 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
An effective male sterility system enables targeted crosses between parent plants with desired and complementary characteristics. The use of chemical hybridising agents (CHAs) to induce male sterility is quicker and more efficient than manual emasculation. This study investigated the concentration, stage of application and frequency of application of ethyl 4'fluorooxanilate (E4FO) for inducing male sterility of sweet stem sorghum without affecting female fertility. In Trial 1, the dose rate of E4FO was determined to optimise male sterility. In this experiment three genotypes were tested at E4FO dose rates. In Trial 2 the frequency of application of E4FO was determined using three sweet stem sorghum genotypes, three E4FO doses, and six frequencies of application. Data on sterility was inferred based on seed set and seed count from the treated plants. Male sterility was achieved when E4FO was applied during heading stage using the following rates: 1 g l−1, 1.5 g l−1 and 2 g l−1, with more than one application. Applying E4FO twice during the heading stage at a rate of 2 g l−1 would induce male sterility in the tested sweet stem sorghum genotypes, a result that could be useful in hybrid breeding programmes.
Introduction
United States of America, Nigeria, and India are the three major producers of sweet stem sorghum, a multi-purpose, annual, C4 crop (REN21 Citation2016). Among its many uses, sweet stem sorghum has emerged as a useful crop for the production of sugar as well as lignocellulosic biofuel feedstock (Mathur et al. Citation2017). Sweet stem sorghum bred for high biomass production can be converted to biofuels (Codesido et al. Citation2013). A lack of self-sufficiency in non-renewable energy resources, import costs of petro-chemicals, and the need to boost agricultural development are some of the reasons that biofuel production has become important during the past 10 years (Kovarik Citation2013; Araújo Citation2017). According to Schaffert (Citation1992), the crisis in the supplies of fuel oil that occurred in the 1970s was the genesis of the production of ethanol from sweet stem sorghum. Since the year 2000, the global biofuels supply has increased by a factor of 8% (BP Citation2016; REN21 Citation2016). The utilisation of sweet stem sorghum for biofuel production has since increased owing to its environment friendliness: low sulfur content, low biological and chemical oxygen demand, and high octane rating (Reddy et al. Citation2006). Enhancing the quantity and quality of the stalk juice are the chief drivers of sweet stem sorghum breeding. Meeting this goal not only requires extensive germplasm screening but also a well-defined strategy that takes less time because global climate change is an serious problem that calls for urgent mitigation strategies.
Sweet stem sorghum is predominately a self-pollinated crop with a low but quantifiable incidences of outcrossing (Schertz and Dalton Citation1980; Pedersen et al. Citation1998). For this reason, male sterility is essential for the production of hybrid sweet stem sorghum cultivars. Replacing sweet stem sorghum inbred lines with hybrid cultivars enables the exploitation of heterosis or hybrid vigour to increase stalk and sugar yields, and to protect breeder’s right. Sweet stem sorghum inbred lines are also known for their high ethanol productivity owing to their inherent genetic potential as pure line cultivars.
Establishment of male sterility systems enables crosses between chosen parents having desirable and complementary characteristics. This is especially important in sorghum and other self-pollinating crops. In sweet stem sorghum both male and female flowers are found in the same spikelet. In the past, development of hybrid sweet stem sorghum cultivars has mainly relied on a cytoplasmic-genetic male sterility system (CMS), which requires male-sterile line (A-line), a sterility-maintainer line (B-line), and a fertility-restorer line (R-line). This system has been used for decades but it has various shortcomings: the non-availability of breeding stocks containing CMS and restorer systems, their instability and the laborious method of heterosis breeding using CMS. Besides being tedious and time-consuming, this technique sometimes becomes untenable because of the lack of a consistent restorer system for the genetic restoration of fertility (Guilford et al. Citation1992; Pfeiffer et al. Citation2010).
A feasible alternative to the male sterile-maintainer-restorer based on three-line hybrid breeding is two-line hybrid breeding that exploits chemical sterilisation (McRae Citation1985; Guilford et al. Citation1992). Chemical hybridising agents (CHAs) can be used to develop a large pool of heterotic combinations expressing various traits (Mogensen and Ladyman Citation1989). Induction of physiological deformities in the male gamete prevent pollen development, pollen shed or pollen viability is the modus operandi of most CHAs (Cross and Ladyman Citation1991). Since the revolutionary studies on the gametocidal property of maleic hydrazide on gladiolus (Moore Citation1950), a wide range of chemicals have been screened. Some of them selectively induce male sterility in crops. Oxanilates have been reported to selectively impair the pollen formation in monoecious and hermaphrodite plants (Batch et al. Citation1980).
Triticum aestivum (Chakraborty and Devakumar Citation2006), Eragrostis tef (Ghebrehiwot et al. Citation2015), Oryza sativa (Ali et al. Citation1999), Helianthus annuus (Tripathi and Singh Citation2008) and Cicer arietinum (Chakraborty et al. Citation2001) are some of the crops that have been emasculated by ethyl 4'fluorooxanilate (E4FO). In contrast, there is no data on its effects on sweet stem sorghum. In order to use CHAs in sorghum hybrid breeding, it is essential to identify effective and safe chemical male gametocides (Amelework et al. Citation2016). The current study aimed at investigating the concentration, stage of application and frequency of application of E4FO to induce male sterility in sweet stem sorghum without affecting female fertility.
Materials and methods
Trial establishment
The trial was conducted at the Controlled Environment Facility (CEF), University of KwaZulu-Natal. Experiments were conducted in an environmentally controlled greenhouse maintained at an air temperature of 28 ± 2.5°C. The sweet stem sorghum plants were grown from seeds sown directly into plastic pots (300 mm in diameter and 280 mm in height) filled with Gromor potting media (http://www.gromor.co.za). The plants were fertilised with Agchem hydroponic water-soluble fertiliser (http://www.agchem.co.za). The plants were irrigated four times a day for 3 min. Four seeds per pot were sown, then thinned-out to two plants per pot at three weeks after germination. All lateral tillers were constantly clipped off, allowing only one main tiller to grow to flowering. Weeds were hand controlled. The control plots were maintained at a distance to circumvent chemical drift and pollen contamination.
Chemical formulation and application
The CHA used in the current trial was E4FO. The E4FO, formulated as a white powder emulsion, was prepared by first dissolving at a 1:6 w/v ratio with dimethyl sulfoxide (DMSO) and adding 2% Tween 80 as a surfactant. Spray emulsions of 1, 1.5, 2, 2.5 and 3 g l−1 concentrations were prepared by diluting the solution with water. Chemical spraying was done with a hollow cone (HCX) 80° nozzle, using sprayer (). Spraying was done in the early morning. The spray mist was directed to the top of the head until run-off occurred. The quantity of the liquid sprayed per plant was approximately 8–10 ml. Distilled water was used to spray the control treatment.
Sweet stem sorghum genotypes and experimental design
Trial 1: Determination of CHA concentration and stage of application
In this trial, the dose of E4FO was determined to discern maximum male sterility. In the trial, the following three sweet stem sorghum genotypes were used: Kari Mtama, Dwarf Wonder and KAT 487, labelled as AS1, AS71, and AS72, respectively. All three genotypes were chosen for their short plant height. The study targeted shorter varieties that could fit in a greenhouse, and for ease of hand spraying the E4FO. This was aimed to and ascertain the most effective concentration of the CHA. Experimental unit comprised of four pots. Each experimental unit was replicated thrice and arranged using a randomised blocks design. Trial 1 had 18 experimental units or treatments (six E4FO concentrations × three sweet stem sorghum genotypes) and the control. The three sorghum genotypes were treated with six different concentrations of E4FO comprising of 0 g l−1 (distilled water), 1 g l−1, 1.5 g l−1, 2 g l−1, 2.5 g l−1, 3 g l−1. The aqueous solution of the E4FO was sprayed during heading.
Trial 2: Determination of the frequency of application of E4FO
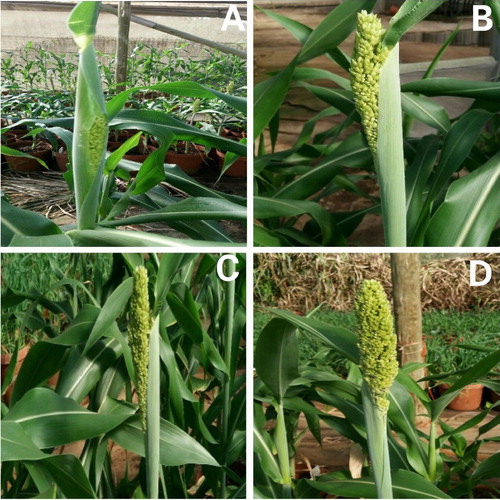
Trial 2 aimed at determining the frequency of application of E4FO required for male sterility using three sweet stem sorghum genotypes, three E4FO doses, and six frequencies of application. Trial 2 was designed based on the results, deductions and conclusions of Trial 1. To establish the frequency of application, AS71 was used together two other sweet stem sorghum genotypes, ICSV 3 and SDSL 89569, denoted as AS17 and AS88 respectively. Each experimental unit comprised of four pots. Each experimental unit was replicated thrice. The experimental units were arranged in a completely random fashion. Trial 2 had 54 experimental units or treatments (three E4FO concentrations × three sweet stem sorghum genotypes × six application frequencies). Three sweet stem sorghum genotypes, AS17, AS71 and AS88, were treated with three concentrations of E4FO of 1 g l−1, 1.5 g l−1and 2 g l−1. All the E4FO applications were carried out during the heading period. illustrates the various E4FO application stages. Three plants of each experimental unit were bagged, and cross pollinated manually two days after the last E4FO application. This was done to test how each treatment affected female fertility of the sweet stem sorghum plants.
Figure 2. Illustration of the different growth stages of the sweet sorghum head at which the E4FO was applied in Trial 2. A, B, C and D are approximately 0, 3, 6 and 9 days in the head protrusion process. E4FO application time combinations were termed as T1 (A + B + C + D); T2 (B + C + D); T3 (A + B + C); T4 (A + C + D); T5 (B + D) and T6 (Control).
Data collection and data analysis
At the end of the trial season, seeds produced from the plants were manually collected, counted and recorded. Data for each replication was collected based on average measurements of four plants. To study female fertility, the number of seeds on cross pollinated plants were counted. Both male sterility and female fertility were calculated by comparison to the control plants. The following formulae were applied:(1)
(1)
(2)
(2) Data was subjected to analysis of variance (ANOVA) using GenStat 17th edition Inc. Means separation of treatments was Fisher’s LSD.
Results and discussion
Determination of CHA concentration and stage of application for effective sterilisation of sweet stem sorghum in Trial 1
Analysis of variance
is a presentation of the analysis of variance (ANOVA) for male sterility. In Trial 1 treatment with six different concentrations of E4FO on three different sweet stem sorghum genotypes showed that concentration of E4FO had a highly significant (p < 0.001) effect on male sterility. However, genotype and the interaction of genotype and concentration had non-significant effects on male sterility. From these results, it can be deduced that E4FO was not genotype specific as all the genotypes had roughly the same levels of male sterility at all the concentrations. This can be attributed to the fact that all the three genotypes are morphologically similar, with short plants because the study targeted shorter varieties that could fit in a greenhouse, and for ease of hand spraying the E4FO. These results are similar to those of Amelework et al. (Citation2016) who studied male emasculation in sorghum and reported highly significant emasculation due to variety and concentration. However, interaction was also significant.
Table 1. Analysis of variance for seed set involving three sweet stem sorghum varieties and six E4FO concentrations in relation to induced pollen sterility.
Mean responses of male sterility and treatment combinations
summarises the degree of male sterility achieved by each treatment combination. As observed by Amelework et al. (Citation2016), levels of pollen sterility increased with an increase in the concentration of test chemicals. Trial 1 showed that almost complete emasculation was achieved with both 2.5 g l−1 and 3 g l−1. However, these concentrations were phytotoxic causing premature senescence of the plants. Similar results were obtained by Ghebrehiwot et al. (Citation2015) on tef, who reported that near complete male sterility was achieved by E4FO at rates ranging between 1.5 g l−1 and 3 g l−1. They also reported floret dryness and early premature senescence when E4FO was applied at 3 g l−1. Amelework et al. (Citation2016) reported that complete pollen sterility was caused by E4FO at concentrations ranging between 2–3 g l−1. No phytotoxicity was observed for concentrations of 1 g l−1, 1.5 g l−1 and 2 g l−1 which is similar to that Ghebrehiwot et al. (Citation2015). However, the male sterility for these concentrations were low (<60%). This can be attributed to the fact that pollen on a sweet stem sorghum head are at different development stages at any given time. Consequently, depending on the application time of the CHA, it can sterilise some of the pollen and leave the rest unsterilised. For this reason, it was essential to conduct Trial 2, which applied three concentrations (1 g l−1, 1.5 g l−1 and 2 g l−1) and apply them more than once.
Table 2. Mean seed set of three sweet stem sorghum genotypes after treatment with E4FO at six concentrations.
Chakraborty and Devakumar (Citation2006) explained that when using E4FO, the CHA technology needed to be optimised in terms of variation in genotype, choice of CHA, stage of spray, numbers of spray, types of formulation, and dose. Although there were no significant differences among the responses of the genotypes, the highest level of male sterility was observed on genotype AS71 for all concentrations except 1 g l−1 and 1.5 g l−1 where AS1 and AS72 had the highest levels of male sterility respectively.
Determination of the optimum frequency of application of E4FO to sterilise sweet stem sorghum in Trial 2
Analysis of variance
Trial 2 is an extension of Trial 1 whereby the frequency of application was increased to more than once. and present the ANOVA for male sterility and female fertility in sweet stem sorghum observed in Trial 2, respectively. It can be observed that all the treatment factors and their interactions were highly significant (p < 0.001). Contrary to Trial 1, in Trial 2 E4FO was a genotype-specific CHA. The change in genotype specificity can be attributed to the fact that genotypes varied morphologically. Morphological variation affects the impact of surfactants that favour the penetration of gametocides into the plant (Parodi and Gaju Citation2009; Amelework et al. Citation2016). Similar female fertility results were obtained by Amelework et al. (Citation2016) who reported that in the bagged panicles, a highly significant difference was observed between varieties, concentrations, and all their interactions.
Table 3. Analysis of variance for seed set among three sweet stem sorghum genotypes to three E4FO concentrations at six frequencies in relation to induced pollen sterility.
Table 4. Analysis of variance of seed set among three sweet stem sorghum genotypes to three E4FO concentrations at six frequencies in relation to female fertility.
Mean responses of pollen sterility and female fertility
Levels of male sterility and female fertility for all treatment combinations are presented in . Male sterility increased with increase in concentration. Moreover, the level of male sterility increased with the frequency of application. The highest level of male sterility observed was 99.6%, a result of applying 2 g l−1 four times (T1) and the lowest level of male sterility was for 1 g l−1 applied twice (T5). Similar results were also found in rice where 100% male sterility was induced by E4FO (Ali et al. Citation1999). For successful hybrid seed production on a commercial level mass emasculation is essential. Ghebrehiwot et al. (Citation2015) noted that the lack of seed production together with high level of pollen sterility found in plants treated with E4FO were indicative of the effectiveness of E4FO in mass emasculation.
Table 5. Male sterility and female fertility as measured by seed set in three sweet stem sorghum genotypes after treatments with three E4FO concentrations at six frequencies.
The high levels of male sterility associated with higher frequency of application, however, came at a cost of lower female fertility. Female fertility increased with a reduction in concentration. The highest levels of female fertility was observed for AS88 sprayed with 1 g l−1 twice (T5), while the lowest female fertility was for AS71 sprayed four times (T1) at 2 g l−1. Applying the CHA twice (T5) resulted in the highest levels of female fertility (desirable) for all genotype x concentration combinations. For genotype AS17, when CHA was applied twice (T5), the highest level of male sterility was observed at 2 g l−1. Hence, the recommendation for high male sterility with functional female fertility for genotype AS17 is 2 g l−1 sprayed twice (T5). The same high level of male sterility with uncompromised female fertility was also achieved by applying the CHA at 2 g l−1 twice for both genotypes AS71 and AS88. Having considered all scenarios, the best balance between high levels male sterility without compromising female fertility were produced by applying the E4FO twice (T5) at a dose of 2 g l−1 (98.6% male sterility and 96% female fertility). Such male sterility percentages comparable to the 95% male sterility considered satisfactory for the production of hybrid seed (Parodi and Gaju Citation2009). Similar results were obtained by Chakraborty and Devakumar (Citation2006) on wheat. They reported that E4FO induced high levels of male sterility (99.76 ± 0.37% at 0.15% concentration over 29 diverse wheat genotypes), and little reduction of female fertility (96.78 ± 2.07% in E4FO treated plants). Ghebrehiwot et al. (Citation2015) reported that 96–99% male sterility was achieved without a significant reduction in female fertility in tef using E4FO at 1 –1.5 g l−1. Research has conveyed that E4FO not only induces a very high degree of male sterility, but also modifies the reproductive biology in such a fashion to ensure cross-pollination in the cleistogamous wheat flowers and increase the probability of the development of hybrids (Chakraborty and Devakumar Citation2006). Ali et al. (Citation1999) concluded that, of the CHAs they tested, E4FO was the most promising and capable of inducing higher levels of pollen sterility than sodium methyl arsenate, their check gametocide.
Acknowledgements
This work was supported by Technology Innovation Agency (South Africa) and National Research Fund (South Africa).
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Precious Mangena is a Doctor of Plant Breeding. She is a co-author for the current study.
Hussein Shimelis is a Professor of Plant Breeding at the University of KwaZulu-Natal in South Africa. He is a co-author for the current study.
Mark Laing is a Professor of Plant Pathology at the University of KwaZulu-Natal in South Africa. He is a co-author for the current study.
Additional information
Funding
References
- Ali AJ, Devakumar C, Zaman FU, Siddiq EA. 1999. Identification of potent gametocides for selective induction of male sterility in rice. Indian J Genet. 59:429–436.
- Amelework A, Laing M, Shimelis H. 2016. Evaluation of effective gametocides for selective induction of male sterility in sorghum. Czech J Genet Plant. 52:163–170. doi: 10.17221/159/2015-CJGPB
- Araújo K. 2017. Low carbon energy transitions: turning points in national policy and innovation. New York, NY: Oxford University Press.
- Batch JJ, Parry KP, Rowe CF, Lawrence DK, Brown MJ. 1980. Plant growth Regulators and Herbicides, Antagonists- Recent Advances: method of controlling pollen formation in monoecious and hermaphrodite plants using oxanilates and their derivatives. New Jersey (NJ): Noyes Data Corporation; p. 82–89.
- [BP] British Petroleum. 2016. Statistical review of world energy. London (UK): British Petroleum.
- Chakraborty K, Devakumar C. 2006. Ethyloxanilates as specific male gametocides for wheat (Triticum aestivum L. Plant Breed. 125:441–447. doi: 10.1111/j.1439-0523.2006.01254.x
- Chakraborty K, Devakumar C, Kumar J, Kumar R. 2001. Effect of anilates on pollen sterility of chickpea (Cicer arietinum L. var. Pusa 240). Trop Agric Res. 13:239–248.
- Codesido V, Vacas R, Macarulla B, Gracia MP, Igartua E. 2013. Agronomic and digital phenotyping evaluation of sweet sorghum public varieties and F1 hybrids with potential for ethanol production in Spain. Maydica. 58:42–53.
- Cross JW, Ladyman JAR. 1991. Chemical agents that inhibit pollen development: Tools for research. Sex Plant Reprod. 4:235. doi: 10.1007/BF00200541
- Ghebrehiwot H, Burgdorf R, Shimelis H, Laing M. 2015. The efficacy of four gametocides for induction of pollen sterility in Eragrostis tef (Zucc.) Trotter. Afr J Biotechnol. 14:774–780. doi: 10.5897/AJB2014.14376
- Guilford WJ, Patterson TG, Vega RO, Fang L, Liang Y, Lewis HA, Labovitz JN. 1992. Synthesis and pollen suppressant activity of phenylcinnoline-3-carboxylic acids. J Agric Food Chem. 40:2026–2032. doi: 10.1021/jf00022a058
- Kovarik B. 2013. Biofuels crops: History of biofuels. Wellington (UK): Center for Bioscience International (CABI).
- Mathur S, Umakanth AV, Tonapi VA, Sharma R, Sharma MK. 2017. Sweet sorghum as biofuel feedstock: recent advances and available resources. Biotechnol Biofuels. 10:146. doi: 10.1186/s13068-017-0834-9
- McRae DH. 1985. Advances in chemical hybridization. Plant Breed Rev. 3:169–191.
- Mogensen HL, Ladyman JAR. 1989. A structural study on the mode of action of CHATM chemical hybridizing agent in wheat. Sex Plant Reprod. 2:173–183. doi: 10.1007/BF00192764
- Moore RH. 1950. Several effects of maleic hydrazide on plants. Science. 112:52–53. doi: 10.1126/science.112.2898.52
- Parodi PC, Gaju MA. 2009. Male sterility induced by the chemical hybridizing agent Colfencent on wheat, Triticum aestivum and T. turgidum var. durum. Ciencia e Investi-gación Agraria. 36:267–279.
- Pederson JF, Toy JJ, Johnson B. 1998. Natural outcrossing of sorghum and sudangrass in the central great plains. Crop Sci. 38:937–939. doi: 10.2135/cropsci1998.0011183X003800040009x
- Pfeiffer TW, Bitzer MJ, Toy JJ, Pedersen JF. 2010. Heterosis in sweet sorghum and selection of a new sweet sorghum hybrid for use in syrup production in Appalachia. Crop Sci. 50:1788–1794. doi: 10.2135/cropsci2009.09.0475
- Reddy BVS, Kumar AA, Ramesh S. 2006. Sweet sorghum: a water saving bioenergy crop. Patancheru (India): ICRISAT. [accessed 2017 August 13] http://www.iwmi.cgiar.org/EWMA/files/papers/PaperforBioenergyandwater.
- [REN21] Renewable Energy Network 21. 2016. Global Status Report: Paris (France): Renewable Energy Network 21.
- Schaffert RE. 1992. Utilization of sorghum and millets: Sweet sorghum substrate for industrial alcohol. Proceedings of the International Workshop on Policy Practice and Potential Relating Uses of Sorghum and Millets. 8–12 Feb 1988 ICRISAT Center Bulawayo, Zimbabwe. p. 131–137.
- Schertz KF, Dalton LG. 1980. Hybridization of crop plants: sorghum. Madison (WI): American Society of Agronomy and Crop Science Society of American.
- Tripathi SM, Singh KP. 2008. Hybrid seed production in detergent induced male sterile Helianthus annuus L. Helia. 31:103–112. doi: 10.2298/HEL0849103T

