ABSTRACT
Purpose: The main aim of this study was to introduce and explore plant growth-promoting bacteria (PGPB) indigenous to ginseng, and to evaluate their ability to improve production and quality, and effect on rhizosphere niche in ginseng.
Materials and methods: Endophytic bacteria were isolated from root, stem, and leaf of ginseng from different sites and genotype in China and Korea, screened based on their beneficial properties as PGPB. Nine bacterial isolates were selected according to their plant growth properties including soluble phosphate and potassium, ammonia, auxin and siderophore producing, ACC deaminase, and antagonistic pathogen as well. Changes in ginseng after PGPB inoculation were evaluated with respect to the non-inoculated control.
Results and Conclusions: The PGPB isolates were identified as genera Bacillus, Lysinibacillus, Rhizobium, Stenotrophomonas, Erwinia, Ochrobactrum, Enterobacter and Pantoea based on 16S rRNA sequences. Inoculation of G209 and G119 increased not only plant height, root length, fresh weight, and dry weight, but also root activity and the amount of ginsenosides significantly. In particular, using the Illumina Miseq platform, the native bacterial community of rhizospheric soil maintained high community diversity and increased abundance of specific bacteria. Therefore, they may be play a crucial role in sustainable ginseng cultivating in farmland.
Introduction
Ginseng (Panax ginseng C.A. Mey.), is an important traditional herbal medicine and is one of the tonics in the Araliaceae, which has beneficial effects on physical and mental activities, immune functions, neuroendocrine system, cell metabolism, etc. (Liu and Xiao Citation1992). As people pay more and more attention to health, the ginseng demand is increasing as a medical treatment and health food. In pursuit of high-yield and high effect for disease control of ginseng, the farmers use large-scale chemical fertilisers and toxic pesticides, which lead to a reduction of soil quality and excessive heavy metal content of ginseng. In recent years, the plant growth promoting rhizospheric and endophytic bacteria (PGPR/PGPB) have been a research focus (Lugtenberg and Kamilova Citation2009). The commercial use of some PGPBs as biofertilisers has become widespread (Timmusk et al. Citation2017), including genera Rhizobium, Bacillus, Pseudomonas, Serratia, Azospirillum, Streptomyces and others, which are accepted widely due to their safety alternatives to chemical fertilisers.
Facing the problem in cultivation of ginseng, the use of PGPB could be a promising alternative. It has been shown that Pseudomonas simiae inoculation of ginseng resulted in better growth under aluminium stress (Farh et al. Citation2017). To our knowledge no studies have considered the effects of PGPB on the indigenous bacterial communities in addition to growth promotion. Therefore the overall aims of this study was to screen and identify endophytic PGPB from different ginseng genotypes and source sites comprehensively; In addition, this study evaluated the effect on ginseng by inoculation with PGPB under filed condition.
Materials and methods
Sample collection
A total of 36 different organs including root, stem and leaf from healthy ginseng of 2, 3, and 4 years old, respectively from the forest in Helong (42.229N, 128.583E) and farmland in Dunhua (43.421N, 128.447) in China. The cultivars of Yunpung and Chunpung were kindly provided by Dr. HyunHo Kim, Chungnam Agricultural Research and Extension Services Cheongyang Boxthorn Experiment Station, 610 Cheongshin-ro, Cheongyang-gun, Chungcheongnam-do, 345-872, Republic of Korea; they were transplanted to the farmland of Yanbian University(42.916N/129.489E) in Yanji, China. Samples were collected simultaneously on June 25, 2017, when it was before flowering period. Soil chemical and physical properties were listed in .
Table 1. The Soil chemical and physical properties of the sampling site in experiment.
Sample pretreatment
Ginseng plants were harvested by hand, and large soil aggregates were removed by shaking the roots. Roots were pooled into a 50 mL Falcon tube by pouring 25 mL of sterilised phosphate buffer (130 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4, pH 7.0), shaken at 180 rpm for 20 min and vortexed at maximum speed for 20 s. The roots were surface-sterilised by running water for 30 min, 70% ethanol for 1 min, 4% sodium hypochlorite for 8 min, 100% ethanol for 30 s, and ddH2O for 1 min. Then roots were transferred to another 50 mL Falcon tube and were sonicated. The roots were grinded by pestle under sterile conditions designated as the root compartment. The other sample processing methods of stem and leaf were the same as the root.
Scanning electron microscopy (SEM)
SEM was performed to check the consequence of surface sterilisation according to a previous report (Timmusk et al. Citation2005). Samples were prepared from plants based on the process previously described, except for the washing procedure of single roots. The roots of inoculated and non-inoculated were respectively fixed in 4% glutaraldehyde of phosphate buffer overnight at 4°C. The dehydration operation was processed termly using an ethanol gradient (20%, 30%, 50%, 75%, and 95% ethanol) after a brief washing. Subsequently, the samples were submerged in water-free ethanol overnight at 4°C. Critical point drying was performed using a critical point dryer, and the pressure was reduced gradually to avoid tissue damage. Finally, the samples were coated with palladium before imaging using scanning electron microscope (SEM; S-3500N, HITACHI, JAPAN).
Isolation of endophytic bacteria
Six kinds of medium were used for isolation of endophytic bacteria. Nutrient broth (NB, Nutrient broth 0.8%; Difco, USA), R2A Agar (R2A, R2A Agar 1.82%; Difco, USA), Tryptic soy broth (TSB, Tryptic soy broth 3%; Difco, USA), KB (g/L): proteose peptone 20.00, glycerol 10.00, K2HPO4 1.50, MgSO4·7H2O 1.50, agar 15.00 and Pikovskaya agar (g/L): yeast extract 0.50, dextrose 10.00, calcium phosphate 5.00, ammonium sulfate 0.50, potassium chloride 0.20, magnesium sulfate 0.10, manganese sulphate 0.0001, ferrous sulfate 0.0001, agar 15.00, and Dworkin-Foster(DF) salt minimal medium containing 0.2% ACC(v/v), which was specific to isolate ACC deaminase producing bacteria by the previous method (Penrose and Glick Citation2003). All plates were incubated at 25°C for 72 h. According to shape, size, colour and texture, different colonies were selected to further identification and screening by stocking in 20% (v/v) glycerol at – 80°C.
Screening of promoting growth properties of bacteria
The ability of phosphate-solubilisation for bacteria isolates was determined on National Botanical Research Institute’s phosphate (NBRIP) growth medium (g/L): glucose 10, Ca3(PO4)2 5, MgCl2⋅ 6 H2O 5, MgSO4⋅ 7 H2O 0.25, KCl 0.2, (NH4)2SO4 0.1, agar 20 (Misra et al. Citation2012). The tricalcium phosphate served as the single inorganic phosphate source in the NBRIP and agar medium with the addition of bromophenol blue. The bacteria isolates were spread on plate and incubated for 7 days at 30°C. The emergence of a transparent halo surrounding the colonies represented the ability of phosphate-solubilisation.
The bacteria isolates were inoculated to a 10 mL tube containing peptone water (10 g/L) and incubated at 25°C for 48 h. In addition, 0.5 mL Nessler’s reagent was added to each tube, and the appearance of brown colour precipitate indicated that the strains had the ability to produce ammonia (Zhao et al. Citation2011).
The siderophores production was determined using the chrome-azurol S(CAS) assay (Schwyn and Neilands Citation1987). The bacteria isolates cultured in TSB were spotted on the CAS-blue agar and incubated 48 h at 30°C. The formation of purple or orange halos around the colonies indicated the siderophores production.
The production of IAA was measured according to previous method (Acuña et al. Citation2011). The bacteria isolates were inoculated to TSB with L-tryptophan as IAA precursor and incubated at 30°C for 24 h. The supernatant after centrifugation was mixed with Salkowski’s reagent(1:4) and incubated at room temperature for 30 min. The existence of a halo zone around the spot indicated the ability to produce IAA. The absorbance of the mixture was determined at 540 nm. Based on the IAA standard curve, the concentration of IAA was obtained.
The activity of 1-aminocyclopropane-1-carboxylate (ACC) deaminase in the bacteria isolates were determined by measuring the amount of α-ketobutyrate produced when ACC cleavage occurred (Timmusk et al. Citation2005).
Antagonistic experiments were conducted on PDA plates, in which four holes of 10 mm diameter were filled with melting TSA. Subsequently, 10 μl of the bacteria suspension was added to the TSA after solidification and incubated at 28°C for 48 h. The inhibition zones were designated to measure the antagonistic effects of bacteria against the pathogen. The pathogens both Alternaria panax Whetz. and Botrytis cinerea Pers. were placed on the centre of the PDA plate respectively.
Determination of all the plant growth promoting traits repeated three times respectively.
Molecular identification of bacteria isolates based on 16s rRNA sequence
Genomic DNA of all bacteria isolates was extracted using the QIAamp DNA mini kit (Mo Bio Laboratories, Inc., New York). The 16S rRNA gene was amplified using the following primers: 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). PCR was carried out using Taq DNA polymerase (Takara Bio, Inc., Japan). The program of thermal cycling was as follows: predegeneration at 94°C for 10 min, 30 cycles at 94°C for 1 min, 56°C for 30 s, 72°C for 1 min, and an extension at 72°C for 10 min. The product of PCR amplification was purified using DNA Fragment Purification Kit (Takara bio, Inc., Japan). The nucleotide sequences were obtained by an ABI 3730xl DNA Analyzer (Applied Biosystems, Forster City, CA, USA) and analysed for Blast in the Genbank database.
Effect of PGPB on ginseng growth
Seeds of ginseng were surface sterilised with 75% ethanol for 5 min and 1% sodium hypochlorite for 5 min successively, followed by rinsing with sterile water thrice. Seeds were sowed in the field. Nine of all PGPBs we obtained were incubated in TSB medium (150 mL) respectively for 48 h at 28°C. Following, the cultures were collected by centrifugation at 8000g for 10 min at 4°C, and pellets were resuspended in sterile distilled water. The final concentration of inoculated solution was regulated to 108 CFU/mL. One hundred millilitres of bacterial suspension was poured around the roots or treatment with sterilised water as a control. The experiment was replicated 10 times for every treatment. After 2 months, all plants were sampled and measure for plant height, root length, fresh weight, and dry weight.
Effect of PGPB on root activity of ginseng
The root system is a basic organ, which serves as receptors and control centres for growth and development. Much information of growth and development are transmitted to the above-ground parts through the root system to regulate the growth and development of crops (Smith et al. Citation2005). Therefore, root activity is an important index that reflects the ability of plants to absorb water and nutrients. Root activity was measured using the redox TTC method. The tripenyltetrazolium chloride (TTC) was reduced to insoluble red triphenylformazan (TF), which is measured in the determination of root activity (Comas et al. Citation2000). The fresh roots 0.5 g were cut into pieces and soaked in 0.6% (w/v) TTC solution (TTC dissolved in phosphate buffer at pH 7.0) in the dark at 30°C for 24 h. Following, they were rinsed twice, dried with filter paper, and soaked into 95% (v/v) ethanol for 30 min at room temperature. The absorbance values of the extraction solution were determined by a spectrophotometer (Hitachi U3010, Tokyo, Japan) at 485 nm.
Determination of ginsenosides contents
High Performance Liquid Chromatographic analysis was carried out using an Agilent 1260 series LC system (Agilent Technologies, USA) (Dong et al. Citation2016), which supplemented an online degassing unit, a binary pump, and an auto sample injector, as well as a controlled column compartment with constant temperature. Agilent Poroshell ZORBAX SB-C18 column (4.6 mm × 150 mm, 5 μm) was used to separate the sample, with a constant flow rate of 0.5 mL/min at 25°C. The mobile phase was comprised of water (A) and acetonitrile (B), using a gradient elution of 22–43% (B) for 0–33 min, 43–65% (B) for 33–47 min, 65–86% (B) for 47–62 min, 86–77% (B) for 62–67 min, 77–40% (B) for 67–72 min, and 40–22% (B) for 72–74 min. The sample was injected at 2 μL.
Effect of PGPB on the rhiziospheric bacteria community of ginseng
The rhizospheric soils of ginseng were eluted in phosphate buffer. The genomic microbial DNA of rhizospheric soil was extracted using the PowerSoil DNA extraction kit (MOBIO Laboratories, Inc.) with the standard MoBio protocol. PCR reaction was performed using Barcode specific primers 341F (5′- CCTAYGGGRBGCASCAG -3′), and 806R (5′- GGACTACNNGGGTATCTAAT -3′), which targets the V3-V4 region of the bacterial 16S rRNA. Phusion®High-Fidelity PCR Master Mix with GC Buffer (New England Biolabs (Beijing) Inc., China) was performed. Sequencing was carried out on an Illumina MiSeq platform as protocols (Caporaso et al. Citation2012).
Sample reads were assembled by using FLASH v1.2.7 (Tanjaand Salzberg Citation2011). Chimeric sequences were removed using the QIIME software (Caporaso et al. Citation2010) software based on the UCHIME algorithm (Edgar et al. Citation2011), and the Gold database, as well as the microbial diversity. Operational Taxonomic Units (OTUs) were clustered based on 97% similarity identity for all effective tags by using the Uparse Software (Edgar Citation2013). Alpha diversity analysis included Chao1, Shannon index, ACE, Simpson, and observed species. Beta diversity included both unweighted and weighted Unifrac distances calculated with 10 times of sampling, and these distances were visualised by non-metric multi-dimensional scaling (NMDS) (Lundberg et al. Citation2012). Taxonomy assignment of OTUs was performed by comparing sequences RDP Classifier v2.2 (Wang et al. Citation2007) and the Greengenes databases (DeSantis et al. Citation2006).
Statistical analysis
Statistical analyses were performed using SPSS statistics version 19.0 (SPSS, Inc., Chicago, IL, USA).
Results
Screening of endophytic bacteria of growth promoting properties and molecular identification
We isolated 128 endophytic bacteria of ginseng from different cultivars and source sites. The scanning electron microscope images illustrated the result of surface sterilisation shown in . Photographs of ginseng roots before and after surface sterilisation were magnified 60-fold and 4000-fold, respectively, which ensured that the bacteria isolated were endophytic bacteria. Among the candidates, 27 endophytic bacteria were screened with at least one kind of growth promoting property. Nine of them were shown in . GT11 and G63 had higher activity ACC deaminase at 3.39 and 3.12 (μmol h−1 mg−1 protein), the IAA concentration of isolate G114, G204, and GT11 were 28.42, 30.22, and 12.40 μg ml−1 respectively, eight bacteria had the ability to suppression one or both pathogenic fungi. Meanwhile, nine bacteria possessed three kinds or more growth promoting properties. The results of blast alignment and the phylogenetic tree based on 16S rRNA sequences indicated that the selected PGPB were chiefly members of genus Bacillus, Rhizobium, Ochrobactrum, Erwinia, Stenotrophomonas, Enterobacter, pantoe and Lysinibacillus, which were shown in .
Figure 1. SEM micrograph of root surface in Panax ginseng. (A) SEM micrograph before root surface sterilisation. Bar = 100 μm. (B) SEM micrograph after root surface sterilisation. No bacteria was detected on the root surface. Bar = 100 μm. (C) SEM micrograph before root surface sterilisation. Many bacteria were adhered to the root surface. Bar = 1 μm. (D) SEM micrograph after root surface sterilisation. Bar = 1 μm.
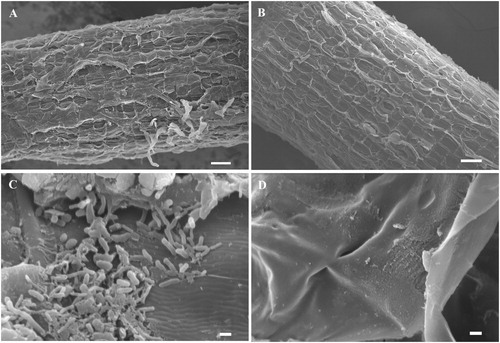
Figure 2. Phylogenetic tree of the PGPB strains isolated from the Panax ginseng based on the sequences of the 16S rRNA gene. “▴” represented the strains isolated in present study along with related sequences of type strain closely obtain from GenBank. The bootstrap was 1000 iterations and the value ≥ 50 were shown at the branching point. The bar indicated sequence divergence of 0.1 nucleotides.
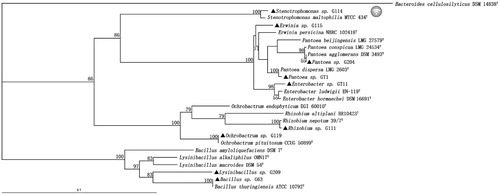
Table 2. Promoting growth properties of bacteria isolated from Panax ginseng.
Plant growth promotion on ginseng
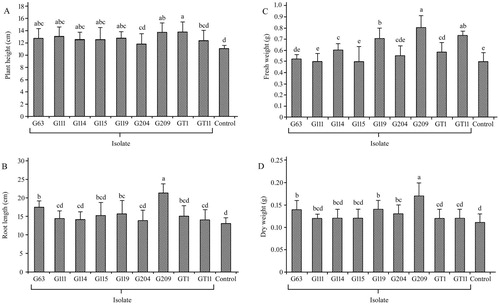
The nine isolates treatment increased the growth parameter of ginseng compared with non-inoculated controls, as shown in . In the matter of plant height, treatment with GT1 performed best at 13.76, which increased 25% compared to that of the control treatment. Specifically, the treatment with G63, G111, G114, G115, G119, G209, and GT1 showed significant differences (p < .05) compared to controls. Similarly for root length, the treatment with G63, G119, and G209 demonstrated significant differences (p < .05). In the case of fresh weight, treatment with G114, G119, G209, GT1 and GT11 indicated significant differences (p < .05). In addition, treatment with G63, G119, G204 and G209 showed significant differences (p < .05) for dry weight. The values of the growth index indicated that inoculated treatment of G209 and G119 performed well.
Figure 3. Effect of plant growth promoting bacteria on growth characteristics in Panax ginseng. Plant height (A), root length (B), fresh weight (C) and dry weight (D) were measured respectively in the non-inoculated (control) and inoculated plantlets. The experiments were repeated twice and 10 plants per set. Values are means ± standard deviation. Different letters denote significant differences (p < .05) comparison between treatments by Duncan’s test.
Root activity of ginseng
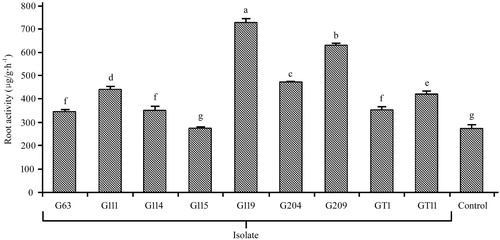
The root activities based on the reducing capacity of TTC were demonstrated in . It was shown that the root activities of treatment with G119 and G209 were maximum comparing with non-inoculated control more than twice. Besides, the root activity of treatment G204, G111, GT11, G114, G63, and GT1 were in descending order and showed significant differences (p < .05) compared to non-inoculated control as well.
Figure 4. Effect of plant growth promoting bacteria on root activity in Panax ginseng. The experiments were repeated twice and 10 plants per set. Values are means±standard deviation. Different letters denote significant differences (p < .05) comparison between treatments based on a one-way ANOVA by Duncan’s test.
Analysis of ginsenoside contents
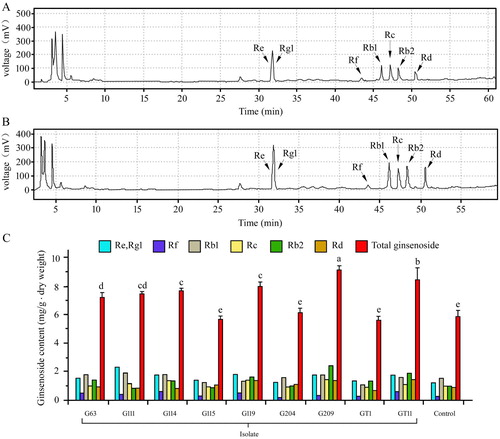
The seven main ginsenosides composed of Re, Rg1, Rf, Rb1, Rc, Rb2 and Rd from ginseng were determined by HPLC, and the chromatogram and column diagram are shown in . The ginsenosides extracted from ginseng were identified by comparing the retention time to those of standard compounds. There are differences of peak area between some treatments with PGPB and control under the same experimental condition. The contents of total ginsenosides in treatment with G63, G111, G114, G119, G209, and GT11 increased in comparison tonon-inoculated control and the variance analysis showed significant differences (p < .05).
Figure 5. Effect of plant growth promoting bacteria on ginsenoside in Panax ginseng. (A) The Chromatogram of ginsenosides of control treatment by HPLC. (B) The Chromatogram of ginsenosides of G209 by HPLC. (C) The content of monomer and total ginsenoside. The experiments were repeated twice and 10 plants per set. Values are means±standard deviation. Different letters denote significant differences (p < .05) comparison between treatments based on a one-way ANOVA by Duncan’s test.
Diversity of rhizospheric bacteria community
Next generation sequencing was carried out, and we assessed the diversity and structure of ginseng rhizospheric soil in four different inoculated treatments with PGPB and non-inoculated control. Across the five rhizospheric soil samples, we obtained 1,032,317 sequences in total with 61,749–74,981 sequences per sample. As shown in , the dominant phyla were Proteobacteria, Actinobacteria and Acidobacteria, accounting for more than 75% of relative abundance from each of the rhizospheric soil. Besides, Verrucomicrobia, Bacterioidetes, Gemmatimonadetes, Chloroflexi and Nitrospirae were present in all samples, but with relatively low abundances. The relative abundance of Proteobacteria in G115, G119, and G209 inoculated treatments increased by 13.76%, 7.52% and 4.77% compared to non-inoculated control, whereas GT11 decreased to 0.87%. The relative abundances of beneficial bacteria by genus level are presented in . The five kinds of beneficial bacteriacomposed of genus Bradyrhizobium, Rhizobium, Mesorhizobium, Pseudomonas and Bacillus. The relative abundances of genus Bradyrhizobium, Rhizobium, Mesorhizobium and Pseudomonas were significantly higher for inoculated G115 treatment than in the control. Similarly, there was also a significant differences in the relative abundance of the genus Rhizobium and Pseudomonas between inoculated G119 and control. In addition, the genus Bacillus increased and showed a significant difference between inoculated G209 and control. The OTUs clustered, indicating greater than 97% of sequence similarity, to count the diversity indicators as shown in . The Shannon, Chao1 and ACE varied by different inoculated and non-inoculated treatments. The mean α-diversity was higher in G119, G209, and control, and lower in G115 and GT11. As shown in , non-metric multi-dimensional scaling (NMDS), the different treatments were shown to be assembled together. Each treatment may form unique bacterial communities. As shown in , clustering and abundance by phylum level (based on weighted and unweighted unifrac distance) denoted that there was more abundance and less community structure differences between the inoculated treatment and non-inoculated control.
Figure 6. Relative abundances of the dominant bacterial phyla in ginseng rhizospheric soils inoculated plant growth promoting bacteria and non-inoculated control. The experiments were repeated triple and 10 plants per set.
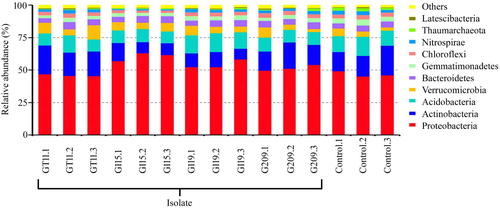
Figure 7. Effect of plant growth promoting bacteria inoculation on relative abundance of beneficial bacteria in ginseng rhizosphere. Values are means ± standard deviation. “*” denote significant differences (p < .05) comparison different inoculated treatment with PGPB and non-inoculated control by Duncan’s test.
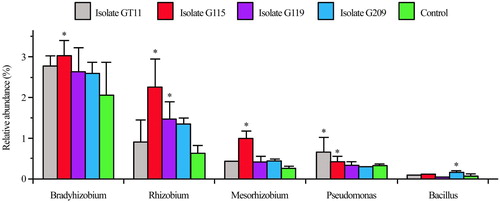
Figure 8. Effect of plant growth promoting bacteria on distribution and clustering of ginseng rhizospheric bacteria community based on Non-metric Multi-dimensional Scaling analysis.
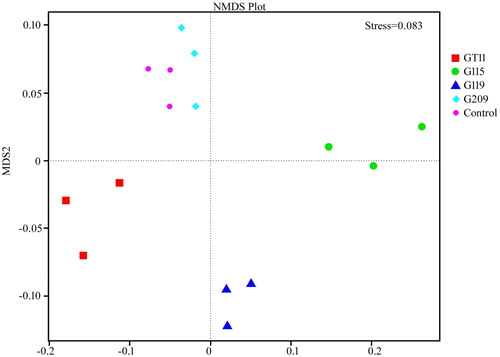
Figure 9. Clustering analysis on bacterial community of different PGPB inoculation of ginseng based on weighted unifrac distance.
Table 3. Diversity of rhizospheric bacteria community of Panax ginseng.
Discussion
PGPB is known to play an important role in the growth and development of host plants (Ahmed et al. Citation2014; Zhao et al. Citation2011; Kumar et al. Citation2015). Among them, endophytes the bacteria living inside plants with certain niches, are less well investigated for their promoting growth properties and metabolic potential than culturable soil bacteria. Most previous researches have focused on the one isolates or little aspects of promoting growth effects, but investigation comprehensively has not been performed. The other important aspect is the evaluation of this study under field conditions, where is more complex.
In the present study, the extent of the effects of PGPB on ginseng growth varied depending on the inoculated isolates. The results of biomass as shown in indicated that isolate G209 and G119 exerted the best growth in terms of plant height, root length, fresh and dry weight compared to control significantly. In line with the previous results, genus Bacillus and Ochrobactrum isolates exhibited the yield promoting compared to the uninoculated control (Hahm et al. Citation2012). Isolate G204 possessed all the promoting growth properties we evaluated, but the effect was not the best, it suggested that the mechanism of plant growth promotion maybe not superimposed, there may also be alternative, similar results were demonstrated on previous literature (Islam et al. Citation2016).
The change of bacterial community structure affects the quality and health of soil directly. The higher the diversity index is, the greater the diversity of soil microbe. In addition, the more complex the soil micro-ecosystem is, the more stable the function becomes (Rutgers et al. Citation1998). Index of diversity reflecting the abundance and distribution of species were analysed in the present study in . The result indicated that Shannon, Chao1, and ACE were different. The diversity of bacteria in the non-inoculated control was the highest, and there was no significant difference between the G119 and G209 and the control. Whereas treatment of G115 and GT11 was lower, and it was significantly different from others. It suggested that PGPB inoculation altered the diversity of rhizobacteria, which may be due to the effects of root exudates on bacteria community and interaction between microbes (). Furthermore, the relative abundance of beneficial bacteria including Bradyrhizobium, Rhizobium, Mesorhizobium, Pseudomonas and Bacillusincreased in present study, as shown in , which was in accordance with previous reports (Prakamhang et al. Citation2015; Hossain and Märtensson Citation2008; Yadav Citation2012), it may become an indirect favourable factors for their application as PGPB. The NMDS analysis and clustering based on weighted unifrac distance in of this study showed that all five treatments were clustered into three parts. The distribution of bacterial community in the rhizosphere soil for different treatments bacteria was clearly separated. Diversity of different treatments denoted that G119 and G209 were clustered together, control and GT11 clustered, and G115 was a cluster. There was little difference between the groups, mainly reflecting differences in quantity. Many reports showed that G115 of blast for genus Ewinia are pathogenic (Wensing et al. Citation2014; Ramírez-Bahena et al. Citation2015). The security of PGPB is crucial in large-scale commercial application, which is one factor for the ideal PGPB (Nakkeeran et al. Citation2005). Nevertheless, this assumption needs further verification. It is very necessary to investigate changes in the rhizobacteria community besides proving the promoting growth abilities.
It is essential to select the sample used for isolation of endophytic bacteria, including not only the soil type where the plant was cultivated, but also the variety and genotype (Edwards et al. Citation2015). In present study, there were 46 isolates from root, stem, and leaf of ginseng which had different extent of plant growth properties, including 15 isolates classified as Bacillus, 7 Stenotrophomonas, 5 Enterobacter, 3 Paenibacillus, 3 Pseudomona, 2 Microbacteriumand 2 Staphylococcus. In addition, one isolate belongs to Lysinibacillus, Arthrobacter, Sphingobacterium, Pantoea, Burkholderia, Ochrobactrum, Rhizobium, Kocuria and Erwinia respectively. The current number of PGPB isolates and plant growth properties were more than previous studies (Vendan et al. Citation2010). showed nine of them were selected which possessed more than four kinds of promoting growth properties. All the selected isolates showed ACC deaminase activity, which is a widely accepted as precursor of ethylene biosynthetic pathway (Glick et al. Citation2007). In agreement with other studies, Bacillus was the predominant genus.
Plant root activity could affect soil enzyme activity due to root exudates or rhizosphere microbe (Kong et al. Citation2009), which indicated it was an important index for interaction between plant and soil. However, the impacts of PGPB on root activity have rarely been reported on previous study. In this study, showed the significant difference of root activity between the root with inoculants and non-inoculants except G115. It is supposed that the application of PGPB promoted the absorption and utilisation of water or nutrient by ginseng root.
The current study was conducted to determine the changes of monomer ginsenoside Re, Rg1, Rf, Rb1, Rc, Rb2, and total ginsenoside as a standard on ginseng quality. The results of showed that PGPB promoted growth of ginseng and increased the content of total ginsenoside in treatments partly, with statistically significant differences. It is widely that bacteria was involved in the transformation of main saponins to rare saponins (Park et al. Citation2017), seldom on the change of ginsenoside concentration. In many reports on medicinal plant, researchers found that Bacillus subtilis GB03 and Pseudomonas fluorescens WCS417r as common PGPB improved the content of essential oil in Peppermint (Del et al. Citation2015). Similarly, Bacillus amyloliquefaciens increased the content of total amino acids in Codonopsis pilosula (Zhao et al. Citation2016). However, the mechanism of PGPB effect on effective constituents has not been clear currently. Previous reports indicated that jasmonic acid, Ca2+, nitricoxide and ethylene production were the well-known signalling molecules that mainly mediate production of ginsenoside (Rahimi et al. Citation2015). Meanwhile, the signalling molecules above related closely to many PGPBs (Ent et al. Citation2009; Shukla et al. Citation2012; Vaishnav et al. Citation2016). As a result, we predicted that these signalling molecules may play a role in the accumulation of ginsenosides by PGPB.
In conclusion, we recomend that PGPB is used for improving ginseng production. In vivo and in vitro indication suggest that the selected isolates benefit ginseng plants by means of multiple modes of action including plant growth promotion, change of rhizosphere community, intervention of ginsenoside synthesis pathway, and antibiosis against phytopathogens. These show the potential of these strains as biofertiliser and commercialised production.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Wenxiu Ji is a PhD Student in College of Agriculture, Northeast Agricultural University. She is presently working as lecturer in College of Agriculture, Yanbian University and her studies mainly focused on quality breeding of medicinal plant and plant growth promoting microbiology.
Xue Leng has obtained a Master Degree in the subject of crop genetic breeding, College of agriculture, Yanbian University. Presently, she is a PhD student in College of Forestry, Northeast Forestry University.
Zhengxun Jin is a professor in College of Agriculture, Northeast Agricultural University. His studies mainly focused on crop molecular genetics and biotechnology.
Hulin Li is a professor in College of Agriculture, Yanbian University. His studies mainly focused on commercial crop breeding and physiology.
References
- Acuña JJ, Jorquera MA, Martínez OA, Menezesblackburn D, Fernández MT, Marschner P, et al. 2011. Indole acetic acid and phytase activity produced by rhizosphere bacilli as affected by pH and metals. J Soil Sci Plant Nutr. 11:1–12.
- Ahmed EA, Hassan EA, Tobgy KMKE, Ramadan EM. 2014. Evaluation of rhizobacteria of some medicinal plants for plant growth promotion and biological control. Ann Agric Sci. 59:273–280.
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 7:335–336. doi: 10.1038/nmeth.f.303
- Caporaso JG, Lauber CL, Walters WA, Lyons DB, Huntley J, Fierer N, et al. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6:1621–1624. doi: 10.1038/ismej.2012.8
- Comas LH, Eissenstat DM, Lakso AN. 2000. Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol. 147:171–178. doi: 10.1046/j.1469-8137.2000.00679.x
- Del RCL, Santoro MV, Reinoso H, Travaglia C, Giordano W, Banchio E. 2015. Anatomical, morphological, and phytochemical effects of inoculation with plant growth-promoting rhizobacteria on peppermint (Mentha piperita). J Chem Ecol. 41:149–158. doi: 10.1007/s10886-015-0549-y
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 72:5069–5072. doi: 10.1128/AEM.03006-05
- Dong WW, Xuan FL, Zhong FL, Jiang J, Wu SQ, Li DH, et al. 2016. Comparative analysis of the rats’ gut microbiota composition in animals with different ginsenosides metabolizing activity. J Agric Food Chem. 65:327. doi: 10.1021/acs.jafc.6b04848
- Edgar RC. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 10:996–998. doi: 10.1038/nmeth.2604
- Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 27:2194–2200. doi: 10.1093/bioinformatics/btr381
- Edwards J, Johnson C, Santosmedellín C, Lurie E, Podishetty NK, Bhatnagar S, et al. 2015. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci USA. 112:911–920. doi: 10.1073/pnas.1414592112
- Ent SVD, Wees SCMV, Pieterse CMJ. 2009. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry. 69(13):1581–1588.
- Farh ME, Kim YJ, Sukweenadhi J, Singh P, Yang DC. 2017. Aluminium resistant, plant growth-promoting bacteria induce over expression of aluminium stress related genes in Arabidopsis thaliana, and increase the ginseng tolerance against aluminium stress. Microbiol Res. 200:45–52. doi: 10.1016/j.micres.2017.04.004
- Glick BR, Cheng Z, Czarny J, Cheng Z, Duan J. 2007. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol. 119:329–339. doi: 10.1007/s10658-007-9162-4
- Hahm MS, Sumayo M, Hwang YJ, Jeon SA, Park SJ, Lee JY, et al. 2012. Biological control and plant growth promoting capacity of rhizobacteria on pepper under greenhouse and field conditions. J Microbiol. 50:380–385. doi: 10.1007/s12275-012-1477-y
- Hossain MS, Märtensson A. 2008. Potential use of rhizobium spp. to improve fitness of non-nitrogen-fixing plants. Acta Agric Scand. 58:352–358.
- Islam S, Akanda AM, Prova A, Islam MT, Hossain MM. 2016. Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol. 6:1360. doi: 10.3389/fmicb.2015.01360
- Kong L, Wang YB, Zhao LN, Chen ZH. 2009. Enzyme and root activities in surface-flow constructed wetlands. Chemosphere. 76:601–608. doi: 10.1016/j.chemosphere.2009.04.056
- Kumar R, Das AJ, Juwarkar AA. 2015. Reclamation of petrol oil contaminated soil by rhamnolipids producing PGPR strains for growing Withania somnifera a medicinal shrub. World J Microbiol Biotech. 31(2):307–313. doi: 10.1007/s11274-014-1782-1
- Liu CX, Xiao PG. 1992. Recent advances on ginseng research in China. J Ethnopharmacol. 36(1):27–38. doi: 10.1016/0378-8741(92)90057-X
- Lugtenberg B, Kamilova F. 2009. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 63:541–556. doi: 10.1146/annurev.micro.62.081307.162918
- Lundberg DS, Lebeis S L, Paredes SH, Yourstone S, Gehring J, Malfatti S, et al. 2012. Defining the core arabidopsis thaliana root microbiome. Nature. 488(7409):86–90. doi: 10.1038/nature11237
- Misra N, Gupta G, Jha PN. 2012. Assessment of mineral phosphate-solubilizing properties and molecular characterization of zinc-tolerant bacteria. J Basic Microbiol. 52(5):549–558. doi: 10.1002/jobm.201100257
- Nakkeeran S, Fernado WGD, Siddiqui ZA. 2005. Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and diseases. In: Siddiqui ZA, editor. PGPR: Biocontrol and Biofertilization. Dordrecht: Springer; p. 257–296.
- Park SE, Na CS, Yoo SA, Seo SH, Son HS. 2017. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. J Ginseng Res. 41:36–42. doi: 10.1016/j.jgr.2015.12.008
- Penrose DM, Glick BR. 2003. Methods for isolating and characterizing of ACC deaminase-containing plant growth promoting rhizobacteria. Plant Physiol. 118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x
- Prakamhang J, Tittabutr P, Boonkerd N, Teamtisong K, Uchiumi T, Abe M, et al. 2015. Proposed some interactions at molecular level of PGPR coinoculated with Bradyrhizobium diazoefficiens, USDA110 and B. japonicum, THA6 on soybean symbiosis and its potential of field application. App Soil Ecol. 85:38–49. doi: 10.1016/j.apsoil.2014.08.009
- Rahimi S, Kim YJ, Yang DC. 2015. Production of ginseng saponins: elicitation strategy and signal transductions. App Microbiol Biotech. 99:6987–6996. doi: 10.1007/s00253-015-6806-8
- Ramírez-Bahena MH, Salazar S, Cuesta MJ, Tejedor C, Igual JM, Fernández-Pascual M, et al. 2015. Erwinia endophytica sp. nov. isolated from potato (Solanum tuberosum L.) stems. Int J Syst Evol Microbiol. 66:975–981. doi: 10.1099/ijsem.0.000820
- Rutgers M, Vańt VI, Wind B, Posthuma L, Breure AM. 1998. Rapid method for assessing pollution-induced community tolerance in contaminated soil. Environ Toxicol Chem. 17:2210–2213. doi: 10.1002/etc.5620171111
- Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 160:47–56. doi: 10.1016/0003-2697(87)90612-9
- Shukla PS, Agarwal PK, Jha B. 2012. Improved salinity tolerance of Arachis, hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria[J]. J Plant Growth Regul. 31:195–206. doi: 10.1007/s00344-011-9231-y
- Smith DM, Inman-Bamber NG, Thorburn PJ. 2005. Growth and function of the sugarcane root system. Field Crops Res. 92:169–183. doi: 10.1016/j.fcr.2005.01.017
- Tanja M, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 27(21):2957–2963. doi: 10.1093/bioinformatics/btr507
- Timmusk S, Behers L, Muthoni J, Muraya A, Aronsson AC. 2017. Perspectives and challenges of microbial application for crop improvement. Front Plant Sci. 8:49. doi: 10.3389/fpls.2017.00049
- Timmusk S, Grantcharova N, Wagner EGH. 2005. Paenibacillus polymyxa invades plant roots and forms biofilms. Appl Environ Microbiol. 71:7292–7300. doi: 10.1128/AEM.71.11.7292-7300.2005
- Vaishnav A, Kumari S, Jain S, Varma A, Tuteja N, Choudhary DK. 2016. PGPR-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J Basic Microbiol. 56:1274–1288. doi: 10.1002/jobm.201600188
- Vendan RT, Yu YJ, Sun HL, Rhee YH. 2010. Diversity of endophytic bacteria in ginseng and their potential for plant growth promotion. J Microbiol. 48(5):559–565. doi: 10.1007/s12275-010-0082-1
- Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 73:5261–5267. doi: 10.1128/AEM.00062-07
- Wensing A, Gernold M, Jock S, Jansen R, Geider K. 2014. Identification and genetics of 6-thioguanine secreted by erwinia, species and its interference with the growth of other bacteria. Mol Genet Genomics. 289:215–223. doi: 10.1007/s00438-013-0805-1
- Yadav J. 2012. Enhancement of nodulation and yield of chickpea by co-inoculation of Indigenous spp. and plant growth-promoting rhizobacteria in eastern Uttar Pradesh. Commun Soil Sci Plant Anal. 43:605–621. doi: 10.1080/00103624.2012.639110
- Zhao Q, Shen Q, Ran W, Xiao T, Xu D, Xu Y. 2011. Inoculation of soil by Bacillus subtilis, Y-IVI improves plant growth and colonization of the rhizosphere and interior tissues of muskmelon (Cucumis melo. L.). Biol Fertil Soils. 47:507–514. doi: 10.1007/s00374-011-0558-0
- Zhao Q, Wu YN, Fan Q, Han QQ, Paré PW, Xu R, et al. 2016. Improved growth and metabolite accumulation in Codonopsis pilosula (Franch.) Nannf. by Inoculation of Bacillus Amyloliquefaciens GB03. J Agric Food Chem. 64:8103–8108. doi: 10.1021/acs.jafc.6b03390
