ABSTRACT
Purpose: Citrus white snail, Helicella candeharica Pfeiffer (Panpulmonata: Helicidae) is one of the most important orchard pests. In this study, the effectiveness of mineral oil was compared with molluscicide baits such as metaldehyde, ferricole (iron phosphate) and a snail-repellent paint in a commercial citrus orchard in northern Iran to reduce access of citrus white snails to citrus trees.
Materials and methods: The number of snails on citrus trees was monitored and counted 10 days after the application of the treatments, and at an interval of 6–8 days up to harvest time.
Results: In the first study, the mineral oil and repellent paint treatments reduced a number of snails best. In the second study, using metaldehyde and mineral oil barrier, again the mineral oil barrier reduced snails best. The cost of each treatment during one season per hectare was calculated at 55, 153, 124 and 120 $/ha for mineral oil, iron phosphate, snail-repellent paint and metaldehyde, respectively.
Conclusions: Mineral oil is an effective alternative for chemical compounds for reducing access by H. candeharica to citrus trees.
Introduction
Snails are one of the important agricultural pests around the world, especially in humid regions (Kiss and Magnin, Citation2003).
Snail control using chemical pesticides is still one of the most effective methods (Heiba et al. Citation2002; El-Shahaat et al. Citation2005, Citation2009). Using molluscicide baits such as metaldehyde and outdated pesticides including carbaryl and lindane are common methods of controlling these pests in citrus orchards (Damavandian Citation2016). Based on Gimingham (Citation1940), metaldehyde has been the most effective chemical control for snails since introduced as molluscicides bait in 1934.
Many species of predators such as beetles in the families, Carabidae, Staphylinidae, Lampyridae and Silphidae feed on snails (Symondson Citation2004). The soil-inhabiting beetles (Carabidae) are the principal predator of snails (Helyer et al. Citation2003). However, due to the high use of pesticides, their residues limit the activity of these predators and ultimately could lead to snail outbreaks (Amiri Besheli Citation2009). The application of mineral oils is mainly because they are effectively, non-toxic to vertebrates (Beattie and Smith Citation1996), degrade relatively quickly in the environment (Davidson et al. Citation1991; Beattie et al. Citation1995) and have never been associated with resistance or outbreaks of secondary pests (Beattie Citation1990; Beattie and Smith Citation1993). Oil spraying is a very important alternative of chemical pesticides for controlling some pests in organic citrus orchards and other organic crops (Davidson et al. Citation1991; Kiss et al. Citation2005; Kim et al. Citation2010). Also, therefore, there is a need for more environmentally friendly methods for control of snails. Here we report the outcome of introducing a low-toxicity alternative to traditional high-toxicity pesticides for preventing damage by snails in citrus orchards in northern Iran.
Materials and methods
A 60 hacitrus orchard, Dasht-e-Naz, Behsat orchard no. 1, located in Sari (36° 42N, 53° 12E), Mazandaran province, in northern Iran was chosen as an experimental orchard. Citrus trees were 5–6-year-old Thomson navel grafted on Citrus aurantium root stock with an inter-row distance of approximately 6 m and the distance between trees within a row about 4 m. The average height of trees was 3.2 m. Two experiments were carried out in two adjacent plots of 1 ha in the mentioned orchard.
Both experiments were conducted in a completely randomised design. In both experiments, all snails were removed from trees before applying the treatments. The treatments included:
Mineral oil; plastic foam sheeting was applied around the tree trunk to a height of about 20 cm above the soil surface. The upper edge of the plastic sheeting was tied to the tree trunks to form a cone shape and the lower 10–15 cm of the plastic sheet was smeared with mineral oil (Tehran oil company®) with a very strong oily smell.
Iron phosphate supplied as pellets (Ferricole) (Kimia Sabzavar Company®): applied at 10 mg/m2 as formulated and consisting of 1% iron phosphate, the pellets were scattered around the trees. These pellets were applied three times during periods of rain.
Snail-repellent paint (Sabzarang) (Kimia Sabzavar Company®): contains copper and iron salts and painted in a 10 cm band around the tree trunk.
Metaldehyde supplied as pellets (Partonar Company®): applied at7 mg/m2 as formulated, pellets were scattered around the trees. After rains more pellets were applied which necessitates three applications during the study.
Control
Suppression of snails with mineral oil, iron phosphate, snail-repellent paint and metaldehyde
The citrus orchard selected for this experiment had 540 trees in 18 rows. The treatments applied in this experiment included mineral oil, metaldehyde, iron phosphate, snail-repellent paint and control (untreated). Each treatment consisted of four replicates and each replicate composed of four trees. Replicates were separated by a buffer zone consisting of two rows of trees. After treatments, all 80 trees and their branches were thoroughly examined for snails, every 7–10 days with a total of five assessments between treatments and harvest time. At each sampling date, snails were removed from the trees after counting, so, snails counted on the next sampling date were those who could pass through the barriers and the treatments did not affect them.
Suppression of snails with mineral oil and metaldehyde
In this experiment, we used a citrus orchard block of 1 ha consisting of 15 rows of trees with 30 trees in each row. One row was used for each replicate. In total, 300 trees were treated and sampled. The treatments included mineral oil, metaldehyde and an untreated control. Each treatment had five replicates (rows) and each replicate consisted of 30 trees. The most common molluscicide for controlling Helicella candeharica in this region is metaldehyde and mineral oil treatment. Since mineral oil is relatively safe for the environment, in this experiment only metaldehyde and mineral oil treatments were applied. At each sampling date, four trees from each row and a total of 60 trees were sampled on each sampling date. On the first sampling date, trees were randomly selected in each row, and then on the next sampling date adjacent to trees previously randomly selected were used. In this experiment, existing snails on the trees were counted only once and then removed, so the results of the sampling date had no effect on the next sampling date.
The cost of each treatment during one season per hectare was calculated as follows:
First step: The cost of each treatment per tree ($/tree) = amount of application(s) for each tree (g) × unit price ($)
Second step: Total cost ($/ha) = the cost of each treatment for each tree ($/tree) × the number of trees in a hectare (approximately 300 trees) × applications + labor cost
Statistical analysis
Data were analysed using one-way analysis of variance (ANOVA). The means of each treatment for each sampling date and the totals over time were compared using Tukey’s range test.
Results
Suppression of snails with mineral oil, iron phosphate, snail-repellent paint and metaldehyde
The results of the ANOVA showed that there were significant differences among the numbers of H. candeharica in the different treatments at all sampling dates (P < .01). According to the results of the mean comparison of the number of snails in the different treatments on different sampling dates (), the lowest number of live snails on all dates occurred in the mineral oil treatment, followed by snail-repellent paint. On the final sampling date, the lowest number of live snails after the mineral oil was found in the metaldehyde treatment, although there were no significant differences between this treatment and the snail-repellent paint andiron phosphate treatments. These results indicate that the greatest efficacy was gained with mineral oil.
Table 1. The effect of various chemical repellent on the presence of snails on citrus trees.
When considering the total number of snails over the period of evaluation, there was a significant difference among the treatments (P = .0001, df = 4, F = 26.101) (). Mineral oil and snail-repellent paint had the greatest efficacy as these treatments had the lowest numbers of snails during the entire period of sampling. Iron phosphate and metaldehyde were less efficacious but were superior to the control.
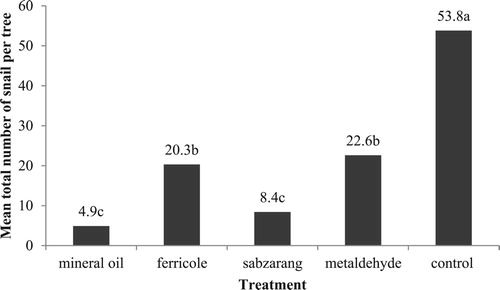
Figure 1. Mean total number of snails found on citrus trees treated with various snail repellents over the experimental period of 30 days in the first study. Different letters above the columns indicate significant difference among treatments at P = .05, using one-way ANOVA followed by Tukey’s test.
Suppression of snails with mineral oil and metaldehyde
There was a significant difference among the numbers of H. candeharica in the different treatments (P < .01). Mineral oil treatments had the lowest number of live snails on the individual sampling dates, opposed to metaldehyde and control with higher numbers ().
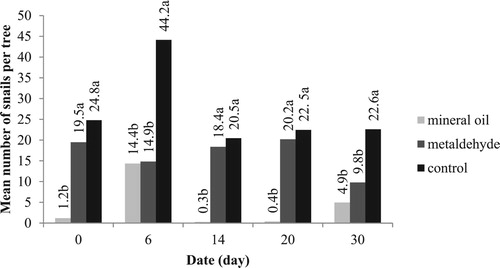
Figure 2. Mean number of snails found on citrus trees treated with various snail repellents on different dates during the second experiment. Different letters above the columns indicate significant differences among treatments on each date at P = .05, one-way ANOVA followed by Tukey’s test.
There were significant differences among the numbers of live snails per tree in the different treatments over the entire period of sampling (P = .0001, df = 2, F = 21.62). The results of mean comparing of the number of live snails per each tree in the treatments are presented in . Mineral oil was more efficient in H. candeharica control than metaldehyde and the control.
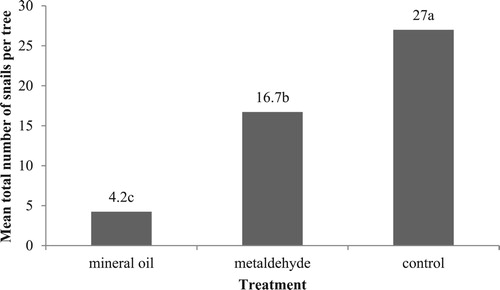
Figure 3. Mean total number of snails found on citrus trees treated with various snail repellents in total sampling time course of second study. Different letters above the columns indicate significant difference among treatments at P = .05, one-way ANOVA followed by Tukey’s test.
The calculation of the cost of each treatment during one season showed that mineral oil treatment had the lowest cost and metaldehyde and snail-repellent paint are costlier. Iron phosphate treatment was the most expensive for controlling H. candeharica ().
Table 2. Cost of each treatment during one season per hectare.
Discussion
Barriers which prevent vertical movement of snails can be chemical or physical (Schüder et al. Citation2003). The plastic foam sheet in cone-like shape mounted around the tree trunk is an example of a physical barrier. Applying strong smelling mineral oil (Tehran oil®) to the internal edges of the cone caused snails to quickly withdraw their tentacles when encountering mineral oil and escape (Personal observation).
Mineral oils also have a repellent effect that discourages egg deposition and feeding. The residual film may inhibit insects from attaching to plant surfaces (Trammel Citation1965). Also, it should be noted that ‘arrested activity’ in insects is one recurrent symptom caused by mineral oil that has been reported directly or indirectly by many authors in laboratory toxicity tests (Taverner Citation2002; Najar-Rodríguez et al. Citation2007; Citation2008; Buteler and Stadler Citation2011).
Separate analysis of each sampling date between treatments was significant (). The effect of various chemical repellents on the presence of alive snail on citrus trees in all dates of sampling () revealed that mineral oil barrier was the most effective treatment giving the lowest mean number of alive snail followed by snail-repellent paint, iron phosphate, metaldehyde and control.
The lowest mean number of H. candeharica on each tree after five sampling dates in the first study was obtained for mineral oil (4.9) followed by snail-repellent paint (8.4), iron phosphate (20.3), metaldehyde (22.6) and control (53.8) (), which is contrary to finding by Amiri Besheli (Citation2009). According to Kheirodin et al. (Citation2012), 30 tons sevin and 40 tons metaldehyde were used to control snails and slugs in citrus orchards in Mazandaran, northern province of Iran.
Mean numbers of snails found on citrus trees treated with various snail repellents on different dates during the second study () indicated that mineral oil barrier was the most effective snail repellent with the lowest mean number of alive snails for each tree followed by metaldehyde and control. The best results were achieved applying mineral oil to internal edges of foam cone mounted around the tree trunk, which confirms the results of Kheirodin et al. (Citation2012), but contradicts those of Gimingham (Citation1940). According to Gimingham (Citation1940), metaldehyde has been the most effective chemical against slugs and snails since introduction as molluscicidal bait in 1934. Pesticide residues from irregular application of pesticides in most citrus orchards of Mazandaran caused pests resistance, natural enemies disruption, environmental imbalance and outbreak of secondary pests (Ahmadi and Arbabi Citation2000; Helyer et al. Citation2003; Armsworth et al. Citation2005; Ahmadi and Hallaji Sani Citation2006; Damavandian Citation2007; Singh and Agarwal Citation2007). So, an alternative solution is the use of mineral oils that are a more natural way of control.
Mean total number of alive snails found on citrus trees treated with various snail repellents in total sampling time of second study () revealed that the mineral oil barrier was the most effective snail repellent giving lowest mean number of alive snails for each tree (4.2) followed by metaldehyde (16.72) and control (27). According to Marcinek et al. (Citation2018), mineral oils have insecticidal, miticidal and fungicidal properties and do not contaminate the soil and do not kill the useful insects (Wilson Citation1999).
Besides contaminating the environment, metaldehyde did not control the snails effectively during the study. According to our results, metaldehyde was even less effective to control H. candeharica as iron phosphate which is contrary to findings of Amiri Besheli (Citation2009), Kheirodin et al. (Citation2012) and Speiser and Kistler (Citation2002).
It is well known that iron phosphate controls snails (Jackel Citation1999; Koch et al. Citation2000) and is not harmful to other organisms in the environment (Roberts et al. Citation1990; Clark Citation1993). This compound is also useful as a nutrient for plants (Rae et al. Citation2009). When snails and slugs eat the pellets, the iron phosphate interferes with their calcium metabolism in their gut, causing the snails and slugs to stop eating almost immediately (Shmuel et al. Citation2004). Ingestion of the iron phosphate bait, even in small amounts, will cause snails and slugs to cease feeding, although it may take several days for the snails to die (Amiri Besheli Citation2009). Besides all its good properties, the only negative is that iron phosphate is somewhat more expensive than metaldehyde ().
Preventing snail’s movement by barriers of copper material is reported by several researchers (Barker Citation2002; Amiri Besheli Citation2009; Kheirodin et al. Citation2012). In this study, snail-repellent paint which contains copper and iron salts caused an overall decrease in the number of H. candeharica and was more effective than metaldehyde and iron phosphate ( and ) which is contrary to findings by Amiri Besheli (Citation2009). He reported that the metaldehyde and iron phosphate were more effective than snail-repellent paint.
Ryder and Bowen (Citation1977) indicated that copper salts can be absorbed by snail’s body and caused damage. However, after rain, the copper salt concentration can decrease so much that control can be ineffective. According to Kheirodin et al. (Citation2012), the copper material around the tree trunk can control Helix aspersa Muller for more than seven months.
Various factors are involved in snails’ movement and activity but the most important factor that caused a reduction in snail activity is cool temperature (Riddle and Miller Citation1988; Ansart et al. Citation2001; Ansart and Vernon Citation2003). In our study, during sampling dates, the average temperature was always more than 10°C. Under these conditions, the environmental moisture and raining probably affect the snail activity. Bedford et al. (Citation1998) stated that H. aspersa activity generally becomes more in raining seasons.
On the one hand, results from the first and second experiment, clearly showed that mineral oil was the most effective treatment (, ) and the most cost-effective (). On the other hand, many researchers stated that mineral oils are effectively non-toxic to vertebrates and degrade relatively quickly in the environment (Beattie and Smith Citation1996; Helmy et al. Citation2012; Damavandian and Kiaeian Moosavi Citation2014). In the other words, they are environmentally friendly. So, it can be concluded that this new method based on a combination of movement barrier and mineral oil is a very powerful physicochemical barrier for several reasons. The most important reasons include the highest rate of pest control and less environmental pollution. Snail-repellent paint and iron phosphate due to their lower efficacy and metaldehyde due to more environmental pollution in controlling H. candeharica are prioritised as the next choices. Meanwhile, other reasons such as no environmental pollution and cost reduction () make mineral oil as the first priority in controlling H. candeharica.
Acknowledgements
We would like to thank Prof. Eddie Ueckermann to edit this manuscript grammatically and thank Mr. Doolatyar who provided citrus orchards for this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mehraneh Sepasi is achieving MSc degree in agricultural entomology from Sari Agricultural Sciences and Natural Resources University, Sari, Iran. She is an agricultural expert in Agricultural Jihad Organization of Mazandaran Province, Iran.
Mohammad Reza Damavandian has an MSc in Agricultural Entomology awarded from Shahid Chamran University of Ahvaz, Iran in 1993; he achieved a PhD in Agricultural entomology seven years later in 2000, from Stellenbosch University, South Africa. He is Associate Professor of Department of Plant Protection in Sari Agricultural Sciences and Natural Resources University. Mohammad Reza has authored or co-authored over 35 scientific publications, of which around 25 were published in journals listed in Journal Citation Reports by Thompson Reuters.
Behnam Amiri Besheli achieved a PhD IN Agricultural Toxicology in 2000, from Reading University, Reading, England. His main research area is agricultural pesticides and primary pest control metabolites. He is Associate Professor of Department of Plant Protection in Sari Agricultural Sciences and Natural Resources University.
ORCID
Mohammad Reza Damavandian http://orcid.org/0000-0002-4403-569X
Additional information
Funding
References
- Ahmadi A, Arbabi M. 2000. Final report of executive research on amount of efficacy of carbaryl pesticide in some citrus orchards of Mazandaran province. Tehran: Iranian Research Institute of Plant Protection.
- Ahmadi E, Hallaji Sani M. 2006. An investigation on effectiveness of copper barrier against Caucasotachea lencoranea (Mussun) in Mazandaran province citrus orchards. Paj Saz J Iran. 76:97–102.
- Amiri Besheli B. 2009. Toxicity appraisement of metaldehyde, ferricole, snail repellent tape and Sabzarang on land snails (Xeropicta derbentina), (Xeropicta krynickii). Afr J Biotechnol. 8:5337–5342.
- Ansart A, Vernon P. 2003. Cold hardiness in molluscs. Acta Oecol. 24:95–102. doi: 10.1016/S1146-609X(03)00045-6
- Ansart A, Vernon P, Daguzan J. 2001. Photoperiod is the main cue that triggers supercooling ability in the land snail, Helix aspersa (Gasteropoda: Helicidae). Cryobiology. 42:266–273. doi: 10.1006/cryo.2001.2332
- Armsworth CG, Bohan DA, Powers SJ, Glen DM, Symondson WOC. 2005. Behavioural responses by slugs to chemicals from a generalist predator. Anim Behav. 69:805–811. doi: 10.1016/j.anbehav.2004.07.009
- Barker GM. 2002. Molluses as crop pests. Wallingford: CABI Publishing.
- Beattie GAC. 1990. Citrus petroleum spray oils. New South Wales: NSW Agriculture Publication.
- Beattie GAC, Liu ZM, Watson DM, Clift AD, Jiang L. 1995. Evaluation of petroleum spray oils and polysaccharides for control of Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae). Austral J Entomol. 34:349–353. doi: 10.1111/j.1440-6055.1995.tb01353.x
- Beattie GAC, Smith D. 1993. Citrus leaf miner. New South Wales: NSW Agriculture Publication.
- Beattie GAC, Smith D. 1996. Integrated pest management: sustainable pest control for the future based on the pest? Proceedings of the 8th International Society of Ctriculture; Nov 18–23, Sun City, South Africa. p. 51–58.
- Bedford ECG, Van Den Bery MA, Villiers EA. 1998. Citrus pests in the republic of South Africa. Nelspruit: Agricultural Research Council Press.
- Buteler M, Stadler T. 2011. A review on the mode of action and current use of petroleum distilled spray oils. In: Stoytcheva M, editor. Pesticides in the modern world – pesticides use and management. London: InTech Press; p. 119–136.
- Clark AM. 1993. Hey’s mineral index. London: Chapman and Hall.
- Damavandian MR. 2007. Laboratory and field evaluation of mineral oil spray for the control of citrus red mite, Panonychus citri McGregor. Acta Agr Scand B-S P. 57:92–96.
- Damavandian MR. 2016. Comparison of mineral oil spray with current synthetic pesticides to control important pests in citrus orchards and their side effects. Arthropods. 5:56–64.
- Damavandian MR, Kiaeian Moosavi SF. 2014. Comparison of mineral spray oil, Confidor, Dursban, and Abamectin used for the control of Phyllocnistis citrella (Lepidoptera: Gracillaridae), and an evaluation of the activity of this pest in citrus orchards in northern Iran. J Plant Prot Res. 54:156–163. doi: 10.2478/jppr-2014-0025
- Davidson N, Dibble J, Flint M, Marer P, Guye A. 1991. Managing insects and mites with spray oils. Oakland: Division of Agricultural and Natural Resources Publication.
- El-Shahaat MS, Al- Nagda A, Eshra EH, Mesbah HA, Ghoneim Emtiaz I. 2009. Toxicity of certain copper fungicides and other pesticides to terrestrial snails. Sci J Agric Mansoura Univ. 34:5501–5507.
- El-Shahaat MS, Eshra EH, Abo-Bakr Y. 2005. Impact of basamide and methomyl bait on non target pests and microbiological processes in soil. Egypt J Agric Res. 83:1007–1016.
- Gimingham CT. 1940. Some recent contributions by English workers to the development of methods of insect control. Ann Appl Biol. 27:161–175. doi: 10.1111/j.1744-7348.1940.tb07486.x
- Heiba FN, Al-Sharkawy IM, Al-Batal AA. 2002. Effects of the insecticide, Lannate, on the land snails, Eopania vermiculata and Monacha contiana under laboratory conditions. J Biol Sci. 2:8–13. doi: 10.3923/jbs.2002.8.13
- Helmy EI, Kwaiz FA, El-Sahn OMN. 2012. The usage of mineral oils to control insects. Egypt Acad J Biol Sci. 5:167–174.
- Helyer N, Brown K, Cattlin ND. 2003. Bioglogical control in plant protection. London: Manson.
- Jackel B. 1999. Methoden des biologischen Pflanzenschutzes als Beitrag zum integrierten Pflanzenschutz in der Grossstadt. Gesunde Pflanz. 51:167–175.
- Kheirodin A, Damavandian MR, Sarailoo MH. 2012. Mineral oil as a repellent in comparison with other control methods for citrus brown snail, Caucasotachea lencoranea. Afr J Agric Res. 7:5701–5707. doi: 10.5897/AJAR12.1452
- Kim DS, Seo YD, Choi KS. 2010. The effects of petroleum oil and lime sulfur on the mortality of Unaspis yanonensis and Aculops pelekassi in the laboratory. J Asia-Pacific Entomol. 13:283–288. doi: 10.1016/j.aspen.2010.06.007
- Kiss L, Labaune C, Magnin F, Aubry S. 2005. Plasticity of the life cycle of Xeropicta derbentina (Krynicki, 1836), a recently introduced snail in mediteranean France. J Mollus Stud. 71:221–231. doi: 10.1093/mollus/eyi030
- Kiss L, Magnin F. 2003. The impact of fire on some mediterranean land snail communities and patterns of post-fire recolonization. J Mollus Stud. 69:43–53. doi: 10.1093/mollus/69.1.43
- Koch R, Jackel B, Plate HP. 2000. Prufung der Effektivitat neuer Beka mpfungsmethoden gegen phytophage Nacktschnecken. Gesunde Pflanz. 52:1–10.
- Marcinek B, Karczmarz K, Szmagara M, Durlak W, Pogroszewska E. 2018. Influence of a prolong application of mineral oils on bulb yield, quality of cut flowers and spread of viruses in tulip cultivation. Acta Sci Pol Hortorum Cultus. 17:115–125. doi: 10.24326/asphc.2018.1.11
- Najar-Rodríguez AJ, Lavidis NA, Mensah RK, Choy PT, Walter GH. 2008. The toxicological effects of petroleum spray oils on insects – evidence for an alternative mode of action and possible new control options. Food Chem Toxicol. 46:3003–3014. doi: 10.1016/j.fct.2008.05.042
- Najar-Rodríguez AJ, Walter GH, Mensah RK. 2007. The efficacy of a petroleum spray oil against Aphis gossypii Glover on cotton. Part 1: Mortality rates and sources of variation. Pest Manag Sci. 63:586–595. doi: 10.1002/ps.1385
- Rae RG, Robertson JF, Wilson MJ. 2009. Optimization of biological (Phasmarhabditis hermaphrodita) and chemical (iron phosphate and metaldehyde) slug control. Crop Prot. 28:765–773. doi: 10.1016/j.cropro.2009.04.005
- Riddle WA, Miller VJ. 1988. Cold-hardiness in several species of land snails. J Therm Biol. 13:163–167. doi: 10.1016/0306-4565(88)90028-9
- Roberts WL, Campbell TJ, Rapp GR. 1990. Encyclopedia of minerals. New York: Van Nostrand Reinhold Company.
- Ryder TA, Bowen ID. 1977. The slug foot as a site of uptake of copper molluscicide. J Invertebr Pathol. 30:381–386. doi: 10.1016/0022-2011(77)90149-5
- Schüder I, Port G, Bennison J. 2003. Barriers, repellents and antifeedants for slug and snail control. Crop Prot. 22:1033–1038. doi: 10.1016/S0261-2194(03)00120-0
- Shmuel M, Yaacov G, Benjamin Y. 2004. Management of land snails in cut green ornamentals by copper hydroxide formulations. Crop Prot. 23:647–650. doi: 10.1016/j.cropro.2003.11.004
- Singh DK, Agarwal RA. 2007. Toxicity of piperonyl butoxide-carbaryl synergism on the snail Lymnaea acuminata. Internationale Revue der gesamten Hydrobiologie und Hydrographie. 74:89–699.
- Speiser B, Kistler C. 2002. Field tests with a molluscicide containing iron phosphate. Crop Prot. 21:389–394. doi: 10.1016/S0261-2194(01)00120-X
- Symondson WOC. 2004. Coleoptera (Carabidae, Staphylinidae, Lampyridae, Drilidae and Silphidae) as predators of terrestrial gastropods. In: Barker GM, editor. Natural enemies of terrestrial mollusks. Wallingford: CABI Publishing; p. 37–84.
- Taverner P. 2002. Drowning or just waving? A perspective on the ways petroleum-based oils kill arthropod pests of plants. In: Beattie GAC, Watson DM, Stevens ML, Rae DJ, Spooner-Hart RN, editors. Spray oils beyond. Sydney: University of Western Sydney Press; p. 78–87.
- Trammel K. 1965. Properties of petroleum oils in relation to performance as citrus tree sprays in Florida [master’s thesis]. University of Florida.
- Wilson CR. 1999. The potential of reflective mulching in combination with insecticide sprays for control of aphid-borne viruses of iris and tulip in Tasmania. Ann Appl Biol. 134:293–297. doi: 10.1111/j.1744-7348.1999.tb05267.x
