ABSTRACT
Purpose: Root and root hairs of plants have been intensively studied in solution culture; however, correlation of such measurements in solution culture with development in soil is poorly understood. Therefore, the aim of this study is to study whether root and root hairs grown in solution culture can predict their behavior in soil and their correlation with macro- and micronutrients uptake of wheat genotypes.
Materials and methods: The growth of roots and root hairs as well as uptake of macro- and micronutrients of six spring wheat varieties was compared in solution culture under P stress and P abundance and in a low fertility soil.
Results and conclusions: Root length and surface area under P stress were significantly positively correlated with that in the low fertility soil, while no such correlation was apparent for root hair length and density. In absolute terms, the root length, surface area, root hair length and density of spring wheat varieties were substantially higher in soil than in solution culture, while the concentration and uptake of macro- and micronutrients in soil differed from solution culture in a complex way. The early uptake of macro- and micronutrients was intimately associated with root length and surface area as well as root hair length and density in soil but not in solution culture. Therefore, root length rather than root hair traits in low-P solution may be used to screen early root growth vigor in soil and thereby high nutrient uptake of wheat in low fertility soil.
Introduction
Plant root systems anchor plants to the soil and serve many essential functions including water and nutrient uptake. They are becoming a research hotspot particularly in the sense of roots to availability of some nutrients which leads to nutrient-specific modifications to root and root hair formation (Giehl et al. Citation2014). Hydroponics is a convenient and quick method for studying root elongation and root hair development as well as whole plant physiology in nutrient solution only or in an inert medium without soil in the laboratory and under controlled climatic environment. Therefore, nutrient solution is widely used for root studies, in particular for root hair research without background interference from soil. The formation of lateral roots and root hairs determines root system architecture and the capacity of plants to absorb water and nutrients from soil in which nutrient resources are limiting (López-Bucio et al. Citation2003). Earlier studies have demonstrated that long and dense root hairs enhance phosphorus (P) acquisition for a wide range of plant species in solution culture (Bates and Lynch Citation1996, Citation2000a, Citation2000b; Ma et al. Citation2001) and in soil (Gahoonia and Nielsen Citation1998, Citation2003) under laboratory conditions, and in the field (Gahoonia et al. Citation1999; Gahoonia and Nielsen Citation2004; Zhu et al. Citation2010; Miguel et al. Citation2015). Root hair development is also regulated in response to nitrate (Bhat et al. Citation1979; Foehse and Jungk Citation1983), iron (Schmidt et al. Citation2000), manganese and zinc (Ma et al. Citation2001).
The uptake of nutrients depends upon both the supply of available nutrients in the rooting media and the root system (Atkinson Citation2000). The factors controlling nutrient uptake are very different in a solution culture where nutrients elements are easily available to the roots for uptake compared to soil condition. In soil conditions, due to the wide spatiotemporal variability of physiochemical conditions, root system properties and thereby the potential uptake of nutrients can differ considerably compared with those in solution culture. The mobility of nutrients in soil solution is strongly restricted and depletion zones form around the roots (Mackay and Barber Citation1984). Nutrients will move from the bulk soil to the root surface to replace those taken up by plants, which never occur in solution culture. Therefore, plant species may show different root and root hair traits when grown in solution compared to soil. In barley (Hordeum vulgare L.) and wheat cultivars (Triticum aestivum L.), Gahoonia et al. (Citation1999) reported that the root hair length was slightly longer in the soil than in solution culture; however, the root hair density was slightly lower in soil than in solution culture, and the variations in root hairs between the cultivars were similar regardless of growth media. In corn (Zea mays L.), Mackay and Barber (Citation1984) found that root length and surface area and the percentage of total root length with root hairs were greater in soil than in solution culture, and they concluded that the usefulness of solution culture was limited in determining P uptake and its correlation with root hair growth. Similarly, Hayes et al. (Citation2004) noted that the uptake of P was significantly different in soil compared with that in solution culture, and they proposed that solution culture was not reliable for investigating P uptake in wheat genotypes.
Studies in solution under laboratory conditions offer the opportunity to investigate root growth traits and root morphology in controlled environments. Large differences in root morphology and root hairs have been observed between genotypes of plant species in experiments with solution culture (Bates and Lynch Citation1996, Citation2000a, Citation2000b; Gahoonia et al. Citation1999; Ma et al. Citation2001). However, root and root hair growth as well as uptake of nutrient elements can be substantially altered in soil where some immobile nutrients, e.g. P cannot easily diffuse to the root surface, and this merit investigation (Niu et al. Citation2013). Therefore, the objective of the present study is to study whether root and root hairs grown in solution culture can predict their behavior in soil and their correlation with macro- and micronutrient uptake of wheat genotypes.
Materials and methods
Solution culture experiment
Spring wheat (Triticum aestivum L.) was cultivated hydroponically in 4-L light-impermeable polyethylene buckets, with three individual plants in each bucket. Seedlings were germinated on vermiculite and then transferred to hydroponic culture. Plants were grown in a complete randomized design in a climate-controlled glasshouse at the Faculty of Science, University of Copenhagen, Frederiksberg with 18/15°C day/night temperature at a photon flux density of 300 μmol m−2 s−1, under a 16-h day/8-h night cycle. The culture solutions were continually aerated and their pH was maintained between 5.8–6.0 with daily additions of KOH or HCl. All nutrient solutions were replaced every five days. Based on an initial screening of 19 genotypes under P stress condition (2 μM P), six spring wheat genotypes were selected for the experiment based on root and root hair: A35-213, April Bearded, Dacke, Farah, Hankkijan Tapio and Hindy62 (Wang et al. Citation2016). Selected varieties were exposed to both P stress (2 μM P) and P abundance (200 μM P) as KH2PO4 for 24 days after transplanting. The nutrient solution was composed of 2 μM or 200 μM KH2PO4, 0.2 mM K2SO4, 0.3 mM MgSO4·7H2O, 0.1 mM NaCl, 0.3 mM Mg (NO3)2·6H2O, 0.9 mM Ca (NO3)2·4H2O, 0.6 mM KNO3, 1 μM MnCl2·4H2O, 0.8 μM Na2MoO4, 0.7 μM ZnCl2, 0.8 μM CuSO4·5H2O, 2 μM H2BO3, 1 μM NiSO4·6H2O and 50 μM Fe-EDTA. Each treatment was replicated four times. Plants were harvested at 25 days after transplanting. The shoots and all the roots of plants were washed in double deionized water. The roots were conserved in 25% v/v ethanol immediately after washing.
Soil experiment
The same six genotypes of spring wheat were grown in a soil column experiment under a glass roof shelter at the experimental farm of Faculty of Science, University of Copenhagen, Taastrup. Soil for the experiment was collected from the upper horizon (0–30 cm) from unfertilized field plots grown with cereals in a long-term experimental farm of University of Copenhagen (Poulsen et al. Citation2013). The unfertilized treatment plots received no external fertilizers, but a clover grass mixture was grown each autumn and incorporated as a green manure since 2003. It was classified as sandy loam containing total C of 14.8 g kg−1, total N of 1.52 g kg−1 and total P of 0.28 g kg−1. The soil taken was air-dried, passed through a 2-mm sieve and thoroughly mixed with an equal amount of purified fine sand. The tubes were 0.5-m high transparent PVC tubes with an inner diameter of 14.5 cm. Tube bottoms were sealed with mesh to allow free drainage. The tubes were filled with 11.7 kg of air-dried soil-sand mixture to arrive at a bulk density of 1.4 g cm−3. To create a soil environment limiting only in P, K, S and micronutrients, nitrogen (N) fertilizer at the rate of 100 kg ha−1 was applied. Each treatment was replicated four times, with two plants in each tube. The tubes were watered twice a week. The plants were harvested at 30 days after germination (DAG) and they were at the similar growth stage as in the solution culture experiment. All root systems were immediately washed out; preliminary tests had shown that it was possible to isolate the roots and root hairs from all adhering soil particles.
Sampling, measurements and analyses
All the roots were scanned using a scanner (Epson Perfection V700, CA, USA) and analyzed by WinRHIZO image analysis system (Regents Instruments Inc., Quebec city, Canada) for root length and surface area. Randomly selected root segments in the root hair zone from each replicate were placed in a film of water in petri dishes. Root hair images with 600 DPI were captured for all the genotypes on the main root axis, first order and second order roots, using a video camera fitted to a microscope (Leica Microsystems GmbH, Wetzlar, Germany) at 2.5 × magnification interfaced with a computer image grabber board. Root hairs were analyzed by recalling the images using ImageJ software. Root hair length (RHL) was measured at more than 100 randomly selected root hairs for each replicate. Root hair density (RHD) was determined as the number of root hairs per mm root length.
The dry biomass (DM) of shoot and root samples was determined after oven drying to constant weight at 70°C. After grinding, the samples were digested in a microwave oven with HNO3 and HCl in a 1:3 v:v mixture, and then analyzed for P, K, Ca, Mg, S, Fe, Mn, Cu, Zn and B using inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Optima 5300 DV, Perkin Elmer Inc., USA). Total N was analyzed using the Dumas dry combustion method in a system consisting of an ANCA-SL Elemental Analyser coupled to a 20–20 Mass Spectrometer (Sercon Instruments, Crewe, UK).
Statistical analysis
The data were analyzed by analysis of variance (ANOVA) using SAS (SAS Institute Inc., 2012) at a significance level of 5%. Linear regression analyses and Pearson correlation were used to determine the relationships between the measured parameters.
Results
There were greater differences in root traits and nutrient uptake among genotypes under the P abundant hydroponic condition; however, these parameters were weakly correlated with the root development in soil. Therefore, the data from the P abundant solution is only indicated specifically when relevant.
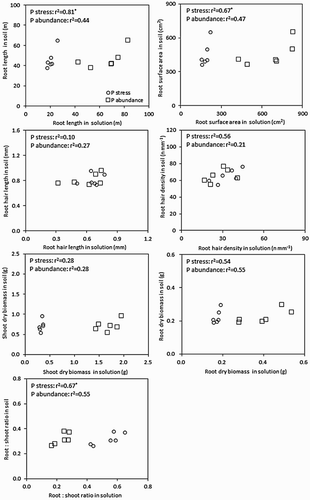
Root and root hair traits
There were significant differences in total root length and surface area as well as root hair length and density among genotypes and between growing media (). Root length and surface area doubled when plants were grown in soil compared to low P solution culture; however, they were higher under P abundant solution than in soil. Root hair length and density was significantly higher in soil than in solution culture. Compared to root hair length, the increase of root hair density was greater going from hydroponic to soil growth condition. There was a significant positive linear relationship in root length and surface area between P stress solution and soil. However, root hair length and density under the P stress or P abundance showed no such linear correlation with those in soil.
Plant growth and biomass allocation
There were significant differences in dry biomass of shoots and roots, and root:shoot ratio among genotypes and between growing media. The dry biomass of shoots and roots were significantly higher in soil than in P stress solution, but they were lower than in P abundant solution ().The root:shoot ratio was increased in P stress solution, while they were decreased in P abundant solution compared with soil growth condition. Significant positive linear relationship was found between root-shoot ratio in P stress solution and soil.
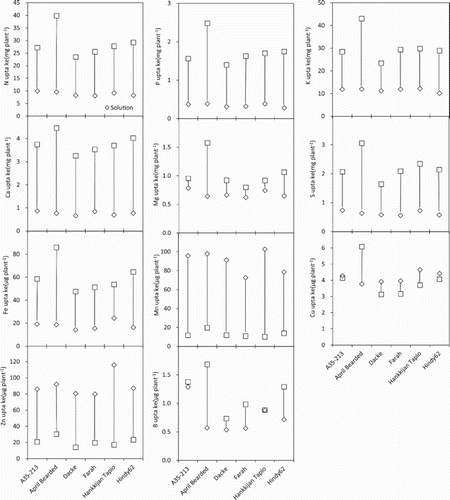
Concentrations and uptake of macro- and micronutrients
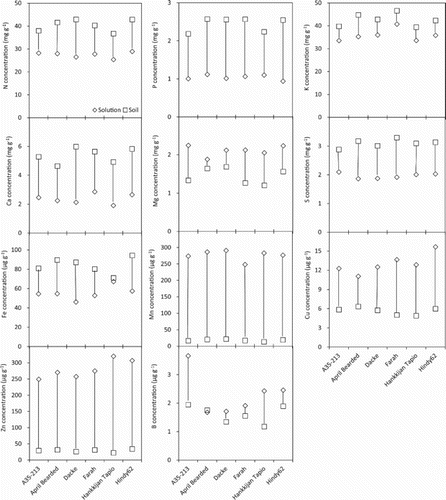
In spite of the higher dry biomass of plants in the soil experiment compared with solution culture, they also showed higher concentrations of some nutrients. Significant differences in concentration of macro- and micronutrients were observed between growth media (). Concentrations of N, P, K, Ca, S and Fe in shoots were significantly higher in soil than in solution culture, whereas the concentrations of Mg, Mn, Cu, Zn and B were significantly higher in solution culture than in soil. In particular the concentrations of Mn and Zn were increased by 10-fold in shoots of plants from solution culture compared to those from soil.
Figure 2. Macro- and micronutrient concentrations in shoot dry biomass of spring wheat genotypes in P stress solution culture and in soil. Lines between the data points denote the variations of nutrient concentrations between solution culture and soil.
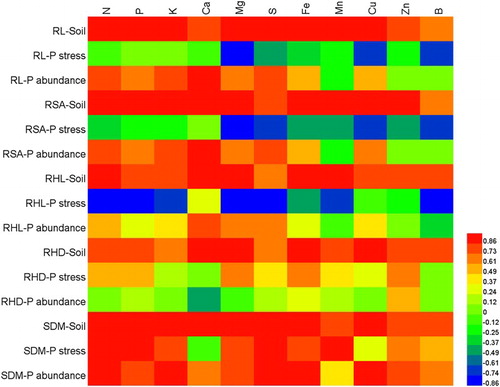
The uptake of all macro- and micronutrients differed significantly among genotypes and between growing media (). The uptake of macronutrient elements as well as Fe and B was significantly higher in soil than in solution culture, whereas significantly higher uptake of Mn, Cu and Zn was observed in solution culture compared with that in soil. In soil, there were significant positive relationships between root length and surface area and uptake of all macro- and micro-nutrients, and also between root hair length and density and uptake of most of the macro- and micronutrients (). However, in solution culture, no such correlation was found between uptake of macro- and micronutrient elements and root length, surface area, root hair length and density. In addition, shoot dry biomass was significantly positively correlated with uptake of most of the macro- and micronutrients in soil and with some of the macro- and micronutrients in solution culture.
Discussion
Can root and root hair growth of wheat genotypes grown in solution culture predict their behavior in soil?
Previous studies have shown that many plant species show reduced root hair development in solution culture when compared to soil (Mackay and Barber Citation1984; Gahoonia and Nielsen Citation1997; Ahn and Schachtman Citation2004; Genc et al. Citation2007). In the current study, a significantly positive linear relationship was found for root length and surface area between wheat plants from P stress solution and soil (); however, the same correlation was not found for root hair length and density between these two growth media. This result indicates that solution culture at low P concentration is able to assist in screening root growth vigor, however, not root hair development among genotypes in less fertile soil for wheat. Therefore, high early root length in low-P solution culture predicts larger early root development in soil, which is positively correlated with nutrient uptake ().
However, in real terms the root length, surface area, root hair length and density of wheat genotypes were significantly higher in soil than in P stress solution culture (). There may be a range of reasons for these differences in root and root hair development between solution and soil culture. Firstly, the differences in root length and surface area could be related to growth environments in solution culture and soil, e.g. duration of plant growth, temperature and light conditions. Secondly, the physical properties, e.g. soil texture, structure, mechanical impedance, aeration and temperature, affect root growth and morphology (Kar et al. Citation1976; Glinski and Lipiec Citation1990; Bengough et al. Citation2006). Thirdly, the distribution of root depends on the distribution and mobility of water and nutrients in the soil (Bengough et al. Citation2006). The concentration of most nutrients in solution culture is generally high despite that in some cases buffer solids have been used to mimic the nutrient release in soil. In contrast, soil solution is highly buffered for many nutrients, maintaining low but stable nutrient concentrations compared to nutrient solution. Fourthly, nutrient supply can affect development of root and root hairs as a result of the differences in ion concentrations in solution and in soil (Forde and Lorenzo Citation2001; Ma et al. Citation2001; Williamson et al. Citation2001). Finally, the roots may sense the water and nutrient conditions, and the developmental responses are adaptive (Forde and Lorenzo Citation2001). When soil nutrient availability is limited, root systems of crops are capable of modifying and acclimating to the surrounding environment (Jackson et al. Citation1990) and thus explore a larger volume of soil; this enable plants to make more efficient use of indigenous soil nutrients (Smith and Smet Citation2012). This adaptation depends on soil water and nutrient heterogeneity and gradients, e.g. soil P concentration (Jackson et al. Citation1990; Williamson et al. Citation2001; Adams et al. Citation2002), which is unlikely to be experienced by plants in solution culture.
Can solution culture be used to identify high nutrient uptake of varieties associated with root and root hairs in soil?
Gahoonia et al. (Citation1999) reported that genotypic variation in root hairs can be assessed with reasonable accuracy in nutrient solution culture, and they proposed that cereal cultivars may be screened using nutrient solution for selecting P efficient barley cultivars in terms of root hairs. However, the current solution based screening of crop genotypes for root and root hair traits was not able to reflect the uptake of nutrients. Hayes et al. (Citation2004) reported that the uptake of P differed significantly when plants were grown in solution culture compared to soil, but the root length of two wheat cultivars was not measured in soil. In the present study, the concentrations and uptake of macro- and micronutrients in soil were significantly different from those in solution culture (). It is noteworthy that the concentrations of almost all the macronutrients were significantly higher in soil than in P stress solution culture, though only the concentration of P was deficient under the P stress solution culture. A possible explanation is that the plants under the P stress condition could accumulate higher amount of starch in the shoots, which can lead to lower nutrient concentrations. On the contrary, the starch content was significantly lower for the plants in P stress solution culture than in soil (data not shown). In addition, the nutrient uptake kinetics might have been modified by both the growing media and low nutrient concentrations (York et al. Citation2016). The specific reasons underlying the lower concentrations of most nutrients for the plants in P stress solution than in soil merit further investigation.
Nutrient uptake is governed by root nutrient uptake capacity, nutrient conditions where plants grow, and above-ground growth creating shoot nutrient demand. The genetic variability in root and root hair traits in wheat plants determines macro- and micronutrient uptake in the low fertility soil. This is evident with April Bearded, which had both the highest total nutrient uptake and the strongest growth of all the genotypes. At the same time it showed some of the highest concentrations of most nutrients, indicating that superior root and root hair traits enable plants to capture higher amounts of macro- and micronutrient elements from the soil in the early growth stages and enhance their uptake in the shoots. Consequently, we observed a significant positive relationship between root and root hair traits and uptake of macro- and micronutrients in soil. In contrast, in solution culture under P stress and P abundant conditions, the correlation between root traits and nutrient uptake was weak, indicating that in the soil where nutrient supply is reduced, root and root hair traits of crop genotypes become critically more important for acquisition of nutrients. Our results have shown that root length rather than root hair traits in low-P solution culture may be able to predict the root system behavior in soil, and that selecting for longer root length in low-P solution may be a way to identify higher growth and nutrient acquisition of plants in moderately fertile soil.
The advantages and disadvantages of solution culture for screening root and root hair traits
The main advantages of hydroponics for root studies are (1) the easy accessibility to investigate root morphology, anatomy, enzymatic activities of roots, root exudates and acidification, and other root and root hair traits as well as root nutrient uptake; since it is difficult and tedious to separate roots from soil particles and accurately measure nutrient concentrations or uptake by roots; (2) a more exact regulation of nutrient profile of the growth medium when compared to soil (3). The effects of individual or multi-nutrient elements or deficiency and their mutual-element interactions or toxicity on plant growth can be determined without interference of soil; and (4) it is an efficient method for screening root and root hair traits with the minimal use of space. However, hydroponics is an artificial system for growing plants, which differs greatly from soil in supplying water and nutrients as well as in the formation of rhizosphere depletion zones of roots. This leads to significant differences in root and root hair behavior associated with nutrient uptake between solution and soil.
Supplementary_material.docx
Download MS Word (34 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Yaosheng Wang is a professor in the Institute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agricultural Sciences (CAAS). The areas of his expertise and main research focus of his group include (1) plant ecophysiology at different levels (molecular, tissue, organ and whole plants) under biotic and abiotic stress, (2) root traits and soil nutrient transformation processes and availability and (3) new agronomic management practices to enhance agricultural water and nutrient use efficiencies. The main research aim is to utilize resources efficiently for agricultural sustainability.
Lars Stoumann Jensen is a professor in the Department of Plant and Environmental Sciences, University of Copenhagen. His major research focus has been on biological soil fertility and the influence of organic matter decomposition processes on nutrient turnover in agroecosystems. He is also working on utilization of agricultural and urban waste, and their fertilizer value and effects on soil quality, gas emission and nutrient losses to the environment.
Jakob Magid is an associate professor in the Department of Plant and Environmental Sciences, University of Copenhagen. His researches mainly focus on nutrient and waste management in land-based production systems, with an emphasis on carbon, nitrogen and phosphorus. His studies also include the possibilities and barriers for increased nutrient and organic matter recycling from urban to peri-urban areas.
ORCID
Yaosheng Wang http://orcid.org/0000-0002-2657-7057
Lars Stoumann Jensen http://orcid.org/0000-0002-1446-2084
Jakob Magid http://orcid.org/0000-0001-5867-0910
Additional information
Funding
References
- Adams MA, Bell TL, Pate JS. 2002. Phosphorus sources and availability modify growth and distribution of root clusters and nodules of native Australian legumes. Plant Cell Environ. 25:837–850. doi: 10.1046/j.1365-3040.2002.00867.x
- Ahn SJ, Shin R, Schachtman DP. 2004. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 134:1135–1145. doi: 10.1104/pp.103.034660
- Atkinson D. 2000. Root characteristics: why and what to measure. In: Smit AL, Bengough AG, Engels C, Noordwijk M, Pellerin S, Van De Geijn SC, editors. Root methods: a handbook. New York: Springer-Verlag Berlin Heidelberg.
- Bates TR, Lynch JP. 1996. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 19:529–538. doi: 10.1111/j.1365-3040.1996.tb00386.x
- Bates TR, Lynch JP. 2000a. Plant growth and phosphorus accumulation of wild type and two root hair mutants of Arabidopsis thaliana (Brassicaceae). Am J Bot. 87:958–963. doi: 10.2307/2656994
- Bates TR, Lynch JP. 2000b. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. Am J Bot. 87:964–970. doi: 10.2307/2656995
- Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA. 2006. Root responses to soil physical conditions: growth dynamics from field to cell. J Exp Bot. 57:437–447. doi: 10.1093/jxb/erj003
- Bhat KKS, Nye PH, Bereton AJ. 1979. The possibility of predicting solute uptake and plant growth responses from independently measured soil and plant characteristics. VI. The growth and uptake of rape in solutions of constant nitrate concentration. Plant Soil. 53:137–167.
- Foehse D, Jungk A. 1983. Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant Soil. 74:359–368. doi: 10.1007/BF02181353
- Forde B, Lorenzo H. 2001. The nutritional control of root development. Plant Soil. 232:51–68. doi: 10.1023/A:1010329902165
- Gahoonia TS, Nielsen NE. 1997. Variation in root hairs of barley cultivars doubled soil phosphorus uptake. Euphytica. 98:177–182.
- Gahoonia TS, Nielsen NE. 1998. Direct evidence on participation of root hairs in phosphorus (32P) uptake from soil. Plant Soil. 198:147–152. doi: 10.1023/A:1004346412006
- Gahoonia TS, Nielsen NE. 2003. Phosphorus (P) uptake and growth of root hairless barley mutant (bald root barley, brb) and wild type in low- and high-P soils. Plant Cell Environ. 26:1759–1766. doi: 10.1046/j.1365-3040.2003.01093.x
- Gahoonia TS, Nielsen NE. 2004. Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil. 262:55–62. doi: 10.1023/B:PLSO.0000037020.58002.ac
- Gahoonia TS, Nielsen NE, Lyshede OB. 1999. Phosphorus acquisition of cereal cultivars in the field at three levels of P fertilization. Plant Soil. 211:269–281. doi: 10.1023/A:1004742032367
- Genc Y, Huang CY, Langridge P. 2007. A study of the role of root morphological traits in growth of barley in zinc-deficient soil. J Exp Bot. 58:2775–2784. doi: 10.1093/jxb/erm142
- Giehl RFH, Gruber BD, von Wirén N. 2014. It’s time to make changes: modulation of root system architecture by nutrient signals. J Exp Bot. 65(3):769–778. doi: 10.1093/jxb/ert421
- Glinski J, Lipiec J. 1990. Soil physical conditions and plant roots. Boca Raton (FL): CRC Press, Inc.
- Hayes JE, Zhu YG, Mimura T, Reid R. 2004. An assessment of the usefulness of solution culture in screening for phosphorus efficiency in wheat. Plant Soil. 261:91–97. doi: 10.1023/B:PLSO.0000035561.00460.8b
- Jackson RB, Manwaring JH, Caldwell MM. 1990. Rapid physiological adjustment of roots to localized soil enrichment. Nature. 344:58–60. doi: 10.1038/344058a0
- Kar S, Varade SB, Subramanyam TK, Ghildyal BP. 1976. Soil physical conditions affecting rice root growth: bulk density and submerged soil temperature regime effects. Agron J. 68:23–26. doi: 10.2134/agronj1976.00021962006800010007x
- López-Bucio J, Cruz-Bamírez A, Herrera-Estrella L. 2003. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 6:280–287. doi: 10.1016/S1369-5266(03)00035-9
- Ma Z, Bielenberg DG, Brown KM, Lynch JP. 2001. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 24:459–467. doi: 10.1046/j.1365-3040.2001.00695.x
- Mackay AD, Barber SA. 1984. Comparison of root and root hair growth in solution and soil culture. J Plant Nutr. 7:1745–1757. doi: 10.1080/01904168409363317
- Miguel MA, Postma JA, Lynch JP. 2015. Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiol. 167:1430–1439. doi: 10.1104/pp.15.00145
- Niu YF, Chai RS, Jin GL, Wang H, Tang CX, Zhang YS. 2013. Responses of root architecture development to low phosphorus availability: a review. Ann Bot. 112:391–408. doi: 10.1093/aob/mcs285
- Poulsen PHB, Magid J, Luxhoi J, de Neergaard A. 2013. Effects of fertilization with urban and agricultural organic wastes in a field trial: waste imprint on soil microbial activity. Soil Biol Biochem. 57:794–802. doi: 10.1016/j.soilbio.2012.02.031
- Schmidt W, Tittel J, Schikora A. 2000. Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol. 122:1109–1118. doi: 10.1104/pp.122.4.1109
- Smith S, Smet ID. 2012. Root system architecture: insights from Arabidopsis and cereal crops. Philos T Roy Soc B. 367:1441–1452. doi: 10.1098/rstb.2011.0234
- Wang YS, Thorup-Kristensen K, Jensen LS, Magid J. 2016. Vigorous root growth is a better indicator of early nutrient uptake than root hair traits in spring wheat grown under low fertility. Front Plant Sci. 7:865.
- Williamson LC, Sebastien PCP, Fitter AH, Ottoline Leyser HM. 2001. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 126:875–882. doi: 10.1104/pp.126.2.875
- York LM, Silberbush M, Lynch JP. 2016. Spatiotemporal variation of nitrate uptake kinetics within the maize (Zea mays L.) root system is associated with greater nitrate uptake and interactions with architecture phenes. J Exp Bot. 67:3763–3775. doi: 10.1093/jxb/erw133
- Zhu J, Zhang C, Lynch JP. 2010. The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Funct Plant Biol. 37:313–322. doi: 10.1071/FP09197