological nitrogen fixation by endophytic has led to investigations on the potential uses of endophytic rhizobia that colonize legume plants. Our present study demonstrated that the two FTR (12531f and gn5f) could endogenously colonize roots, stems, and leaves of alfalfa seedlings. The key point of the two FTR is efficient colonization of root, since the densities remain stable till D28 (), which provides a good condition for the two FTR migrate to and colonize alfalfa aerial tissues.
The two FTR exhibited the same spatial heterogeneity that high population densities were detected in the roots, while lower densities were found in aerial tissues. In aerial tissues, the population densities in lower tissues were higher than upper tissues and also in stems were higher than that in leaves (). These results are in line with Wei et al. (Citation2014) that most green fluorescent protein (GFP) tagged nitrogen fixing bacteria in sugarcane plants were located in the roots, followed by in the leaf sheaths and finally in the leaves. It indicates that there may exist selection barrier when FTR migrated from roots to stems and leaves, the first barrier exists between roots and stems that fewer FTR in stems than in roots; the second barrier exists between stems and leave that fewer FTR in leaves than in stems; these results also suggest that the two FTR migration channel may from roots to stems finally to leaves, but need to be demonstrated the exact channel by confocal fluorescent microscopy. The larger densities of FTR in stems than in leaves may also because of the leaves are not suitable for inhabiting or the detection times are not suitable for FTR colonizing leaves. The two FTR also exhibited the same temporal heterogeneity that most densities were detected before D21, then the densities decreased and few were noticeable in aerials tissues. In our previous study (Miao et al. Citation2017), we also found most FTR (12531f and gn5f) could colonize lower leaves at D15 then the densities declined. Chi et al. (Citation2005) also found the same temporal heterogeneity that the gfp-tagged rhizobia A.caulinodans ORS571 densities reached highest at D15, and followed by maintenance of persistent or declining within rice roots and leaves. Matrine could degrade after D14 (Yao Citation2016), so weakened the inhibition effect on air-oriented and resident micro-organisms, thus increased the competition between endophytic rhizobia (12531f and gn5f) and resident plant-associated microbial community for nutrition and space, thus lead to lower rhizobia colonization densities. May also because of the slow growth of the two FTR after D14 (supplemental Figure). These results indicate that the colonization of rhizobia in alfalfa seedlings is a dynamically distributed process, the rhizobia entrance roots and can ascend migrate to aerial stems and leaves and maintain a certain population densities.
Among all the treatments, the addition of matrine enhanced the migration ability and colonization densities of FTR in alfalfa tissues compared with control and single inoculation treatments. Matrine at 100 mg L−1 and 300 mg L−1 induced more gn5f and 12531f, respectively, to migrate from the roots and colonize the aerial stems and leaves (). Matrine promoted the two FTR colonizing and allocating into the plant tissues of alfalfa, which might through the following mechanisms of action, for example, (i) increased the production of secondary metabolites such as indoleacetic acid (IAA) and gibberellin (GA), (ii) weakened the competition between rhizoia (12531f and gn5f) and resident plant-associated microbial community for nutrition and space.
Xiong (Citation2015) had speculated that the mechanisms of matrine promoting the elongation of cells were to increase the content of IAA and GA of tomato seedlings. Nevertheless, for matrine exactly effect on plants hormone, there is no any report yet. Adding GA to plant could enhance the content of IAA, while IAA is not only beneficial to plant growth, but also beneficial to microbes colonizing plant tissues. IAA could inhibit the activity of plant defense system enzymes, such as chitinase, β-1,3 glucanase, which make bacteria more likely to colonize plants (Fuentes-Ramirez et al. Citation1993). Zhang et al. (Citation2013) had found IAA could improve the growth of rhizobia (12531f and gn5f) and Li et al. (Citation2015) also found IAA is helpful for them to migrate and colonize inside alfalfa tissues. We had also found GA could improve the migration and colonization of 12531f and gn5f in various alfalfa tissues (unpublished).
Plants are normally associated with diverse microorganisms, such as bacteria and fungi (Liu et al. Citation2016); they may compete with the effective endophytic rhizobia for the limited space and nutrients provided by the host plants. These competitions will decrease the colonization densities and the proliferation ability of the effective endophytic rhizobia. Matrine has the ability to inhibit soil-oriented and air-oriented micro-organisms (Huo Citation2014). In this paper, the matrine levels were lower than 400 mg L−1, the two rhizobia (12531f and gn5f) could grow without significant inhibition, while the microbes in the air and other endogenous microbes in alfalfa might be inhibited. That is, matrine weakens the competition between endophytic rhizobia (12531f and gn5f) and resident plant-associated microbial community for nutrition and space, thus lead to high rhizobia colonization densities.
When rhizobia infect host plant, the defense mechanical barrier, the adaptability of microbes to the plant microhabitat, and the signal recognition all induce the plant’s defense responses (Li et al. Citation2015) and resulting in decrease the ascending migration and colonization densities. In this experiment, matrine might also decrease the selection barrier of the plants to ward off the two FTR, but the mechanisms of matrine improving FTR migration and colonization are needed to confirm.
Legumes under natural growth conditions could develop nodules, but many of which are ineffective (Huang et al. Citation2005). In this study, the alfalfa inoculated with the two FTR with or without the addition of matrine could grow better than the un-inoculated control alfalfa. Among all the inoculation treatments, with the addition of 100 mg L−1 and 300 mg L−1 matrine into gn5f and 12531f, respectively, enhanced alfalfa seedling nodules (), leaf chlorophyll content () and total N percentage (), with a concomitant increase in relative growth rate (), root length () and biomass () more than did the other inoculation treatments, even when the rhizobia cannot be found within these same plant tissues. Endophytic rhizobia are known to synthesize growth stimulating plant hormones. Enhanced plant growth could be attributed to the production of phytohormones, such as IAA and GA, which increase photosynthesis and enhance the biomass (Yanni et al. Citation2001; Chi et al. Citation2005). These results were consistent with Huo (Citation2014), who reported that matrine had the same effect as rhizobia inoculation alone in increasing total nitrogen content, nitrogenase activity, nodule number, root length, leaf number, and chlorophyll content. The two FTR expressed their nitrogen fixation potential better when colonized alfalfa tissues with matrine treatment, the results were consistent with the above speculates that matrine could increase the content of IAA and GA and decrease the competition between rhizobia (12531f and gn5f) and indigenous plant-associated microbial community for nutrition and space, thus promoted FTR colonizing alfalfa tissues and enhanced alfalfa growth.
In this experiment, matrine at 100 mg L−1 and 300 mg L−1 induce more gn5f and 12531f, respectively, to migrate from the roots and colonize the aerial stems and leaves, and also enhanced alfalfa growth. The different matrine levels for the two FTR might result from the different genotype and metabolism. Some studies have been conducted to explore the inhibitory effect of matrine on microbes and found that the inhibitory effect of matrine on microbe growth varied from each strains, and is based on microbe type. Yang et al. (Citation2008) found that EC50 (50% effective concentration) values of matrine on Marssonina brunnea, Cladosporium oxysporum and Sphaeropsis sapinea were 123, 272 and 428 ug mL−1, respectively. The other reason is that the symbiosis capability and matching degree between rhizobia and alfalfa plants were different, gn5f was isolated from the alfalfa seeds used in the experiment, which is more adapt to the alfalfa plant environment; whereas 12531f was obtained from melilotus which may encounter more plant defenses and other stress responses. Therefore, 300 mg L−1 of matrine enhanced more 12531f migrate and colonize alfalfa tissues and alfalfa plant growth, while for gn5f only needed 100 mg L−1.
It should be noted that the results of this study were only based on alfalfa seedlings cultured in vitro condition, the migration and colonization dynamics in field alfalfa, especially reproductive stage alfalfa, should be further studied. Only two rhizobia strains and one alfalfa variety (Gannong No.5) were used in this study, a rhizobium strain cannot result in the same inoculation outcome to all alfalfa varieties, even under the same culture conditions and a strain might be proven beneficial to some varieties, but poor to others (Kan and Chen Citation2002). Thus, additional rhizobia strains and alfalfa varieties need to be investigated for a more comprehensive characterization of rhizobia migration and colonization dynamics in alfalfa. Such researches will be important for understanding of plant microbe interaction between endophytic rhizobia and host plants.
In summary, several important features of endophytic migration and colonization by FTR have been characterized in this work. The exogenous application of suitable matrine levels can promote the two rhizobia (gn5f and 12531f) migration and colonization from roots to aerial tissues. The best matrine levels for different FTR might greatly differ. 100 mg L−1 matrine for gn5f and 300 mg L−1 matrine for 12531f were more suitable for their migrating from the roots to the aerial tissues and also promoting alfalfa seedling growth compared with control and single inoculation treatment. Therefore, matrine is an essential material at the inoculation of rhizobia for better enhancement of migration and colonization in seedling alfalfa and promoting alfalfa growth.
Geolocation information
The greenhouse experiment was conducted College of Grassland Science, GSAU, on 23 November 2014. The greenhouse was located in northwestern Lanzhou (E103°41′, N36°05′) at an altitude of 1525 m above sea level (mean annual precipitation: 330 mm; mean annual sunshine duration: 2446 h; average annual evaporation: 1650 mm; annual frost-free period: > 200 days). The average temperature of the house was 20.0°C.
Acknowledgements
We want to thank Lin Xu from Agriculture and Biotechnology Department, Hexi University for revised the manuscript; Tou Zhou, Yan Li, Wen-juan Kang from the College of Grassland Science, Gansu Agricultural University (GSAU), who helped with lab work; Jian-feng Li, Shu-qing Zhang from the Institute of Soil and Environment Bioremediation in Karst Habitats, Guizhou Normal College, and Ping-hui Huo from the Institute of Tropical Pratacultural Science, Lingnan Normal University for their early involvement in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Yang-yang Miao is a Ph.D. candidate at Key Laboratory for Grassland Ecosystem of Ministry of Education, Pratacultural Engineering Laboratory of Gansu Province, Sino-U.S. Centers for Grazing Land Ecosystem Sustainability, College of Grassland Science, Gansu Agricultural University, Lanzhou 730070, People’s Republic of China. She has co-authored over 5 scientific papers on the fields of plant endophytic rhizoia, soil and plant azotobacter, salt stress. Presently, she is involved in research in migration and colonization of rhizobia in various alfalfa plant tissues.
Shang-li Shi is a professor at Key Laboratory for Grassland Ecosystem of Ministry of Education, Pratacultural Engineering Laboratory of Gansu Province, Sino-U.S. Centers for Grazing Land Ecosystem Sustainability, College of Grassland Science, Gansu Agricultural University, Lanzhou 730070, People’s Republic of China. He has co-authored over 5 scientific papers on the fields of germplasm resources and breeding of forage grass varieties, alfalfa cultivation technique, alfalfa endophytic rhizobia exploiture and application. Presently he is involved in mechanism of alfalfa drought, diversity of alfalfa endophytic rhizobia, mechanism of alfalfa rotation and continuous cropping.
Jian-guo Zhang is a professor at the Department of Grassland Science, South China Agricultural University, Guangzhou 510642, People’s Republic of China. He has co-authored over 5 scientific papers on the fields of plant forage production, forage processing, and applied microbiology. Presently he is involved in research in microbiology improving the quality of silages.
Osama Abdalla Mohamad is visiting scholar at Key Laboratory of Biogeography and Bioresource in Arid Land, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi, 830011, People’s Republic of China. He has co-authored over 5 scientific papers on the fields of microbiology, Agricultural plant science, environmental science, and waste management. Presently, he is involved in research in plant endophytic bacteria.
ORCID
Shang-li Shi http://orcid.org/0000-0002-3304-8030
Additional information
Funding
References
- Afzal M, Khan S, Iqbal S, Mirza MS, Khan MQ. 2013. Inoculation method affects colonization and activity of Burkholderia phytofirmans PsJN during phytore mediation of diesel-contaminated soil. Int Biodeter Biodegr. 85:331–336. doi: 10.1016/j.ibiod.2013.08.022
- Bertrand A, Prevost D, Bigras FJ, Castonguay Y. 2007. Elevated atmospheric CO2 and strain of rhizobium alter freezing tolerance and cold-induced molecular changes in Alfalfa (Medicago sativa). Ann Bot. 99:275–284. doi: 10.1093/aob/mcl254
- Chi F, Shen SH, Cheng HP, Jing YX. 2005. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl Environ Microbiol. 71:7271–7278. doi: 10.1128/AEM.71.11.7271-7278.2005
- Dang MT. 2003. [Nitrogen fertilizer pollution and the countermeasures]. J Weinan Teachers College. 18:50–51. Chinese.
- Fuentes-Ramirez EL, Jimenez-Salgado T, Abarca-Ocampo R, Caballero-Mellado J. 1993. Acetobacter diazotrophicus, an indoleacetic acid producing bacterium isolated from sugarcane cultivars of Mexico. Plant Soil. 154:145–150. doi: 10.1007/BF00012519
- Gao SH, Xu HY, Wang HG, Zhao BG. 2013. [Research progress of the growth promoting effect of alkaloids]. Forestry Sci Technol. 40:39–42. Chinese.
- Guo LZ, Zhang HT, He YH, Cai Q, Huang GB. 2012. [Effect of rhizobium on crop growth and nitrogen nutrition of a pea/maize intercropping system]. Acta Pratac Sin. 21:46–47. Chinese.
- Hartley EJ, Gemell LG, Deaker R. 2012. Some factors that contribute to poor survival of rhizobia on pre-inoculated legume seed. Crop Pasture Sci. 63:858–865. doi: 10.1071/CP12132
- Hoagland DR, Arnon DS. 1950. The water culture method for growing plants without soil. Berkeley, CA: University of California, College of Agriculture, Agricultural Experiment Station.
- Hu YS, Li CX, Sun FL, Wu K, Jia X C. 2007. [Improved culturability of soil bacteria using proper combination with various culturing medium]. Acta Microbiol Sin. 47:882–887. Chinese.
- Huang X, Wang YQ, Liu JX, Yu ZZ, Bao MD, Chen WH, Shi JT, Sun HX. 2005. [Effect of inoculating rhizobial strains on nodulation and biomass yield of alfalfa, Medicago sativa]. Acta Agriculturae Zhe jiangensis. 17:391–394. Chinese.
- Huo PH. 2014. Antimicrobial resistant rhizobia screening and effect verification of undesired microbe control in the prepared rhizobia inoculant [Dissertation]. Lanzhou: Gansu Agricultural University.
- Ji KX, Chi F, Yang MF, Shen SH, Jing YX, Dazzo FB, Cheng HP. 2010. Movement of rhizobia inside tobacco and lifestyle alternation from endophytes to free-living rhizobia on leaves. J Microbiol Biotechnol. 20:238–244. doi: 10.4014/jmb.1003.03014
- Kan FL, Chen WX. 2002. [Comparative analysis of numerical taxonomy and 16S rDNA PCR-RFLP of fast growing rhizobia isolated from western China]. Microbiol. 29:1–8. Chinese.
- Li JF, Shi SL, Zhang SQ. 2010. [Effects of the pH value of an acid environment on early growth and physiology of Medicago sativa]. Acta Pratac Sinca. 19:47–54. Chinese.
- Li JF, Zhang SQ, Shi SL, Huo PH. 2009. [Position and quantity of endogensis rhizobia in alfalfa plant]. Chin J Eco-Agr. 17:1200–1205. Chinese. doi: 10.3724/SP.J.1011.2009.01200
- Li JF, Zhang SQ, Shi SL, Miao YY, Huo PH. 2015. [Infection and migration of marked rhizobia in alfalfa bud seedlings under the action of exogenous substance]. Acta Agres Sin. 23:1259–1264. Chinese.
- Liu PF, Wang HY. 2012. [Effects of organic and chemical fertilizer applications on the diversity of soybean rhizobia]. Chin J Ecol. 31:1468–1472. Chinese.
- Liu TZ, Zhang JM, Mao ZW, Li RJ. 2016. Influence of endophytic diazotroph and nitrogen fertilization on the growth and turf quality of ‘TifEagle’ bermudagrass. Eur J Hortic Sci. 81:227–233. doi: 10.17660/eJHS.2016/81.4.6
- Miao YY, Zhou T, Shi SL, Kang WJ, Zhang YT. 2017. [Effect of boron on migration and colonization by rhizobia and seedling growth in Medicago sativa]. Acta Pratac Sin. 26:120–133. Chinese.
- Mohamed AB, Haddad M, Ferchichi A. 2009. Diversity of lucerne (Medicago sativa L.) populations in south Tunisia. Pak J Bot. 41:2851–2861.
- Ortiz-Santaliestra ME, Marco A, Fernndez MJ, Lizana M. 2006. Influence of developmental stage on sensitivity to ammonium nitrate of aquatic stages of amphibians. Environ Toxix Chem. 25:105–111. doi: 10.1897/05-023R.1
- Peoples MB, Brockwell J, Herridge DF, Rochester IJ, Alves BJR, Urquiaga S, Boddey RM, Dakora FD, Bhattarai S, Maskey SL, et al. 2009. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis. 48:1–17. doi: 10.1007/BF03179980
- Peralta H, Aguilar A, Díaz R, Mora Y, Martínez-Batallar G, Salazar E, Vargas-Lagunas EC, Martínez E, Encarnación S, Girard L, Mora J. 2016. Genomic studies of nitrogen-fixing rhizobial strains from Phaseolus vulgaris seeds and nodules. BMC Genomics. 17:711. doi: 10.1186/s12864-016-3053-z
- Shetta ND, Alshahrani TS. 2016. The symbiotic efficiency of legume tree rhizobia for host range legumes in central Saudi Arabia. Int J Agri Biol. 18:851–857. doi: 10.17957/IJAB/15.0183
- Wang L, Bai YL. 2005. [Correlation between corn leaf spectral reflectance and leaf total nitrogen and chlorophyll content under different nitrogen level]. Scientia Agri Sin. 38:2268–2276. Chinese.
- Wei CY, Xing YX, Mo Y, Lin L, Yang LT, Hu CJ, Li YR. 2014. [Colonization of nitrogen fixing bacterial strain klebsiella sp. DX120E labeled with green fluorescent protein (GFP) gene within sugarcane plants]. Acta Agron Sin. 40:1132–1139. Chinese. doi: 10.3724/SP.J.1006.2014.01132
- Xie H, Hu X, Zhang CR, Chen YF, Huang X, Huang XL. 2013. Molecular characterization of a stress-related Gene MsTPP in relation to somatic embryogenesis of alfalfa. Pak J Bot. 45:1285–1291.
- Xiong X. 2015. Regulation of tomato growth by alkaloids contained matrine [Dissertation]. Yangling: North West Agriculture and Forestry University.
- Yang XY, Zhao BG, Ju YW. 2008. [Antifungal and activities and synergetic tests of matrine and oxymatrine to some tree pathogens]. J Nanjing For U (Nat Sci eds). 32:70–82. Chinese.
- Yanni YG, Rizk RY, El-Fattah FKA, Squartini A, Corich V, Giacomini A, de Bruijn F, Rademaker J, Maya-Flores J, Ostrom P P, et al. 2001. The beneficial plant growth-promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Aust J Plant Physiol. 28:845–870.
- Yao M. 2016. Behavors of matrine in soil and water environments [Dissertation]. Yangling: North West Agriculture and Forestry University.
- Zhang JY. 2008. Soil microbial mass and diversity under long-term different fertilization utilizations [Dissertation]. Wuhan: Huazhong Agricultural University.
- Zhang LJ, Wang TT, Wen XM, Wei Y, Peng XC, Li H, Wei L. 2007. Effect of matrine on HeLa cell adhesion and migration. Eer J Pharmacol. 563:69–76. doi: 10.1016/j.ejphar.2007.01.073
- Zhang SQ, Li JF, Chen LY, Shi SL, Miao YY. 2015. [Establishment and screen of Cyan Fluorescent Protein labeled strains of alfalfa rhizobia]. Pratac Sci. 32:711–718. Chinese.
- Zhang SQ, Shi SL, Chen LY, Miao YY, Li JF, Huo PH. 2013. [The effect of LaCl3, IAA and plant sap on the growth and proliferation of fluorescence marked rhizobia]. Grassland Turf. 33:57–61. Chinese.
- Zhang ZM, Chen HK, Li FL. 1991. [Construction of gene library and isolation of praz15 containing complete nodulation genes in rhizobium astragali]. Chin J Biotechnol. 7:213–219. Chinese.
- Zhou MM, Jiang X, Jin DY, Lv JZ. 2016. [Effects on seed germination and growth with matrine in wheat]. Hortic seed. 2:28–29. 45. Chinese.
