ABSTRACT
Four vineyards from the eastern foot of Helan Mountain within the same climate classification, where the planted grapes were 4-year-old Cabernet Sauvignon, were selected for investigating the difference in grape and wine properties. Results showed that the grapes of Lilan vineyard had a higher sugar content and sugar–acid ratio than Huida, Yuquan and Zhihuiyuanshi vineyards. The grapes of Zhihuiyuanshi vineyard had the highest tannin and total phenols content. Concerning the wines, the wine of Yuquan vineyard had low pH but had a great ageing potential, and wine of Zhihuiyuanshi vineyard had highest tone, chroma, tannins, total phenols and alcohol content than other vineyards. Soil pH was positively correlated with anthocyanins in the grapes and negatively correlated with total acid in wine. The microbial biomass carbon (MBC) was correlated with the anthocyanins in grape. Microbial biomass nitrogen (MBN) was positively correlated with soluble solid in grapes, and positively correlated with tannin and total phenols in the wine. The MBC/MBN ratio was negatively correlated with tone in the wine. Our findings indicate that adjusting soil pH and choosing microbial fertiliser with high soil microbial carbon and nitrogen nutrients are effective ways to improve the quality of regional grapes and wine.
Introduction
According to the statistical data of the International Organization of Vine and Wine, the total area of grape planting in the world has increased rapidly by 5.8 million ha in 1997, through 6.7 million ha in 2007, to 7.6 million ha in 2017, and the output of wine was keeping in 24–27 billion litres, which was the second-largest yield in the world for beverage wine, and indicating beverage wine have great developmental potential (OIV Citation2017). With the increase in global grape wine consumption, wine quality and wine production have paid extensive attention (Picard et al. Citation2018). Terroir is the main basis for representing regional wine quality, and it is the overall characteristics of regional climate, soil, and management measures (Ricardo-Rodrigues et al. Citation2019). Combined with different management measures, including fertilization, irrigation, pests and diseases controlling (Jermini et al. Citation2010; Žežlina et al. Citation2010, Citation2013) and pruning (Cornelis van et al. Citation2004), distinctive grapes and wine with special local flavour are produced. The formation of the terroir by natural and artificial factors is a comprehensive interaction process. In a large spatial scale, climate conditions are the main factors determining the quality and terroir of wine grapes and wine in an area. However, on a microscale in small vineyards, some factors may cause considerable differences in local soil, leading to variations in the characteristics of vineyards in different locations within a small area (Bramley et al. Citation2011; Wang et al. Citation2015). With basically the same management measures, soil condition has become the decisive factor of vineyard terroir, thereby causing a small region to produce wine with different terroirs (Rankine et al. Citation1971; Prado et al. Citation2007).
The terroir of wine grapes is a complex problem. Current studies focus on terroir differences caused by the effect of climate and terrain, and further studies should explore vineyard terroir caused by soil under consistent local climatic conditions (Bramley Citation2005; Trought et al. Citation2008; Bramley et al. Citation2011). The eastern foot of Helan Mountain (Latitude in 37°43′N–39°23′N, Longitude in 105°45′E–106°47′E and Altitude in 1100–1120 m) in Ningxia Hui Autonomous Region has a flat terrain, sufficient sunshine with annual sunshine duration of 2850–3110 h, and gravel in soil. Some experts and scholars have considered this site to be one of the rare production areas for high-end wines worldwide (Wang et al. Citation2015; Jiang et al. Citation2016; Ma et al. Citation2018). Although the vineyards along the eastern foot of Helan Mountain were classified as the same climate and hydrology type, differences were observed in the properties of local soil among the vineyards (Wang et al. Citation2015). Therefore, it would be of interest to study the impact on the terroir of vineyards. Consequently, we selected Qingtongxia, Hongsibao, Yuquanying and Yinchuan production areas situated at the eastern foot of Helan Mountain as our study sites. One vineyard was chosen in each of the four sites respectively, and the most widely cultivated 4-year-old Cabernet Sauvignon was taken as the reference of grape quality in the area. The soil properties and quality indicators of grape and wines were subjected to correlation analysis to explore the specific sensitive soil quality factors affecting the wine quality, and to provide a basis for implementing the refined management of vineyards in Helan Mountain, the cultivation of wine grapes, and the development of the wine industry.
Materials and methods
Study area
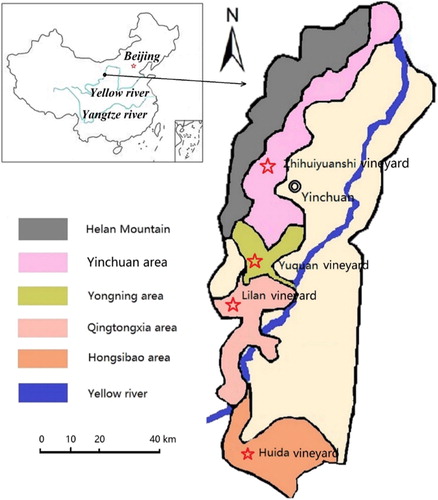
The eastern foot of Helan Mountain in Ningxia is located in the 37°43′N–39°23′N and 105°45′E–106°47′E, which is in the midtemperate semi-arid climate zone. This area is the alluvial fan in front of Helan Mountain and in the transition zone of Helan Mountain and Yinchuan Basin. Helan Mountain is approximately 200 km long from north to south with a total area of approximately 2,00,000 ha. It is tilted from the southwest to the northeast, and the ground is relatively flat, with an altitude of 1100–1120 m. The average annual temperature is 8.6°C to ≥10°C, the effective accumulated temperature is 3135–3272°C, the diurnal temperature change is 10–15°C, sunshine lasts 3032 h with the sunshine rate of 67%, and the annual precipitation is 180–200 mm. The soil of this area is corroded slightly, with a thick soil layer. The soil types are mainly ustic cambosols with aeolian sandy texture, and some soil contains gravels, which are suitable for the growth of wine grapes. The production area is flat, with sufficient sunshine, convenient irrigation conditions, and abundant heat. The diurnal temperature change from August to September is large, which is conducive to the accumulation of sugar in grapes. With Helan Mountain in the west as the natural barrier against cold current and Yellow River irrigation channel crossing in the east, the water needs of each period of grape growth can be met. The wine industry in the eastern foot of Helan Mountain in Ningxia started up in the 1980s and has entered a rapid development. To date, the planting area of grapes here has reached more than 40,000 ha, accounting for a quarter of the country’s total acreage and is the largest production area in China. The wine grape planting at the eastern foot of Helan Mountain can be divided into four production areas: Hongsibao area, Qingtongxia area, Yinchuan area, and Yongning area (). Nearly 100 wineries, which correspond to more than one-third of the total number of wineries in China, have been built in this area.
Experimental design
In 2013, four vineyards, Lilan (LL) vineyard distributed in Qintongxia production area with an area of 2.5 ha, Huida (HD) vineyard distributed in Hongsibao production area with an area 3.2 ha, Yuquan (YQ) vineyard distributed in Yongning production area with an area 2.9 ha, and Zhihuiyuanshi (ZH) vineyard distributed in the Yinchuan production area with an area 4. ha, were selected. Three sets of repetitions were set in each vineyard, and each set had area of approximately500m2. The four vineyards all planted Cabernet Sauvignon, which were planted in north and south direction. The vine form was tilted to the shelves, with the row space of 0.8 m × 3 m. The irrigation way was trickle irrigation, and the manuring was by root application. The fertilization of all the vineyards used the same type, amount, and method. After the grape was unearthed, 120 kg ha−1 urea (with 55.2 kg ha−1 N) was applied at the end of April, and urea and diammonium phosphate were applied at 225 kg ha−1 (N: 40.5 kg ha−1; P2O5: 108 kg ha−1) after the flowering in late May. Nitrogen, phosphorus, and potassium ternary compound fertilizer was applied at 375 kg ha−1 (N: 56. kg ha−1; P2O5: 56.3 kg ha−1; K2O: 56.3 kg ha−1) in the second half of June, and additional fertilizer of top dressing potassium sulphate was applied at 450 kg ha−1 (K2O: 225 kg ha−1) at the end of August. All land parcels were managed in the same way.
Sample collection
In 2017, the soil samples were collected during germination, anthesis, swelling, veraison, and maturation stages of grape growth in the vineyards. Given that grapes are perennial plants, the roots are mainly distributed within 50 cm from the horizontal trunk. Therefore, the sampling site was set at 30–40 cm from the grape trunk horizontally. The grape root system was within 60 cm from the soil surface, providing the sampling depth of vineyards of 60 cm. In the study area, the five-point sampling method was adopted to collect 0–60 cm soil samples by using an auger. Samples were taken at intervals of 20 cm, and the soil samples at each soil layer were uniformly mixed and then packed in two ziplock bags. One bag was stored in a refrigerator as fresh soil sample for soil microbial biomass properties measurement. After air dried, another bag sample was passed through a 2 mm sieve to separate the gravels (>2 mm) and soil (<2 mm) and to determine the mass percentage of gravels in the sample by weighing method. At the time of harvest, 10 clusters of representative ears of the same part of grapes in each set of vineyards, which was consistent with soil sample sits, were randomly collected and washed by distilled water and frozen and stored for later analyses.
Soil, grape and wine properties measurement
Measurement of soil physical and chemical properties
The soil particle size distribution was determined by the Master Sizer2000 ultrasonic particle size analyzer. Soil cores (volume = 100 cm3) were oven-dried at 105°C for 24 h and dry weight of soil was monitored. Soil bulk density was calculated as the ratio of dry soil weight and the volume of the soil. The soil porosity was obtained from known bulk density and soil particle density (ds = 2.65 g cm−3) (Qi et al. Citation2015). A glass electrode was used to measure the soil pH in 1:5 soil:water suspension. Soil organic matter (SOM) was determined by the dichromate-wet combustion method (Nelson and Sommers Citation1982), total nitrogen (TN) by the Kjeldahl method (Bremner and Mulvaney Citation1982), and available nitrogen (Av-N) by the alkali diffusion method. Soil available phosphorus (Av-P) content was extracted with 0.5 mol L−1 NaHCO3 at a pH of 8.5, and P was obtained colorimetrically by the molybdate method (Olsen et al. Citation1954). Soil available potassium (Av-K) content was determined by extraction with 1N ammonium acetate and using an atomic absorption spectrometer (AAS) (Lu Citation2000).
Measurement of soil microbial biomass properties
The chloroform fumigation extraction method was applied to measure microbial biomass carbon (MBC), microbial biomass nitrogen (MBN) and microbial biomass phosphorus (MBP). About 20 g of fresh soil sample were exposed to alcohol-free chloroform (CHCl3) vapour in a vacuum desiccator containing soda lime at 25°C for 24 h. Fumigated soil was then transferred into an empty desiccator, and residual CHCl3 was removed from the fumigated soils by repeated evacuations. The fumigated soil was extracted immediately following CHCl3 removal by shaking for 30 min with 50 mL 0.5 mol L−1 K2SO4. The unfumigated samples were extracted at the time of fumigation commencement. Automatic analyzers were used to measure total soil organic carbon (Shimadzu, TOC-500) and soil nitrogen (Astoria, Pacific-Inc.) (Guan Citation1986; Li et al. Citation2008).
By using the dried soil sample, urease was determined by indophenol blue colorimetric method. Alkaline phosphatase was determined by sodium phenyl phosphate colorimetry. Sucrase was determined by the 3,5-dinitrosalicylic acid colorimetric method. Catalase was determined by potassium permanganate titration, and protease was determined by the ninhydrin method (Guan Citation1986).
Determination of properties of grape and wine
All the grapes were collected exactly same sites as the soil sample. After squeezed, 30 mL grape juice was used to record the pH by using a Metmorph 702SM automatic neutralizer (Titrino, Herisau, Switzerland). Brix was determined using a Brix refractometer (PR32 Atago Co. Ltd., Japan). The content of reducing sugar was measured using titration with Fehling reagent, and titratable acidity was determined using standardized 0.1 N NaOH (end-point pH 8.2). The total phenols content was determined by the Folin–ciocalteu method by using a gallic acid meter (Rapisarda et al. Citation1999). The tannin content was determined by Fehling reagent method by using a tannic acid meter (Wang et al. Citation2013). The anthocyanin content was determined by the pH differential method by using a malvidin-3-glucosinometer (Orak Citation2007).
For the collected grape fruits, the stems were cut and squeezed to expose the inside, then poured into a 2.5 L fermentor, and added 20 mg L−1 pectinase and 50 mg L−1 partial potassium sulphite. The fermentation was carried out at 25–30°C with Saccharomyces cerevisiae (LAFFORT from France) (Wang Citation1999). After 3 weeks, the fermentation was completed. The wine was separated from the skin residue of grapes, filtered, and stored in bottles. The pH value of 30 mL wine was determined by a pH meter. The alcohol content was determined by the alcohol method in the National Standard GB 5009.225–2016 ‘Determination of Ethanol Concentration in Wine’ (NHCPR Citation2016). The spectrophotometer was adopted to measure the absorbance values at wavelengths of 420, 520, and 620 nm (A420, A520, and A620 nm), wherein the sum of the three was the chroma, and the ratio of A420 to A520 nm was the tone (Pérez-Magariño and González-Sanjosé Citation2003). The total phenols and tannin contents were determined using the same methods as in the grape. The total acid was determined using standardized 0.1 N NaOH (end-point pH 8.2) (Wang et al. Citation2017).
Data analysis
Statistical analysis was conducted using DPS9.5 software, and Duncan’s multiple range was used to carry out the significance test on the physical and chemical properties of soil, biological characteristics, and the properties of grapes and wine. The correlation analysis between soil physical and chemical properties and properties of grapes and wine was performed by the SPSS software.
Results
Soil properties of different wineries
showed that the soil was coarse. The soil of the four vineyards did not differ in texture, which were all gravel soil containing 5–13% gravel (). With the mountain body distant from Helan Mountain decreased, the gravel content tended to increase. The sand content of the four vineyards all exceeded 85%, and the content of silt and clay was less than 8.5%. The soil of the four vineyards had tighter structure, with the soil bulk density of 1.36–1.66 g cm−3 and soil porosity of 37–48%.
Table 1. Soil physical properties in different vineyards.
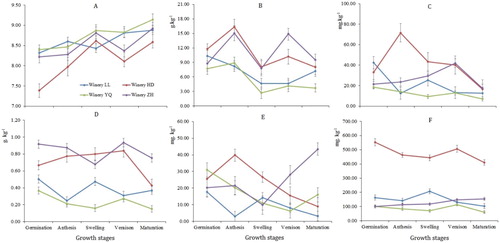
According to , soil pH, SOM and nutrients changed greatly during the growth period. Soil pH showed an increasing trend during the growth process. Soil pH was 7.39–8.47 in the germination stage in the four vineyards, while it increased to 8.59–9.20 in the maturation stage. Huida vineyard had the lowest soil pH and Yuquan vineyard had the highest soil pH. The SOM content showed variation during the growth process with lower content in the swelling stage, and it was higher in the Huida and Zhihuiyuanshi vineyards than in Lilan and Yuquan vienyards. The content of TN, Av-N and Av-P showed the similar distribution with SOM and were higher in the Huida and Zhihuiyuanshi vineyards wineries than in Lilan and Yuquan vineyards in the most of the growth stages, while the variation became more complex during the growth process. The available potassium content of Huida vineyard was higher than that of other vineyards and smoothly varied during the growth process.
Figure 2. Soil chemical properties at different vineyards and grape growth stages. A = Soil pH, B = soil organic carbon, C = available nitrogen, D = total nitrogen, E = available phosphorus, F = available potassium. ![]()
shows that the four vineyards did not differ with respect to microbial biomass carbon (MBC). The microbial biomass nitrogen (MBN) of Zhihuiyuanshi vineyard was higher than the others. The microbial biomass phosphorus (MBP) of Yuquan vineyard was the highest of 1.53 mg kg−1, and Lilan vineyard was the lowest of only 0.43 mg kg−1. shows that the MBC/MBN ratios were low in the Zhihuiyuanshi vineyard (3.22) and Huida vineyard (5.53), and they were remarkably lower than those of Lilan vineyard (8.77) and Yuquan vineyard (8.97).
Table 2. Soil microbial biomass in different vineyards.
shows that the contents of alkaline phosphatase of the four vineyards were all above 5 mg g−1, indicating that despite the low phosphorus supply ability of the soil, its activity was high. In particular, the phosphatase of Huida vineyard and Zhihuiyuanshi vineyard reached 15.62 and 12.80 mg g−1, respectively. The sucrase content in Zhihuiyuanshi vineyard was higher than those in the three other vineyards. The urease was high in Huida vineyard and low in Zhihuiyuanshi vineyard, and protease was high in Lilan vineyard, but that in Yuquan vineyard and Zhihuiyuanshi vineyard was significantly low. Catalase was high in Huida vineyard and low in Lilan vineyard and Yuquan vineyard.
Table 3. Soil enzymatic activity of the vineyards.
Grape properties in different vineyards
As shown in , the grape soluble solids ranged from 22 to 28 0Brix, and differences were detected among the selected four vineyards in the order of Zhihuiyuanshi vineyard > Lilan vineyard > Yuquan vineyard > Huida vineyard. No differences in the pH of grape juice were detected among the vineyards.
Table 4. Properties of grapes from the vineyards.
shows that the titratable acid of the grapes from the four vineyards differed. Grapes from Yuquan vineyard showed the highest value of 7.02 g L−1, which was higher than those of the three other vineyards. The content of reducing sugar in the grapes grown at Huida vineyard was the highest of 45.21 g L−1. The contents of reducing sugar in the grape from Yuquan and Zhihuiyuanshi vineyard did not differ, but were lower in Lilan vineyard. The ratios of sugar to acid in the four vineyards were 49–88, the lowest in Lilan and the highest in Huida.
From , the anthocyanin content of grapes from Yuquan vineyard was the highest of 5.16 mg g−1; the total phenols content of grapes from Lilan vineyard was the highest of 3.75 g g−1 and it was the lowest of only 1.13 g g−1 in the Huida vineyard. The tannin content of grapes grown in Zhihuiyuanshi vineyard was the highest of 18.38 mg g−1, and the second was Lilan vineyard of 16.04 mg g−1. The tannin content of grapes from Yuquan vineyard was the lowest of only 13.60 mg g−1.
Wine properties in different vineyards
According to , the wine chroma in the four vineyards differed, and the chroma of Zhihuiyuanshi vineyard was the highest of 2.80. The tone of wine produced from vineyards differed, wherein the tone of Yuquan vineyard was the smallest of 0.49, and that of Zhihuiyuanshi vineyard was the largest of 0.57. The pH values of the wine varied between 3.59–3.77. The value is shown in the following order: Zhihuiyuanshi > Lilan > Huida > Yuquan. The tannin contents of wine from the vineyards all above 2 g L−1, and the value of Zhihuiyuanshi vineyard of 2.5 g L−1 was highest.
Table 5. Properties of wine produced from the vineyards.
In , similar to the tannin content, the total phenols of wine from Lilan vineyard, Huida vineyard, and Yuquan vineyard did not differ. Conversely, the total phenols content of Zhihuiyuanshi vineyard (2.04 g L−1) was highest. The alcohol content ranged from 12.10% to 13.40% and differed among the vineyards. Consistent with the soluble solids in grapes, the alcohol content was highest in the Zhihuiyuanshi vineyard and lowest in Yuquan vineyard. The total acid content in the wine ranged from 6.7 to 7.0 g L−1, with small differences among the vineyards, and with the highest content in Huida vineyard.
Correlation between soil and grapes and wine properties
From and , no correlations between soil properties and grapes and wine properties were deserved. However, soil pH was positively correlated with total anthocyanin content and negatively correlated with total acid content of wine (p < 0.05). The soil microbial biomass carbon, nitrogen, and phosphorus had a remarkable effect on the properties of grapes and wine, in which the microbial biomass carbon was positively correlated with the anthocyanin (p < 0.01). The microbial biomass nitrogen was positively correlated with the tannin content (p < 0.05) and positively correlated with the total fentanyl content (p < 0.01). The C/N ratio of microbial biomass was negatively correlated with the chrome (p < 0.05).
Table 6. Correlation between soil factors and properties of wine grapes.
Table 7. Correlation between soil factors and wine properties factors.
Discussion
Even climate was considered to be the main constraining effect on wine properties (Muscas et al. Citation2017), soil provides the vine with nutrients and water, and any chemical composition imbalance will affect vine growing and wine quality (Prado et al. Citation2007; Wang et al. Citation2012). As shown in , profited from favourable climate and soil properties, four high-end wine production areas have established by the government and nearly 100 wineries have been invested by the vineyard owners along the eastern foot of Helan Mountain. Soil characteristics can help to explain differences in wine quality even within the same region or climate classification (Prado et al. Citation2007), this has been proved by the significant differences of most of soil properties as shown in and , as well as partly variation of grape and wine properties as shown in and . In this paper, the correlation analysis of the soil, grapes, and wine properties showed that most of the indicators were not correlated as shown in and . However, some soil properties, which were sensitive to the properties of grapes and wine, were found. As shown in and , the data support the observations made by Noble (Citation1979) and Song et al. (Citation2018) that soil pH affected the total anthocyanin of grapes and the total acidity of wine. This is possible because a high pH suggests a high CaCO3 content, and Ca content can affect the total anthocyanin in grapes and wine and the acidity of wine. In addition, pH can influence the effectiveness of some nutrients in the soil, thus affecting the quality of grapes. The negative correlation between soil pH and total acidity of wine indicated that adjusting soil pH is an effective way to improve wine quality in the vineyards with alkaline soil by decrease soil CaCO3 content.
Another factor sensitive to the properties of grapes and wine is soil microbial nutrients. and showed that microbial biomass carbon significantly affected the total anthocyanin of grapes. Microbial biomass nitrogen influenced the tannin content and the total phenols content of wine. In addition, the C/N ratio of microbial biomass significantly affected the tone of wine. The total amount of biomass in the soil except those less than 5 ×103 μm is called soil microbial biomass, which is the active part of soil organic matter. Soil microbial biomass accounts for only approximately 3% of the total soil organic matter. Although microbes are not directly involved in the growth of grapes, they directly influence various biochemical processes, including organic material decomposition in soil, humus formation, nutrient conversion and circulation (Jin et al. Citation2009; Jiang and Zhang Citation2012; Qi et al. Citation2018). Thus, soil microbial biomass nutrients content affects the activity of microbes in soil and further influences the quality of plant products. The reported research results showed that the microbial biomass nutrient content in soil, especially nitrogen content, might change the microenvironment of leaf curtain and the phenols content in grape fruits (Downey et al. Citation2006; Cohen and Kennedy Citation2010; Schreiner et al. Citation2013).
Fertilization was considered to be one of the most important management measures during vine growth. The chemical fertilizer was regarded as the straight fertilizer by providing macronutrients for plant growth directly, and this has been emphasized by the vineyard to achieve higher yield (Arrobas et al. Citation2014). In recent years, researchers have recognized that soil is a living environment, the habitat of tens of thousands of species, and many of these are very important to plant health (Jiang et al. Citation2016). While, very little about the interactions between different species of soil microorganisms, or between microorganisms and crop species has been understood. However, we are becoming increasingly aware that biologically active soil helps control soil-borne diseases and promotes plant vigour. In this research, the positive correlation between soil microbial nutrients and some important grapes and wine properties as shown in and indicating that higher microbial biomass content responding for improvement of grapes and wine quality, therefore, applying microbial fertilizer is a straight measure to improve wine quality in the vineyard.
Acknowledgments
Authors thank professor Shukla, New Mexico State University, for review the English and editing the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Yanbing Qi is an associate professor at the College of Natural Resources and Environment, Northwest A&F University, and researches on GIS and soil quality evaluation.
Rui Wang is an associate professor at the School of Agricultural, Ningxia University, and researches on plant nutrition.
Qianru Qin is a master student at the College of Natural Resources and Environment, Northwest A&F University.
Quan Sun is a professor at the School of Agricultural, Ningxia University, and researches on plant nutrition.
Additional information
Funding
References
- Arrobas M, Ferreira IQ, Freitas S, Verdial J, Rodrigues MA. 2014. Guidelines for fertilizer use in vineyards based on nutrient content of grapevine pares. Sciential Hortic. 172:191–198. doi: 10.1016/j.scienta.2014.04.016
- Bramley RGV. 2005. Understanding variability in winegrape production systems. 2. Within vineyard variation in quality over several vintages. Aust J Grape Wine Res. 11:33–42. doi: 10.1111/j.1755-0238.2005.tb00277.x
- Bramley RGV, Ouzman J, Boss PK. 2011. Variation in vine vigour, grape yield and vineyard soils and topography as indicators of variation in the chemical composition of grapes, wine and wine sensory attributes. Aust J Grape Wine Res. 17:217–229. doi: 10.1111/j.1755-0238.2011.00136.x
- Bremner JM, Mulvaney CS. 1982. Nitrogen-total. In: Page AL, et al. editor. Methods of soil analysis. Part 2 Chemical and microbiological properties. Madison, WI: American Society of Agronomy; p. 595–624.
- Cohen SD, Kennedy JA. 2010. Plant metabolism and the environment: implications for managing phenolics. Crit Rev Food Sci. 50:620–643. doi: 10.1080/10408390802603441
- Cornelis van L, Philippe F, Xavier C, Oliver T, Stephanos K, Denis D. 2004. Influence of climate, soil, and cultivar on terroir. Am J Enol Viticult. 55:207–217.
- Downey MO, Dokoozlian NK, Krstic MP. 2006. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: a review of recent research. Am J Enol Viticult. 57:257–268.
- Guan SY. 1986. Research methods on soil enzyme activity. Beijing: China Agricultural Press. (in Chinese with English abstract).
- International Organisation of Vine and Wine (OIV). 2017. Globe economic vitiviniculture data [EB/OL]. http://oiv.int/public/medias/5686/ptconj-octobre2017-en.pdf.
- Jermini M, Blaise P, Gessler C. 2010. Quantitative effect of leaf damage caused by downy mildew (Plasmopara viticola) on growth and yield quality of grapevine ‘Merlot” (Vitis vinifera). Vitis. 49(2):77–85.
- Jiang B, Zhang ZW. 2012. Comparison on phenolic compounds and antioxidant properties of Cabernet Sauvignon and merlot wines from four wine grape-growing regions in China. Molecules. 17:8804–8821. doi: 10.3390/molecules17088804
- Jiang P, Wang R, Ji LD, Sun Q, Xu XR, Li L. 2016. Study on the application effect of the trace elements on wine grapes in the eastern foot of the Helan mountain. North Horticul. 40(5):22–27. (in Chinese with English abstract).
- Jin ZZ, Lei JQ, Xu XW, Li SY, Fan JL, Zhao SF, Zhou HW, Gu F. 2009. Relationships of soil microbial biomass with soil environmental factors in Tarim Desert highway shelter-forest. Chin J Appl Ecol. 20:51–57. (in Chinese with English abstract).
- Li ZG, Luo YM, Teng Y. 2008. Research methods for soil and environmental microorganism. Beijing: China Agricultural Press. (in Chinese with English abstract).
- Lu RK. 2000. Soil and agro-chemical analytical methods. Beijing: China Agricultural Science and Technology Press. (in Chinese).
- Ma LW, Li JP, Han YJ, Li WC. 2018. Meteorological conditions and rating method of quality formation of ‘Cabernet Sauvignon’ grape in eastern foothills of Helan Mountain. Chin J Eco-Agric. 26(3):453–466. (in Chinese with English abstract).
- Muscas E, Cocco A, Mercenaro L, Cabras M, Lentini A, Porqueddu C, Nieddu G. 2017. Effects of vineyard floor cover crops on grapevine vigor, yield, and fruit quality, and the development of the vine mealybug under a Mediterranean climate. Agric Ecosyst Environ. 237:203–212. doi: 10.1016/j.agee.2016.12.035
- National health commission of the people’s republic of China (NHCPR). 2016. GB 5009.225-2016: Ethyl alcohol measure method for liquor. Beijing: Standards Press of China. (in Chinese with English abstract).
- Nelson DW, Sommers LE. 1982. Total carbon, organic carbon, and organic matter. In: Page AL, RH Keeney DR, editor. Methods of soil analysis, part 2-chemical and microbiological properties. Madison, WI: ASA-SSSA; p. 539–594.
- Noble AC. 1979. Evaluation of Chardonnay wines obtained from sites with different soil compositions. Am J Enol Viticult. 30:217–217.
- Olsen SR, Cole CV, Watanable FS, Dean LA. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Washington, DC: USDA Circular 939.
- Orak HH. 2007. Total antioxidant activities, phenolics, anthocyanins, polyphenol oxidase activities of selected red grape cultivars and their correlations. Sci Hortic. 111:235–241. doi: 10.1016/j.scienta.2006.10.019
- Pérez-Magariño S, González-Sanjosé ML. 2003. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem. 81:301–306. doi: 10.1016/S0308-8146(02)00509-5
- Picard D, Moreira CN, Loloum T. 2018. Wine magic consumer culture, tourism, and terroir. J Anthropol Res. 74:526–540. doi: 10.1086/699943
- Prado RA, Yuste-Rojas M, Sort X, Andreä S-Lacueva C, Torres M, Rosa ML. 2007. Effect of soil type on wines produced from Vitis vinifera L. Cv. Grenache in commercial vineyards. J Agric Food Chem. 55:779–786. doi: 10.1021/jf062446q
- Qi YB, Chen T, Pu J, Yang FQ, Shukla MK, Chang QR. 2018. Response of soil physical, chemical and microbial biomass properties to land use changes in fixed desertified land. Catena. 160:339–344. doi: 10.1016/j.catena.2017.10.007
- Qi YB, Yang FQ, Shukla MK, Pu J, Chang QR, Chu WL. 2015. Desert soil properties after thirty years of Vegetation Restoration in Northern Shaanxi Province of China. Arid Land Res Manage. 29:454–472. doi: 10.1080/15324982.2015.1030799
- Rankine B, Fornachon B, Boehm E, Cellier K. 1971. Influence of grape variety, climate and soil on grape composition and on the composition and quality of table wines. Vitis. 10:33–50.
- Rapisarda P, Tomaino A, Lo Cascio R, Bonina F, De Pasquale A, Saija A. 1999. Antioxiadant effectiveness as influenced by phenolic content of fresh orange juices. J Agri Food Chem. 47:4718–4723. doi: 10.1021/jf990111l
- Ricardo-Rodrigues S, Laranjo M, Coelho R, Martins P, Rato AE, Vaz M, Valverde P, Shahidian S, Vestia J, Agulheiro-Santos AC. 2019. Terroir influence on quality of ‘Crimson’ table grapes. Sci Hortic. 245:244–249. doi: 10.1016/j.scienta.2018.10.035
- Schreiner RP, Scagel CF, Lee J. 2013. N, P and K supply to Pinot noir grapevines: impact on berry phenolics and free amino acids. Am J Enol Viticult. 65:43–49. doi: 10.5344/ajev.2013.13037
- Song JQ, Qu HG, Liang HZ, Zhai RY, Jiang WG, Shen ZY, Duan CQ, Li JM. 2018. Correlation analysis between soil indicators and physicchemical indexes of wine composition in “Cabernet Gernischt” vineyard. Acta Agric Bor Sin. 27:863–870. (in Chinese with English abstract).
- Trought MCT, Dixon R, Mills T, Greven M, Agnew R, Mauk JL, Praat JP. 2008. The impact of differences in soil texture within a vineyard on vine vigour, vine earliness and juice composition. J Int Sci Vigne Vin. 42:67–72.
- Wang H. 1999. Experimental handle book for vine and wine detection. Xi’an: Xi’an Maps Press. (in Chinese with English abstract).
- Wang HA, Li JM, Jiang WG, Gao M, Liang HZ. 2013. Effects of different soil textures on wine quality of Cabernet Gernischt. Sino Grape Wine. 4:24–27.
- Wang R, Ma L, Li L, Gu X, Sun Q. 2017. Influence of different fertilization treatments on soil fertility and wine grapes composition. North Horticul. 18:121–126. (in Chinese with English abstract).
- Wang R, Sun Q, Chang QR. 2015. Soil types effect on grape and wine composition in Helan mountain area of Ningxia. PLoS ONE. 10:e0116690. doi: 10.1371/journal.pone.0116690
- Wang R, Sun Q, Guo J, Zhang XJ, Chang QR. 2012. Influence of irrigation and fertilization on the wine grape growth, yield and quality. Agric Res Arid Area. 30:123–127. (in Chinese with English abstract).
- Žežlina I, Skvarc A, Bohinc T, Trdan S. 2013. Testing the efficacy of single applications of five insecticides against Scaphoideus titanus on common grapevines. Int J Pest Manage. 59(1):1–9. doi: 10.1080/09670874.2012.735378
- Žežlina I, Skvarc A, Rusjan D, Trdan S. 2010. The efficacy of different spraying programs against two fungal pathogens in organic grape production. J Plant Dis Prot. 117(5):220–225. doi: 10.1007/BF03356364