?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Nitrogen uptake being part of nitrogen use efficiency (NUE) is largely decided by root traits. Root traits variability has hardly been explored by breeders mainly because of difficulties in scoring. The hydroponic system requiring lesser space for precise phenotyping of large numbers of genotypes independently of the growing season can be a suitable alternative. However, the effectiveness of hydroponic screening methods needs to be verified under the soil condition of the field or pot. In the present study, root traits and NUE were investigated in 19 genotypes under two conditions (hydroponic and pipe filled with soil). Both environments revealed large variability for root traits and NUE under high and low N conditions establishing the absence of any direct selection for these traits in the past. Under both sets of experimentation, NUpE was largely responsible for improved nitrogen efficiency mainly because of higher root biomass. The significant association between the two screening methods i.e. hydroponic and pot filled with soil under both low and high N condition support large scale screening for root traits under hydroponic condition.
Introduction
Increased nitrogen (N) fertilisation besides irrigation and dwarf varieties was an important factor for increasing wheat yield during the twentieth century (Yadav et al. Citation2010) and have further scope for yield consolidation through genetic manipulation and selection under improved environment (Yadav et al. Citation2017). The continuous response of improved germplasm to the higher application of N pushed the crop producers for higher and higher N applications to realise better yield. However, a large portion of applied N (around 60%) is lost in the ecosystem largely through volatilisation, runoff, denitrification and leaching (Malyan et al. Citation2016). To meet the projected demand for food with minimum footprints of N fertiliser on the environment, improving nitrogen use efficiency (NUE) of cropping systems is need of the time (Ranjan and Yadav Citation2019).
N uptake efficiency (NUpE) and N utilisation efficiency (NUtE) are the two primary components of NUE. Theoretically, NUE can be enhanced by improving either NUpE or NUtE or both. Genetic variation for NUE parameters in cereal crops has been reported in several studies (Ortiz-Monasterio et al. Citation1997; Foulkes et al. Citation1998; Le Gouis et al. Citation2000, Barraclough et al. Citation2010; Fageria and Santos Citation2014, Yin et al. Citation2018 and Ranjan et al. Citation2019). Root being the first point of contact for plants with soil solution, the primary root traits are important to improve N captures (Foulkes et al. Citation2009). Selection for root length, lateral root dispersion, root biomass and root density improved the water and nutrient uptake in upland rice (Price et al. Citation2002), wheat (Hurd Citation1964) and maize (Lynch Citation2007). All of these root architectural traits are under multigenic control with very large environmental impact and therefore have poor selection efficiency (Hall and Richards Citation2013).
However, due to difficulties in large scale phenotyping of root traits, genetic progress by exploring these traits is largely limited. Recent advancement in screening techniques of root phenotype such as rhizotron, digital imaging technique (Manschadi et al. Citation2006), hydroponics and pots screening, have some limitations. Hydroponic screening requiring limited space, less labour, its amenability for creating variable environments, less error prone and its computability for large screening required in the most of breeding programme is attracting a lot of attention of researchers in recent years. However, its reliability of screening under hydroponic needs to be verified in other suitable media like a pot filled with soil. Therefore, the present experiment was carried out to examine critically the ability of hydroponic screening to select N use efficient genotypes as effectively as a pot filled with soil.
Materials and methods
Plant materials
A subset of 19 genotypes (Table S2) was picked from the initial screening of 175 genotypes (Table S1). Extreme seven genotypes for shoot dry weight and six each for root dry weight and gN in the shoot were picked to constitute the subset of 19 genotypes.
gN in shoot = N % in shoot × shoot dry weight (g). Where, N % in shoot was estimated by the Kjeldahl method (Jackson Citation1967).
Experiment I: evaluation of 19 genotypes under hydroponic conditions
Hydroponic screening was carried out under controlled condition of National Phytotron Facility at IARI, New Delhi, India under high N as well as low N conditions with two replication during 2015–2016. A temperature regime of 25/22°C (day/night), the light intensity of 300 µmol m−2 s−1 using cool florescent lamps in 10/14 h of dark and light and relative humidity of 65–70% were maintained for growing the plants (Ayalew et al. Citation2015). Uniform plump seeds of each genotype were surface sterilised with 1% sodium hypochlorite for 2 min and then thoroughly washed with distilled water and germinated on towel paper in seed incubator. One week old seedlings were transferred to the hydroponic system. A hydroponic system was developed from plastic boxes of 18 l capacity with ceramic lid. Holes of around 8 mm diameter were drilled on the lids. Two seedlings wrapped in cotton plug were placed in each hole of lid in a way that their roots remained immersed in hydroponic solutions tank. The pH of the solution was maintained to 6–6.5 by using 1M HCl or 1M KOH and was continuously aerated with aquarium air pump. The nutrient solution used in hydroponic system for non-limiting N (HN) environment was developed by using 0.4 mM NH4NO3, 10 mM KNO3, 2 mM CaNO3, 2 mM MgSO4, 0.1 mM KH2PO4, 1.5 mM CaCl2 and micronutrient: 0.1 mM Fe-EDTA, 12.5 µM H3BO3, 2 µM MnCl2, 3 µM ZnSO4, 0.5 µM CuSO4, 0.1 µM Na2MoO3, 0.1 µM NiSO4 and 25 µM KCl. For creating nitrogen limiting environment (LN), N containing compound (NH4NO3, KNO3, CaNO3) of this solution were reduced by 1/4 th of non-LN. The solution was replaced every week to maintain the normal status of the nutrients. The experiment was terminated after eight weeks of growth under hydroponic condition.
After eight weeks of plant growth, the data were recorded on maximum root length (RL), root dry weight (RDW), shoot dry weight (SDW) and shoot nitrogen (N) % for each independent plant. The maximum root length (cm) was measured at harvest from the shoot base to longest root tip. Root and shoot portion were separated after harvest and were dried in oven at 60°C for 4 days before measuring the dry weights (g). Shoot N% was estimated by Micro- Kjeldahl method. Other data too derived from above recorded traits like root-shoot ratio (R: S) which is the ratio of RDW to SDW and total dry weight (TDW), which is the sum of SDW and RDW. NUE and its component traits i.e. NUpE (Nitrogen uptake efficiency) and NUtE (Nitrogen utilisation efficiency) were calculated using the following three formulas (Moll et al. Citation1982).
where Total N in g per plant (gN) = N % in shoot × Shoot dry weight (g).
Thus, and N supply (g/plant) was measured as the ratio of total N supplied through N source by the total number of plants in a hydroponic tray.
Experiment II: generation of root data in PVC pipe
The same set of 19 genotypes were also raised on soil filled one metre tall PVC pipes of 12 cm radius mounted in open field during rabi 2015–2016. Sandy loam soil used for the experimentation was first solarised and then sieved. Repeated random sampling was done to estimate N content in the soil and was found 58 kg/ha of available N. The fine sieved soil was then weighed and mix properly with P @ 100 kg/ha and K @ 60 kg/ha and then filled to top of PVC pipe. Surface sterilised uniform two seeds of each genotype were sown in individual PVC pipe. To create N limited environment, no additional N was added, whereas for non-limiting N environment(200 kg/ha) additional N was supplied in two split dose (½ doses at sowing + ½ doses at 30 DAS) in the form of urea solution. After terminating the experiment at flowering stage, the soil of pipe was carefully removed to harvest intact root. Plant characters like plant height (cm), maximum root length (cm), number of tillers per plant, number of spikes per plant, spike length (cm), shoot dry weight (g), root dry weight (g) and N content in shoot. N content was estimated as per the Kjeldahl method.
Results
Evaluation of subset genotypes for NUE up to 8 weeks under HN and LN of hydroponic screening
The mean performance and range for different characters under hydroponic condition are presented in (). Significant variation was observed for most of the NUE traits under both N environments. The overall mean for SDW trait was found to be 0.733 g under HN and 0.44 g under LN condition. The range for this character varied from 0.326 g to1.276 g under HN and from 0.235 to 0.684 g under LN. RDW varied from 0.018 g to 0.179 g under HN and 0.053–0.148g under LN with the overall mean value being lesser under HN. Similarly, RL was also higher under LN condition. NUpE, NUtE were comparatively higher under LN condition than HN and sufficient variability was indicated for both of these traits by large range observed.
Table 1. Mean of 2 years for characters for subset genotypes.
Correlation between different traits under the hydroponic condition
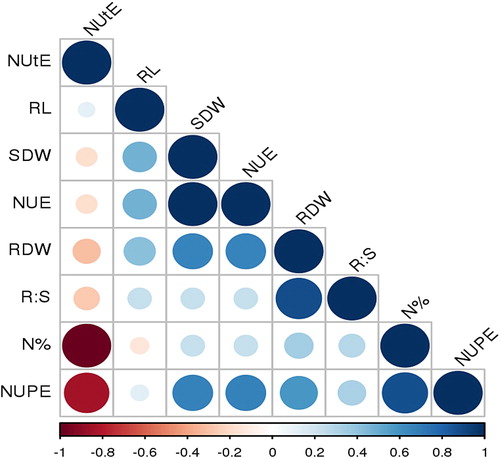
A correlation coefficient is presented for LN and HN condition in . In the present investigation, under HN environment (below diagonal), NUE has a positive and significant correlation with NUpE (0.961), RDW (0.769) and RL (0.588) and with positive and non-significant correlation with R:S (0.32) and Shoot N% (0.253). NUtE showed negative correlation with NUE. Similarly, RDW shows positive correlation with R:S (0.841), RL (0.823) and NUpE (0.793). RL shows positive and significant correlation with R:S (0.732), NUpE (0.548) and NUE (0.588). N% in shoot shows positive correlation with RDW (0.346), R:S (0.288), NUpE (0.501) and significant negative with NUtE (−0.994). NUpE showed negative association (−0.231) with NUtE. Under LN environment (above diagonal), NUE showed positive and significant association with RDW (0.745) and NUpE (0.967). NUE association with R:S was negative (−0.323) and no association was found with RL, N% in shoot and NUtE respectively. RDW showed positive correlation with R:S (0.387) and NUpE (0.767), and no correlation among RL, N% in shoot and NUtE were shown. Here, NUpE showed no relation with NUtE respectively. The validation of the previous experiment with subset genotypes for NUE traits too revealed that the trait RDW show significant and maximum correlation with NUE compared to other component traits under both HN and LN (Ranjan et al. Citation2019).
Table 2. Phenotypic correlation under LN (above diagonal) and HN (below diagonal) for different root traits in the subset genotypes under hydroponic screening.
Evaluation of subset genotypes in pipe under HN and LN condition
The data for agronomic and NUE traits are presented in . More SDW was observed under HN than LN. RDW under both environments has almost similar mean value. RL under pot culture was more under LN than HN. Mean value for plant height (PH), numbers of tiller (TN), length of spike (SL), numbers of spike (SN) and N% in shoot were at par under both N environments. The mean value for NUE and NUtE was comparatively more under LN whereas NUpE was more under HN. The result presented here under LN in pipe has been published and the data presented here is only for comparison for traits under HN and LN environments (Ranjan et al. Citation2019).
Table 3. Mean of characters under study for subset genotypes in pipe under HN and LN.
Correlation between different traits under pipe filled with soil
The results obtained for correlation among different root traits and NUE under HN are presented in . Association analysis in pipe experiment too revealed that NUE was significantly and positively correlated to RDW (0.659), RL (0.469), PH (0.534), TN (0.543), SN (0.528) and NUpE (0.674). The data are more or less in confirmation with hydroponic results under HN. Positive non-significant correlation was also found with R:S (0.224), SL (0.2) and N% (0.23) and negative non signification association with NUtE (−0.23). Under pipe experiment too, RDW is positively associated with SDW (0.804), R:S (0.879), RL (0.407), PH (0.324), TN (0.497), SN (0.461), N% (0.341) and NUpE (0.579) respectively. RL too show positive association with PH (0.341), TN (0.478), SL (0.547), SN (0.343) and NUpE (0.113) respectively. NUpE shows positive association with N% (0.873) and negative association with NUtE (−0.841) respectively.
Correlation between the results under two condition
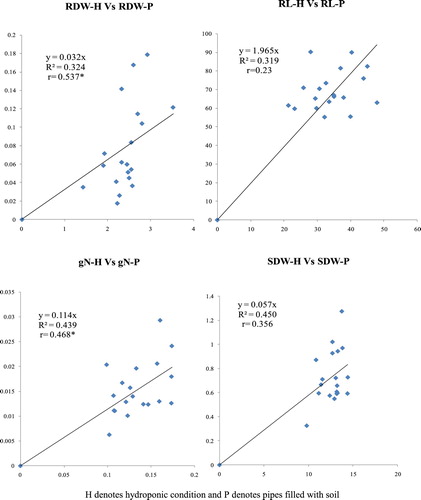
The NUE traits observed both under hydroponic condition and pipe experiment were SDW, RDW, RL, N% in shoot. Other traits were derived traits from these parameters. Correlation analysis of values observed in hydroponic and pipe experiment in the subset genotypes showed broad agreement between themselves. RDW observed under hydroponic and pipe showed a positive and significant association with a correlation coefficient value of 0.537 as shown in (). Similarly, gN in plant under hydroponic and pipe also showed positive and significant correlation (0.46). Similar SDW and RL data were in the broad agreement under two set of conditions with correlation coefficient value of 0.356 and 0.230 respectively ().
Discussion
The material chosen for the study was largely selected from a breeding programme in which the genotypes were being picked up and advanced to next generation on the basis of their adaptability, phenology, yielding ability and disease resistance and therefore had no direct selection history for nitrogen response and root traits. As shown in and ; our experiment revealed the presence of enough genetic variability for most of the traits related to N uptake and utilisation both under LN and HN. However, the response of the genotype was influenced by the environment because of strong interaction with genotypes (Ranjan Citation2018). The presence of strong interaction of genotype with N environment suggests for a separate breeding programme for their improvement depending upon the objectives of the crop improvement programme.
A large variation for the NUE and root traits indicate almost absence of any direct selection for and provide opportunities to the breeders for further yield consolidation without any negative footprints on the environment by exploiting these variabilities. Significant variation for root traits has also been reported by Narayanan et al. (Citation2014), Petrarulo et al. (Citation2015), Ahmadi et al. (Citation2018) and Ranjan et al. (Citation2019). The presence of sufficient genetic variation for the most of traits under low and high N coupled with high heritability is promising since, direct selection under low N by Presterl et al. (Citation2003) in maize and Laperche et al. (Citation2006) in wheat, yielded desired results. To further improve the selection efficiency, selection for the component traits like root biomass, root-shoot ration, shoot biomass and root length will be more rewarding than direct selection of NUE and NUpE as reported by Sathisha and Desai (Citation2016) and Naveen Kumar and Uma (Citation2016). Despite strong selection history of studied genotypes under high input condition, the presence of variability under high and low N conditions for the most of root traits both under hydroponic and pipe filled with soil condition indicates inherent large variation for root traits as well as nitrogen uptake.
Though the association between size of the root system and N uptake and thereby utilisation seems to be obvious, lack of consensus on this relationship (Palta et al. Citation2011) prompted us to characterise the component of NUE and explore their relationship with different root traits. In present investigation as shown in and , sufficient genetic variability with a positive and significant correlation of NUE with NUpE, RDW and RL under hydroponic as well as under pot condition. Irrespective of N supply, which indicated N supply had a specific response to NUpE than NUtE as suggested by Ortiz-Monasterio et al. (Citation1997), Foulkes et al. (Citation1998) and Brancourt-Hulmel et al. (Citation2003). Our study also corroborates the earlier finding of Sadras and Lawson (Citation2013) which indicate more importance of NUpE than NUtE in Australia.
Absence of correlation between NUtE with NUE ( and ) in our study was probably because shorter vegetative phase available in spring wheat genotypes might have imposed the restriction of ammonium assimilation in amino acids. Nitrate reductase (NR), nitrite reductase (NiR) and glutamine synthetase (GS) are key enzymes in the reduction and incorporation of N in organic compounds. Indepth study on genetic variability for key enzymes like NR, nitrite reductase (NiR) and GS available in spring wheat genotypes and their association with key growth regulatory genes like Vrn1 may throw further light on the poor association of NUtE with NUE.
Our studies indicate more association between NUE and RDW under LN than HN largely because when mineral elements are limiting, plants often adapt by allocating a higher proportion of their assimilate toward root system (Hermans et al. Citation2006) to maintain a balance between the roots and shoot growth (Robinson Citation2001). Foulkes et al. (Citation2009) suggested that deep rooting systems may be favoured by later anthesis date, thus affecting NUpE. Therefore, examining the root structure of cultivars under LN environments may help define traits associated with NUpE and overall NUE. The genetic variability of the root system (architecture and biomass) and its relationship with NUpE have been the subject of only a few studies (Ju et al. Citation2015; Pan et al. Citation2016; Nehe et al. Citation2018) and require further investigation.
Negative association of NUpE and NUtE under both LN and HN in the present study () might be because the activities of the N transporters and enzymes involved in the assimilation of N occur at different proportions, degradation of the leaf proteins as DoVale et al. (Citation2012) or as we carried out experimentation up to flowering the mobilisation of N toward sink remained largely unexplored.
Breeding generally require large scale screening at least in early generation and broad correlation between hydroponic and pot data as shown in , confirmed with the result by Mian et al. (Citation1993) in wheat; Ogbonnaya et al. (Citation2003) in cowpea and Girdthai et al. (Citation2010) in peanut indicates suitability of hydroponic screening as it is independent of season and numbers with less labour requirement.
Thus, to minimise the N footprint on the environment and to lessen the economic burden due to over N fertilisation, increasing NUE of cropping system is the present day need. Root trait exploration under field condition is labour intensive and largely affected by environment hence is largely ignored by breeders. The presence of huge variability for these traits and facilitation of large scale screening under condition offers an opportunity to the breeders to break the yield barrier through increasing the NUE efficiency. Good agreement between hydroponic data and pipe filled with soil for root and NUE traits support the large scale screening and selection of segregating material under hydroponic condition for making the genetic gain.
Supplimentary_Table_final.docx
Download MS Word (19.7 KB)Acknowledgements:
The author acknowledge Indian Council of Agricultural Research- New Delhi, India for providing scholarship for Ph.D. at IARI, New Delhi under Netaji Subash ICAR international fellowship program, International Plant Nutrition Institute, Georgia, U.S.A. for honouring with IPNI Scholar Award, Indo-U.K. centre for the improvement of Nitrogen Use Efficiency in Wheat (INEW), Rothamsted Research, Harpenden, United Kingdom for providing a training on genotyping and phenotyping on Nitrogen use efficiency at Rothamsted Research, University Bristol, University of Nottingham and John Inn Center, Norwich, United Kingdom and Division of Plant Physiology IARI, New Delhi for providing PVC pipe for conducting the pot experiment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Rumesh Ranjan holds a Ph.D. (Genetics and Plant Breeding) from Indian Agricultural Research Institute, New Delhi. His dissertation was on Genetic analysis and identification of QTLs influencing nitrogen use efficiency in wheat. He was NetaJi ICAR-International Fellow, 2015, India, and was awardee of International Plant Nutrition Institute (IPNI) Scholar, 2016, USA. He has been graduated his PhD in 2019 and will be serving Borlaug Institute of South Asia, CIMMYT, Ludhiana, India, as a Plant Breeding Consultant from June 2019.
Dr Rajbir Yadav is Principal Scientist in Genetics and wheat breeder in Indian Agricultural Research Institute, working on development of high yielding, disease resistant wheat varieties keeping cropping system and conservation agriculture production environment in view for Indian subcontinent.
Mr Ashish Kumar is a PhD scholar in the Division of Genetics, IARI, New Delhi. His PhD research is on high throughput genotyping and MAS along with Conventional breeding approaches.
Mr Swarupa Nanda Mandal is Assistant Professor in Genetics and Plant Breeding at Bhidhan Chandra Krishi Viswavidyalaya, Burdwan, West Bengal. He is doing his PhD at the Department of Plant and Soil Science, Texas Tech University, Lubbock under Netaji ICAR International Fellowship program.
References
- Ahmadi J, Pour-Aboughadareh A, Fabriki-Ourang S, Mehrabi AA, Siddique KH. 2018. Screening wheat germplasm for seedling root architectural traits under contrasting water regimes: potential sources of variability for drought adaptation. Arch Agron Soil Sci. 64(10):1351–1365. doi: 10.1080/03650340.2018.1432855
- Ayalew H, Ma X, Yan G. 2015. Screening wheat (Triticum spp.) genotypes for root length under contrasting water regimes: potential sources of variability for drought resistance breeding. J Agron Crop Sci. 201(3):189–194. doi: 10.1111/jac.12116
- Barraclough PB, Howarth JR, Jones J, Lopez-Bellido R, Parmar S, Shepherd CE, Hawkesford MJ. 2010. Nitrogen efficiency of wheat: genotypic and environmental variation and prospects for improvement. Eur J Agron. 33(1):1–11. doi: 10.1016/j.eja.2010.01.005
- Brancourt-Hulmel M, Doussinault G, Lecomte C, Berard P, Le Buanec B, Trottet M. 2003. Genetic improvement of agronomic traits of winter wheat cultivars released in France from 1946 to 1992. Crop Sci. 43(1):37–45. doi: 10.2135/cropsci2003.3700
- DoVale JC, DeLima RO, Fritsche-Neto R. 2012. Breeding for nitrogen use efficiency. In: Fritsche-Neto R, Borém A, editors. Plant breeding for abiotic stress tolerance. Berlin: Springer; p. 53–65. doi: 10.1007/978-3-642-30553-5_4
- Fageria NK, Santos AD. 2014. Lowland rice genotypes evaluation for nitrogen use efficiency. J Plant Nutr. 37(9):1410–1423. doi: 10.1080/01904167.2013.868482
- Foulkes MJ, Hawkesford MJ, Barraclough PB, Holdsworth MJ, Kerr S, Kightley S, Shewry PR. 2009. Identifying traits to improve the nitrogen economy of wheat: recent advances and future prospects. Field Crops Res. 114(3):329–342. doi: 10.1016/j.fcr.2009.09.005
- Foulkes MJ, Sylvester-Bradley R, Scott RK. 1998. Evidence for differences between winter wheat cultivars in acquisition of soil mineral nitrogen and uptake and utilization of applied fertilizer nitrogen. J Agric Sci. 130(1):29–44. doi: 10.1017/S0021859697005029
- Girdthai T, Jogloy S, Kesmala T, Vorasoot N, Akkasaeng C, Wongkaew S, Holbrook CC, Patanothai A. 2010. Relationship between root characteristics of peanut in hydroponics and pot studies. Crop Sci. 50(1):159–167. doi: 10.2135/cropsci2008.09.0529
- Hall AJ, Richards RA. 2013. Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops. Field Crops Res. 143:18–33. doi: 10.1016/j.fcr.2012.05.014
- Hermans C, Hammond JP, White PJ, Verbruggen N. 2006. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 11(12):610–617. doi: 10.1016/j.tplants.2006.10.007
- Hurd EA. 1964. Root study of three wheat varieties and their resistance to drought and damage by soil cracking. Can J Plant Sci. 44(3):240–248. doi: 10.4141/cjps64-046
- Jackson ML. 1967. Soil chemical analysis. New Delhi: Prentice Hall of India, Pvt. Ltd. Vol. 1, pp. 111–203.
- Ju C, Buresh RJ, Wang Z, Zhang H, Liu L, Yang J, Zhang J. 2015. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crops Res. 175:47–55. doi: 10.1016/j.fcr.2015.02.007
- Laperche A, Brancourt-Hulmel M, Heumez E, Gardet O, Le Gouis J. 2006. Estimation of genetic parameters of a DH wheat population grown at different N stress levels characterized by probe genotypes. Theor Appl Genet. 112(5):797–807. doi: 10.1007/s00122-005-0176-z
- Le Gouis J, Béghin D, Heumez E, Pluchard P. 2000. Genetic differences for nitrogen uptake and nitrogen utilisation efficiencies in winter wheat. Eur J Agron. 12(3–4):163–173. doi: 10.1016/S1161-0301(00)00045-9
- Lynch JP. 2007. Roots of the second green revolution. Aust J Bot. 55(5):493–512. doi: 10.1071/BT06118
- Malyan SK, Bhatia A, Kumar A, Gupta DK, Singh R, Kumar SS, Tomer R, Kumar O, Jain N. 2016. Methane production, oxidation and mitigation: a mechanistic understanding and comprehensive evaluation of influencing factors. Sci Total Environ. 572:874–896. doi: 10.1016/j.scitotenv.2016.07.182
- Manschadi AM, Christopher J, Hammer GL. 2006. The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol. 33(9):823–837. doi: 10.1071/FP06055
- Mian MAR, Nafziger ED, Kolb FL, Teyker RH. 1993. Root growth of wheat genotypes in hydroponic culture and in the greenhouse under different soil moisture regimes. Crop Sci. 33(2):283–286. doi: 10.2135/cropsci1993.0011183X003300020014x
- Moll RH, Kamprath EJ, Jackson WA. 1982. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization 1. Agron J. 74(3):562–564. doi: 10.2134/agronj1982.00021962007400030037x
- Narayanan S, Mohan A, Gill KS, Prasad PV. 2014. Variability of root traits in spring wheat germplasm. PLoS One. 9(6):e100317. doi: 10.1371/journal.pone.0100317
- Naveen, KVM, Uma MS. 2016. Genetic variability for nitrogen use efficiency and yield-related traits in rice under low nitrogen and available soil nitrogen conditions. Int J Appl Pure Sci Agricul. 2(7):83–90.
- Nehe AS, Misra S, Murchie EH, Chinnathambi K, Foulkes MJ. 2018. Genetic variation in N-use efficiency and associated traits in Indian wheat cultivars. Field Crops Res. 225:152–162. doi: 10.1016/j.fcr.2018.06.002
- Ogbonnaya CI, Sarr B, Brou C, Diouf O, Diop NN, Roy-Macauley H. 2003. Selection of cowpea genotypes in hydroponics, pots, and field for drought tolerance. Crop Sci. 43(3):1114–1120. doi: 10.2135/cropsci2003.1114
- Ortiz-Monasterio R, Sayre KD, Rajaram S, McMahon M. 1997. Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Sci. 37(3):898–904. doi: 10.2135/cropsci1997.0011183X003700030033x
- Palta JA, Chen X, Milroy SP, Rebetzke GJ, Dreccer MF, Watt M. 2011. Large root systems: are they useful in adapting wheat to dry environments? Funct Plant Biol. 38(5):347–354. doi: 10.1071/FP11031
- Pan S, Liu H, Mo Z, Patterson B, Duan M, Tian H, Hu S, Tang X. 2016. Effects of nitrogen and shading on root morphologies, nutrient accumulation, and photosynthetic parameters in different rice genotypes. Sci Rep. 6:32148. doi: 10.1038/srep32148
- Petrarulo M, Marone D, Ferragonio P, Cattivelli L, Rubiales D, De Vita P, Mastrangelo AM. 2015. Genetic analysis of root morphological traits in wheat. Mol Genet Genom. 290(3):785–806. doi: 10.1007/s00438-014-0957-7
- Presterl T, Seitz G, Landbeck M, Thiemt EM, Schmidt W, Geiger HH. 2003. Improving nitrogen-use efficiency in european maize. Crop Sci. 43(4):1259–1265. doi: 10.2135/cropsci2003.1259
- Price AH, Townend J, Jones MP, Audebert A, Courtois B. 2002. Mapping QTLs associated with drought avoidance in upland rice grown in the Philippines and West Africa. Plant Mol Biol. 48(5–6):683–695. doi: 10.1023/A:1014805625790
- Ranjan R. 2018. Genetic analysis and identification of QTLs influencing nitrogen use efficiency in wheat. PhD dissertation. Indian Agricultural Research Institute, New Delhi.
- Ranjan R, Yadav R. 2019. Targeting nitrogen use efficiency for sustained production of cereal crops. J Plant Nutr. 42(9):1086–1113. doi: 10.1080/01904167.2019.1589497
- Ranjan R, Yadav R, Pandey R, Jain N, Bainsla NK, Gaikwad KB, Singh AM. 2019. Variation in wheat (Triticum aestivum) advance lines and released cultivars for traits associated with nitrogen use efficiency under N limiting environment. Indian J Agric Sci. 89(1):99–104.
- Robinson D. 2001. Root proliferation, nitrate inflow and their carbon costs during nitrogen capture by competing plants in patchy soil. Plant Soil. 232(1–2):41–50. doi: 10.1023/A:1010377818094
- Sadras VO, Lawson C. 2013. Nitrogen and water-use efficiency of Australian wheat varieties released between 1958 and 2007. Eur J Agron. 46:34–41. doi: 10.1016/j.eja.2012.11.008
- Sathisha TN, Desai SA. 2016. Genetic variability for nitrogen use efficiency (NUE) and yield attributing traits in wheat. Int J Agric Sci. 8(48):2010–2014.
- Yadav R, Gaikwad KB, Bhattacharyya R. 2017. Breeding wheat for yield maximization under conservation agriculture. Indian J Genet Plant Breed. 77(2):185–198. doi: 10.5958/0975-6906.2017.00026.8
- Yadav R, Singh SS, Jain N, Pratap Singh G, Prabhu KV. 2010. Wheat production in India: technologies to face future challenges. J Agric Sci. 2(2):164.
- Yin L, Dai X, He M. 2018. Delayed sowing improves nitrogen utilization efficiency in winter wheat without impacting yield. Field Crops Res. 221:90–97. doi: 10.1016/j.fcr.2018.02.015