ABSTRACT
Genetic diversity is a pre-requisite for rice (Oryza sativa L.) breeding and population development. Hence, the objective of this study was to assess the genetic diversity and population structure of 54 rice accessions using 14 polymorphic simple sequence repeats (SSR) markers to select unique parents for breeding. Data analysis was based on marker and population genetic parameters. The mean polymorphic information content (PIC) was 0.61 suggesting high polymorphisms for the selected SSR markers among the rice accessions. The population structure revealed a narrow genetic base with only two major sub-populations. Analysis of molecular variance revealed that only 30% of the variation was attributed to population differences while 47% and 23% were due to variation among individuals within populations and within individual variation, respectively. The genetic distance and identity among genotypes varied from 0.083 to 1.834 and 0.159 to 0.921, respectively. Dendrogram grouped the genotypes into three clusters with wide variation among the accessions. The study established the existence of considerable genetic diversity among the tested 54 accessions. The selected genetic resources will be useful resources for rice breeding in Tanzania or other African countries.
Introduction
In sub-Sahara Africa (SSA), rice (Oryza sativa L.; 2× = 24) has become a pivotal crop in ensuring food security and in sustaining the livelihoods of millions of people. In Tanzania, rice is the second most important food and cash crop after maize (Bucheyeki et al. Citation2011). Tanzania is the second largest rice producer in East and Central Africa after Madagascar, with an annual production of 1.2–1.4 million tons (Nkuba et al. Citation2016; FAO Citation2017). The majority of rice production in Tanzania is carried out by small-scale farmers using landrace varieties, which have low yield potential (Mogga et al. Citation2018). There is need to develop modern and improved varieties to serve the diverse needs of the rice value chains. There is an evident lack of adoption of improved rice cultivars because they lack the taste or aroma preferred by farmers and consumers (Mogga et al. Citation2018). Hence, most farmers opted to grow landraces, which harbour important attributes such as aroma and good cooking qualities that are absent in the introduced cultivars. There is an urgent need to develop cultivars that incorporate farmer and consumer-preferred traits.
Previous studies on rice focused on evaluations for agronomic performance and value for cultivation (Mligo and Msuya Citation2015; Ansah et al. Citation2017) with less emphasis on breeding for improved yield and related traits. Progress in rice breeding is strongly related to the genetic variation within the germplasm resources (Yan et al. Citation2016). Therefore, understanding the population structure and genetic variation in germplasm is a prerequisite for crop genetic improvement (Xiao et al. Citation2016). Genetic diversity in rice has been investigated using morphological, biochemical and DNA markers (Palanga et al. Citation2016; Luther et al. Citation2017). However, both morphological and biochemical traits are highly influenced by environments, genotype × environment interaction effects, and may not provide accurate genetic classification of the crop (Aljumaili et al. Citation2018; Mulualem et al. Citation2018). Moreover, morphological traits cannot define the exact level of genetic diversity among germplasm, because of the presence of polygenic control on the expression of traits. Therefore, rice genetic resources should be effectively characterised using genomic tools for efficient utilisation and conservation.
A range of DNA techniques, including amplified fragment length polymorphism (AFLP) (Sorkheh et al. Citation2016), restriction fragment length polymorphism (RFLP) (Sun et al. Citation2000), random amplified polymorphic DNA (RAPD) (Ali et al. Citation2014), microsatellites (Liu et al. Citation2015; Chen et al. Citation2017), single nucleotide polymorphisms (SNP) (Sun et al. Citation2013) markers have been applied in rice genetic diversity studies. However, the choice of markers depends on the availability of genetic information on the genome sequence, cost of marker development, ease of documentation and level of polymorphism (Mittal and Dubey Citation2009). The SSR markers are widely used because of their high degree of polymorphism, multi-allelic variation, co-dominance, high reproducibility, and ease of detection by PCR, and relatively abundance with a uniform coverage. Moreover, SSR markers have remarkable potential to discriminate rice genotypes due to their high polymorphic nature and transferability (Islam et al. Citation2012; Mousavi et al. Citation2017). Further, SSRs markers that are linked to major genes could increase the efficiency of classical breeding by significantly reducing the number of selection generations required to identify superior and stable progenies. Recently, Yelome et al. (Citation2018) used SSR markers to assess the extent of genetic divergence among O. sativa and West African rice O. glaberrima accessions. To develop breeding populations, a panel of 54 genetically diverse rice genotypes including landraces were collected from farmers’ and different research organisations in Tanzania. Based on agro-morphological classification, these accessions were found to be phenotypically distinct. However, the extent of genetic diversity and genetic relationships present in these collections has not been rigorously studied using molecular markers. Knowledge of genetic diversity and relationships among the rice germplasm will play a significant role in local and regional breeding programmes. Therefore, the objective of the present study was to determine the genetic relationship and population structure present among 54 rice collections using SSR markers to identify genetically divergent genotypes for breeding.
Materials and methods
Plant materials
The study used 54 rice genotypes acquired from Tanzania Agricultural Research Institute (TARI), International Rice Research Institute (IRRI)/Philippines, Africa Rice/Benin, Sokoine University of Agriculture (SUA)/Tanzania and farmer fields in Tanzania. The details of the germplasm are described in .
Table 1. List and sources of the rice genotypes used in the study.
DNA extraction
Prior to DNA extraction, seeds of 54 rice genotypes were planted at University of KwaZulu-Natal (latitude 29°37’51.75″ S; longitude 30°23’59.10″ E), South Africa. All genotypes were established under glasshouse condition. Four seeds of each rice genotype were sown in a plastic pot, and from each pot, three healthy and vigorous plants were randomly selected and fresh young leaves collected for DNA extraction. The DNA was extracted following the Cetyl-tetramethyl ammonium bromide (CTAB) method. Approximately 200 mg of ground plant tissue combined with 500 µL of CTAB buffer, was incubated for one hour at 65°C, and subjected to centrifugation at 3500 rpm for 10 min. The supernatant was then transferred into new micro-tubes, and 400-µl chloroform: iso-amyl alcohol (24:1) was added into the tubes and mixed gently. After a second centrifugation (centrifuged at 3500 rpm for 30 min), the DNA was precipitated from the aqueous layer by the addition of salt and ethanol. The upper aqueous phase containing DNA was transferred to a clean microfuge tube. The resulting pellet was dried and re-suspended in TE buffer.
The PCR amplification reaction contained a total volume of 12 µl of PCR mix. The PCR mix contained 0.72 µl Magnesium chloride (50 mM MgCl2), 1.2 µl dNTPs (25 µM), 0.12 µl Taq (5U/uL), 0.06 µl forward primer (10 µM), 0.3 µl reverse primer (10 µM), 1.2 µl of 1× reaction buffer, 6.16 µl PCR grade water and 0.24 µl dye. A PCR profile of initial denaturation for 2 min at 94°C, and 33 cycles of denaturation for 1 min at 55–60°C, an annealing temperature of 63°C for 2 min, and an extension for 2 min at 72°C was used. The PCR products (DNA samples) were fluorescently analysed using a Genetic Analyzer 3130xl labelled and separated by capillary electrophoresis on an ABI 3013 automatic sequencer. Analysis of the electrophrograms was performed using Gene Mapper 4.0 and the marker data was presented as fragment sizes in an Excel spreadsheet.
Microsatellite analysis
Fourteen simple sequence repeats (SSRs) distributed on the 12 chromosomes of rice were used in this study and chosen based on their use in published rice diversity analysis reports (Chen et al. Citation1997; Ashfaqa and Khan Citation2012; Ashraf et al. Citation2016). Forward and reverse primers of the SSR markers are presented in .
Table 2. Sequence of SSR markers used for rice genetic diversity analysis.
Data analysis
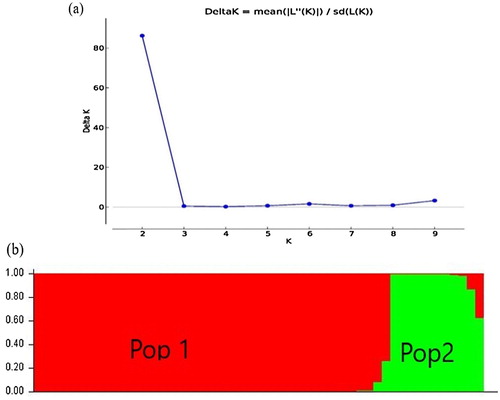
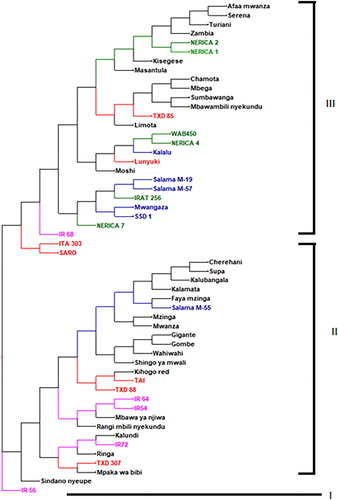
Genetic diversity was assessed using GenAlex version 6.5 (Peakall and Smouse Citation2007). The following parameters were computed: total number of alleles per locus (Na), number of effective alleles per locus (Ne), Shannon’s information index (I), observed heterozygosity (Ho), gene diversity (He), and inbreeding coefficient (FIS) were determined using the protocol of Nei and Li (Citation1979). The Polymorphic information content (PIC) values were calculated for each SSR locus as PIC = 1– Σ (pi2), where pi is the frequency of ith allele. An analysis of molecular variance (AMOVA) was performed to test the degree of differentiation among and within the sources of collection of the rice genotypes. The population structure of the 54 rice accessions was established using the Bayesian clustering method in STRUCTURE version 2.3.4 (Pritchard et al. Citation2000). The length of the burn-in period and Markov Chain Monte Carlo (MCMC) were set at 10,000 iterations (Evanno et al. Citation2005). To obtain an accurate estimation of the number of populations, 20 runs were performed for each K-value (assumed number of subpopulations), ranging from 1 to 10. Further, Delta K values were calculated and the appropriate K value was estimated by implementing Evanno et al. (Citation2005) method using STRUCTURE Harvester program (Earl and von Holdt Citation2012). The genetic relationships or relatedness (cluster analysis) of the 54 genotypes were estimated using the genetic dissimilarity coefficients and the dendrogram were drawn using the unweighted pair group method (UPGMA) in DARwin 6.0 (Perrier and Jacquemoud-Collet Citation2006).
Results
Genetic variability of 54 rice accessions based on SSR markers
The number of alleles scored per locus ranged from 2 for the markers RM319 and RM338, to 20 for marker RM206 with a mean of 7.43 per locus (). The number of effective alleles (Ne) per locus varied from 1.43 to 9.57 with a mean of 3.97 and markers RM319 and RM206 had the lowest and highest numbers of effective alleles respectively. Expected heterozygosity (He) ranged from 0.30 (M319) to 0.90 (RM206 and RM235) with a mean of 0.62 (). The observed heterozygosity (Ho) values had a mean of 0.18 and a range of 0.00 (RM319) to 0.80 (RM125 and RM235). The inbreeding coefficient (FIS) ranged from 0.10 to 0.93 with a mean of 0.74 (). The PIC values of the 14 SSR markers ranged from 0.30 (RM319) to 0.90 (RM206 and RM235) with a mean of 0.61.
Table 3. Genetic parameters generated by 14 SSR markers on 54 rice genotypes.
Genetic relationship among 54 rice accessions based on source of collection
The genetic variability among rice genotypes based on source of collection is presented in . The mean values of observed (Na) and effective (Ne) number of detected alleles were 3.47 and 2.36, respectively. IRRI and Africa Rice recorded the lowest Na (2.53) and Ne (1.87), respectively. Similarly, the highest Na and Ne values of 5.67 and 3.26 were recorded for landrace collections. The mean observed Ho and He across rice genotypes were 0.17 and 0.47, respectively. The lowest values of Ho (0.12) and He (0.36) were observed from rice genotypes collected from Africa Rice and IRRI, respectively. The highest value of Ho = 0.22 and He = 0.53 was recorded from TARI and SUA, genotypes, respectively (). Shannon’s information index ranged from 0.65 to 1.05 with a mean of 0.82. High heterozygosity values recorded were associated with F values ranging from 0.38 (TARI) to 0.77 (SUA), with a mean of 0.63 at population level ().
Table 4. Genetic diversity of 54 rice genotypes classified by areas of collection.
The genetic differentiation (Fst) ranged from low (0) between IRRI and TARI accessions, while a large Fst (0.49) was observed between IRRI and Africa Rice collections (). Gene flow ranged between 0.05 and 1.06. The average Nei’s unbiased genetic distance showed that the greatest genetic distance (1.84) was between genotypes collected from Africa Rice and landraces followed by Africa Rice and IRRI (1.74), SUA and IRRI (1.46), Africa Rice and TARI (1.41), SUA and TARI (1.32). The lowest genetic distance (0.08) was observed between TARI and IRRI rice genotypes. The genetic identity among varied from 0.16 to 0.92 (). The highest genetic identity (0.92) was between TARI and Africa rice, followed by IRRI and SUA (0.88), TARI and SUA (0.81) genotypes and the lowest (0.16) observed between landraces and Africa rice.
Table 5. Pair-wise estimates of gene flow (above diagonal off brackets), genetic identity (above diagonal within brackets) and genetic differentiation (lower diagonal off brackets), genetic distance (lower diagonal within brackets).
Population structure of 54 rice accessions
The population structure analysis of the 54 genotypes grouped the population into two sub-populations (). Forty-one rice genotypes, representing 76% of the population, were assigned into sub-population 1 (Pop 1), and the remaining 13 were grouped into sub-population 2 (Pop 2). Results showed that sub-population 1 comprised of genotypes from SUA, landraces, TARI and Africa Rice, while population 2 consisted of landraces and IRRI genotypes.
Genetic cluster analysis of 54 rice accessions
The UPGMA cluster analysis based on genetic dissimilarity using the neighbour-joining method grouped the 54 genotypes into three major clusters (). The distribution of the genotypes into the three main clusters was not homogeneous. Cluster I consisted one genotype. Cluster II composed of 25 (46.30%) of the rice genotypes studied (). Cluster III comprised of 28 (51.85%) genotypes (). The genotypes IR56 and Mwanza, Salama M-55 and Sindano nyeupe, SARO and Gigante, Mwanza and SARO, Lunyuki and Zambia, Rangimbili and IRAT 256, Zambia and Salama M-19 were highly distinct based on genetic makeup. The lowest genetic dissimilarity among rice genotypes was between Cherehani and Supa, Afaa Mwanza and Serena, Nerica 1 and Nerica 2, ITA 303 and SARO and IR 54 and 64 (). These landraces may have the same genetic background but collected under different names in different locations.
Analysis of molecular variance (AMOVA)
The results from AMOVA displayed highly significant genetic differences (P ≤ 0.001) among populations, among individuals and within individuals (). Thirty percent of the variance was due to genetic differentiation among the populations, while 47% of the variance was accounted for by individuals within populations. The remaining 23% of the variance was due to the differences within individuals.
Table 6. Analysis of molecular variance among and within the 54 rice genotypes.
Discussion
Identification of genetic relationship and divergence of genetic resources is a useful step for parental choice for breeding. This will assist in minimising the use of closely related parents in breeding programs, which would otherwise lead to genetic depression and reduced genetic variation. The current study was therefore carried out to establish genetic diversity, relationship and population structure among selected rice genotypes to identify appropriate parents for hybridisation. The present study utilised 14 microsatellite markers to reveal genetic polymorphism of 54 rice accessions collected from four different sources. The genetic improvement of yield and other economically important traits in crop depends upon the genetic diversity available within the crop species and the rice genotypes with high levels of genetic variation found in this study are beneficial resources for broadening the genetic base and for achieving rapid gains during rice breeding in Tanzania. A wide genetic diversity translates to a potentially high variation in morphological traits.
The number of alleles investigated ranged from 2 to 20, with a mean value of 7.48 per locus similar to 7.8 and 7.7 alleles per locus reported by Jain et al. (Citation2004) and Zeng et al. (Citation2007), respectively. This suggests that there is favourable allelic diversity, which is essential for assessment of genetic diversity. The mean number of alleles (7.48) obtained in the study was significantly higher than 6.4 alleles per locus reported by Chemutai et al. (Citation2016). Rahman et al. (Citation2012) detected even lower number of allele of 4.18 using 34 SSR markers. In contrast, the number of alleles detected in the present study was lower than the average number of alleles (11.85) reported by Prathepha (Citation2012). The variability in the number of alleles detected per locus might be due to the use of diverse genotypes. The number of effective alleles per locus ranged from 1.43 to 9.57 with a mean of 3.97 close to 3.77 previously reported by Chen et al. (Citation2017). Greater number of alleles generated by SSR markers suggests the usefulness of this marker system for detecting genetic polymorphisms. In contrast, Aljumaili et al. (Citation2018) detected 1.48 effective number of allele per SSR locus among 53 rice cultivars. On the contrary, effective number of alleles detected in the present study was lower than the average number of effective alleles (5.51) reported by Yelome et al. (Citation2018), among West African rice accessions.
The mean expected gene diversity was 0.62 (), which was similar to value reported by Wang et al. (Citation2014). This was comparable to the findings of Aljumaili et al. (Citation2018) who reported a gene diversity of 0.60 in a microsatellite-based study that involved 53 rice accessions. However, the mean gene diversity recorded in the present study was higher than that reported by Anh et al. (Citation2018) and Islam et al. (Citation2018). Further, the gene diversity obtained in the present study was higher than the findings of Chemutai et al. (Citation2016), Choudhary et al. (Citation2013), and Nachimuthu et al. (Citation2015) who reported values of 0.54, 0.52 and 0.42 respectively in rice. This could be attributed to high rate of exchange of genetic materials among the sources of germplasm collection.
The mean observed heterozygosity () of the genotypes of 0.18 was similar to low heterozygosity reported by Yelome et al. (Citation2018) among 42 rice accession from six West African countries using 20 polymorphic SSR markers. The low level of heterozygosity has also been reported in other studies on rice (Choudhury et al. Citation2014; Nachimuthu et al. Citation2015) and this could be attributed to its autogamous mode of reproduction.
Over 60% of the tested primers in the present study were highly polymorphic with mean PIC value of 61 implying the high discriminating ability of the SSR markers. This indicates that the selected microsatellites were highly informative in distinguishing the test genotypes. The PIC value of a marker is the probability of the marker to be detected in the progeny and is a good measure of a marker's usefulness for linkage analysis. It is also a reflection of allelic diversity among varieties (Meti et al. Citation2013). The PIC and inbreeding coefficient (FIS) are the functions of how heterozygosity is partitioned within and among genotypes, based on differences in allele frequencies (Mulualem et al. Citation2018). Furthermore, high PIC value implies that the SSR markers were informative. A similar PIC value of 0.61 among rice genotypes was reported by Jain et al. (Citation2004). In addition, the PIC values observed in this study were comparable to 0.60 and 0.62 reported by Meti et al. (Citation2013) and Ashraf et al. (Citation2016) using 12 and 24 SSR markers, respectively. On the contrary, the present study reported higher mean PIC value compared to 0.48 and 0.37 reported by Ashfaqa and Khan (Citation2012) and Chemutai et al. (Citation2016), respectively. The differences in PIC values maybe linked to the selection of different markers and the diversity of test genotypes.
Seven percent of the markers in the present study had negative inbreeding coefficient values (). FIS represents the average deviation of the population’s genotypic proportions from Hardy-Weinberg equilibrium for a locus. The FIS values revealed that, one of the 14 markers (RM125) showed higher heterozygotes (−0.27). Populations differ with respect to richness of allelic diversity, distribution and frequency (Rao and Hodgkin Citation2002). Variation in population may be attributed to the breeding system of the species and the ecological factors such as latitude, altitude, temperature, and moisture availability and other soil-related factors. Shannon’s information index (I) ranged from 1.05 to 6.65 with an average of 0.82 (). This is in agreement with finding of Aljumaili et al. (Citation2018), who reported an index of 0.88. The high value of Shannon’s information index in the present study was another indication of the presence of genetic diversity of the rice germplasm used in the study.
Population structure analysis revealed two sub-populations (Pop 1 and Pop 2) () indicating that a narrow genetic base exists among the studied rice genotypes. This result is consistent with the population structure of West African rice accessions reported by Yelome et al. (Citation2018). Further, the population structure analysis confirmed the clustering of the sampled genotypes in a similar group, suggesting the need for crosses using genetically unrelated parents to develop breeding populations. However, AMOVA revealed highly significant genetic differences (P ≤ 0.001) among the populations, among individuals and within individuals (). Of the total genetic variation in the 54 genotypes, 47% of the variation was contributed by the genetic differentiation among individuals within populations indicating that there is adequate variation among the studied genotypes useful for breeding. Variation of similar pattern has been reported in previous studies on rice germplasm (Aljumaili et al. Citation2018; Yelome et al. Citation2018). AMOVA results suggest that a small collection within a given source will capture the genetic diversity present in the test genotypes. The presence of variability within and between the populations represents the possibility of making wide crosses for population development and to enhance genetic divergence in rice.
Genetic distance is a measure of the genetic divergence between pairs of genotypes or populations. The present study revealed average genetic distance estimate of 1.84, which is higher than previous reports. Becerra et al. (Citation2015) reported a genetic distance of 0.87 in elite rice genotypes from Chile. Similarly, a mean genetic distance of 0.86 was reported in Ugandan rice genotypes (Mogga et al. Citation2017). In addition, Ndjiondjop et al. (Citation2018) reported a genetic distance of 0.01–0.76. The high genetic distance (1.84) for the genotypes studied could be attributed to the uniqueness of Tanzania rice germplasm collections, which seems to be different from other regions. According to Nei (Citation1972), genetic distance is linearly related to geographical distance. However, the genetic distance values of rice germplasm (1.74 and 1.84) require further confirmation using additional SSR primers.
According to standard interpretation of genetic differentiation, 0.0–0.005 shows little, 0.05–0.15 moderate, 0.15–0.25 large, and above 0.25 very large genetic differentiations (Wright Citation1978). The lower genetic variance among sources of collection in this study can also be associated with the observed low gene differentiation and high gene flow. According to Morjan and Rieseberg (Citation2004), gene flow <1 is considered to be low (Nm), while Nm = 1 is considered to be moderate and Nm > 1 is considered to be high. The occurrence of high gene flow in the germplasm studied could be attributed to the evolutionary history of these populations, out-crossing between rice genotypes or effects of spontaneous mutations (Nuijten et al. Citation2009). Further, exchange of rice genotypes among farmers and traders may have enhanced gene flow across rice growing regions of Tanzania.
The UPGMA cluster analysis based on genetic dissimilarity using the neighbour-joining method grouped the 54 genotypes into three major distinct clusters. The clustering pattern in the present study indicated the existence of variability among rice genotypes. Chemutai et al. (Citation2016) also grouped 50 rice genotypes into three clusters using SSR markers. However, the cluster patterns did not correspond to the predefined population structure based on the area of collection. According to Mulualem et al. (Citation2018), this may be due to the fact that genotypes collected from similar areas belong to the same gene pool or they may have similar ancestral relationships. Conversely, genetic dissimilarity among the rice genotypes studied could arise due to the diverse ancestral origin, high gene flow caused by cross-pollination and possible gene/chromosomal mutation. In the present study, rice genotypes collected from similar regions were grouped together in the same cluster such as Gigante and Gombe, and Salama M-55 and Faya mzinga. These results are in agreement with earlier studies, which reported that geographical separation did not affect genetic distance among genotypes (Zhang et al. Citation2012). According to Ganesamurthy et al. (Citation2010), geographic location should not be used as a measure of genetic diversity during genotype selection. This could be a consequence of exchange of genetic materials among the neighbouring farmers as well as traders in the region. Besides, farmers’ selections and management practice affect the patterns of genetic diversity (Barnaud et al. Citation2008). Tanzanian rice farmers’ recycled seed as a source of planting material, which in turn increases the genetic similarity among landraces. Mekbib (Citation2007) reported that farmers selected, and preserved genotypes are based on the phenotypic and agronomic traits. The study suggests that parents used in breeding should be chosen following assessment of genetic diversity based on molecular markers.
In conclusion, the current study found the existence of reasonable variability among rice genotypes, which could be exploited for future breeding. The results revealed that nine of the 14 selected SSR markers were highly polymorphic and sufficiently distinguished the tested rice genotypes. The cluster analysis classified the 54 rice genotypes into three major distinct genetic groups irrespective of the source of collection. Genotypes IR56, Mwanza, Salama M-55, Sindano Nyeupe, SARO, Gigante, Mwanza, SARO, Lunyuki, Zambia, Rangimbili, IRAT 256, Zambia and Salama M-19 showed unique genetic pattern and relationship suggesting that they may have different genetic makeup. These can be used as sources of novel genes in rice breeding programs. Hence, the information generated will contribute significantly to rice improvement in Tanzania and other related environments in east Africa.
Acknowledgements
The authors acknowledged the African Centre for Crop Improvement, University of KwaZulu-Natal, Pietermaritzburg, South Africa for their technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
William Titus Suvi is a Ph.D. fellow in Plant Breeding at the University of KwaZulu-Natal in South Africa. He is the main author for the current study.
Hussein Shimelis is a Professor of Plant Breeding at the University of KwaZulu-Natal in South Africa. He is a co-author for the current study.
Mark Laing is a Professor of Plant Pathology at the University of KwaZulu-Natal in South Africa. He is a co-author for the current study.
Isack Mathew is a Post-Doctoral Research Fellow in Plant Breeding at the African Centre for Crop Improvement (ACCI), University of KwaZulu-Natal in South Africa. He is a co-author for the current study.
Admire Isaac Tichafa Shayanowako is a Post-Doctoral Research Fellow in Plant Breeding at the African Centre for Crop Improvement (ACCI), University of KwaZulu-Natal in South Africa. He is a co-author for the current study.
ORCID
Isack Mathew http://orcid.org/0000-0001-7358-4690
Additional information
Funding
References
- Ali MA, Islam MS, Mandal SK, Nasrin Z, Rahman MM, Kuddus RH, Prodhan SH. 2014. Genetic diversity among salt-tolerant rice (Oryza sativa L.) landraces cultivated in the coastal districts of Bangladesh. J Biosci Biotech. 3:15–22.
- Aljumaili SJ, Rafii MY, Latif MA, Sakimin SZ, Arolu IW, Miah G. 2018. Genetic diversity of aromatic rice germplasm revealed by SSR markers. BioMed Res Int. 2018:1–11.
- Anh TTT, Khanh TD, Dat TD, Xuan TD. 2018. Identification of phenotypic variation and genetic diversity in rice (Oryza sativa L.) mutants. Agriculture. 8(2):30. doi:10.3390.
- Ansah T, Dogbe W, Cudjoe S, Abdul-Basit IAR, Eseoghene AS. 2017. Agronomic performance of five rice varieties and nutritive value of the straw from these varieties. West African J Appl Ecol. 25:1–10.
- Ashfaqa M, Khan AS. 2012. Genetic diversity in basmati rice (Oryza sativa L.) germplasm as revealed by microsatellite (SSR) markers. Russ J Genet. 48(1):53–62.
- Ashraf H, Husaini AH, Bhat MA, Parray GA, Khan S, Ganai NA. 2016. SSR based genetic diversity of pigmented and aromatic rice (Oryza sativa L.) genotypes of the western Himalayan region of India. Phys Mol Bio Plants. 22:547–555.
- Barnaud A, Trigueros G, McKey D, Joly HI. 2008. High outcrossing rates in fields with mixed sorghum landraces: how are landraces maintained? Heredity. 101:445–452.
- Becerra V, Paredes M, Gutierrez E, Rojo C. 2015. Genetic diversity, identification, and certification of Chilean rice varieties using molecular markers. Chilean J Agri Res. 75:267–274.
- Bucheyeki LK, Shennkawa E, Kadadi D, Lobulu J. 2011. Assessment of rice production constraints and farmers preferences in Nzega and Igunga district, Tanzania. J Adv Dev Res. 2:30–37.
- Chemutai LR, Musyoki MA, Kioko WF, Mwenda NS, Muriira KG. 2016. Genetic diversity studies on selected rice (Oryza sativa L.) genotypes based on gel consistency and alkali digestion. J Rice Res. 4:192.
- Chen C, He W, Nassirou TY, Nsabiyumva A, Dong X, Adedze YMN, Jin D. 2017. Molecular characterization and genetic diversity of different genotypes of Oryza sativa and Oryza glaberrima. Electron J Biotechnol. 30:48–57.
- Chen X, Temnykh S, Xu Y, Cho YG, McCouch SR. 1997. Development of a microsatellite framework map providing genome-wide coverage in rice (Oryza sativa L.). Theor Appl Genet. 95:553–567.
- Choudhary G, Ranjitkumar N, Surapaneni M, Deborah DA, Vipparla A, Anuradha G. 2013. Molecular genetic diversity of major Indian rice cultivars over decadal period. PLoS One. 8(6):e66197.
- Choudhury DR, Singh N, Singh AK, Kumar S, Srinivasan K, Tyagi RK. 2014. Analysis of genetic diversity and population structure of rice germplasm from North-eastern region of India and development of a core germplasm set. PLoS One. 9(11):e113094.
- Dixit S, Swamy BPM, Vikram P, Ahmed HU, Cruz MTS, Amante M, Atri D, Leung H, Kumar A. 2012. Fine mapping of QTLs for rice grain yield under drought reveals sub-QTLs conferring a response to variable drought severities. Theor Appl Genet. 125:155169.
- Earl DA, von Holdt BM. 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genetic Res. 4:359–361.
- Evanno G, Regnaut S, Goudet J. 2005. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 14:2611–2620.
- [FAO] Food and Agriculture Organization of the United Nations. 2017. Global paddy production. http://faostat.fao.org/default.aspx.
- Ganesamurthy K, Punitha D, Elangovan M. 2010. Genetic diversity among the land races of sorghum collected in Tamil Nadu. Electron J Plant Breeding. 1:1375–1379.
- Islam MR, Gregorio GB, Salam MA, Collard BCY, Singh RK, Hassan L. 2012. Validation of SalTol linked markers and haplotype diversity on chromosome 1 of rice. Mol Plant Breeding. 3:103–114.
- Islam MZ, Khalequzzaman M, Prince MFRK, Siddique MA, Rashid MSMH, Ahmed MSU, Pittendrigh BR, Ali MP. 2018. Diversity and population structure of red rice germplasm in Bangladesh. PLoS ONE. 13(5):e0196096.
- Islam MR, Singh RK, Salam MA, Hassan L, Gregorio GB. 2008. Molecular diversity of stress tolerant rice genotypes using SSR markers. SABRAO J Breeding Genet. 40(2):127–139.
- Jain S, Jain RK, McCouch SR. 2004. Genetic analysis of Indian aromatic and quality rice (Oryza sativa L.) germplasm using panels of fluorescently labelled microsatellite markers. Theory Appl Genet. 109(5):965–977.
- Lee HH, Neoh PPN, Bong WST, Puvaneswaran J, Wong SC, Yiu PH, Rajan A. 2011. Genotyping of Sarawak rice cultivars using microsatellite markers. Pertanika J Trop Agric Sci. 34(1):123–136.
- Liu D, Wang JY, Wang XX, Yang XL, Sun J, Chen WF. 2015. Genetic diversity and elite gene introgression reveal the japonica rice breeding in northern China. J Integrative Agri. 14:811–822.
- Luther Z, Akromah R, Nyadanu D, Tokpah DP, Page Z, Voor VM, Kwaloe AD. 2017. Evaluation of genetic diversity in rice (Oryza sativa and Oryza glaberrima) germplasm from Liberia and Ghana using simple sequence repeat (SSR) markers. Afr J Biotechnol. 16:1990–1996.
- Mekbib F. 2007. Infra-specific folk taxonomy in sorghum (Sorghum bicolor L.) Moench] in Ethiopia: folk nomenclature, classification, and criteria. J Ethnobiol Ethnomed. 3:1746–4269.
- Meti N, Samal KC, Bastia DN, Rout GR. 2013. Genetic diversity analysis in aromatic rice genotypes using microsatellite based simple sequence repeats (SSR) marker. African J Bio Technol. 12(27):4238–4250.
- Mittal N, Dubey AK. 2009. Microsatellite markers – A new practice of DNA based markers in molecular genetics. A review. Plant Breed. 3:235–246.
- Mligo FEI, Msuya CP. 2015. Farmer’s adoption of recommended rice varieties: A case of Kilombero district of Morogoro region of Tanzania. Southern African J Agri Extens. 43(1):41–56.
- Mogga M, Sibiya J, Shimelis H, Lamo J, Ochanda N. 2018. Appraisal of major determinants of rice production and farmers choice of rice ideotypes in South Sudan: implications for breeding and policy interventions. Exp Agric. 55:1–14.
- Mogga M, Sibiya J, Shimelis H, Lamo J, Yao N. 2017. Diversity analysis and genome-wide association studies of grain shape and eating quality traits in rice (Oryza sativa L.) using DArT markers. PLoS ONE. 13(6):e0198012..
- Morjan CL, Rieseberg LH. 2004. How species evolve collectively: Implications of gene flow and selection for the spread of advantageous alleles. Mol Ecol. 13:1341–1356.
- Mousavi S, Mariotti R, Regni L, Nasini L, Bufacchi M, Pandofi S, Baldoni L, Proietti P. 2017. The first molecular identification of an Olive collection applying standard simple sequence repeats and novel expressed sequence tag markers. Front Plant Sci. 8:1283. doi:10.3389/fpls.2017.01283.
- Mulualem T, Mekbib F, Shimelis H, Gebre E, Amelework B. 2018. Genetic diversity of yam (Dioscorea spp.) landrace collections from Ethiopia using simple sequence repeat markers. Aust J Crop Sci. 12(8):1223–1230.
- Nachimuthu VV, Muthurajan R, Duraialaguraja S, Sivakami R, Pandian BA, Ponniah G. 2015. Analysis of population structure and genetic diversity in rice germplasm using SSR markers: An initiative towards association mapping of agronomic traits in Oryza sativa. Rice. 8:30–54.
- Ndjiondjop MN, Semagin K, Warburton ML. 2018. Assessment of genetic variation and population structure of diverse rice genotypes adapted to lowland and upland ecologies in Africa using SNPs. Front Plant Sci. 9:446.
- Nei M. 1972. Genetic distance between populations. Am Nat. 106:283–292.
- Nei M, Li W. 1979. Mathematical method for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 76:5256–5273.
- Nkuba J, Ndunguru A, Madulu R, Lwezaura D, Kajiru G, Babu A, Chalamila B, Ley G. 2016. Rice value chain in Tanzania: identification of constraints, opportunities and upgrading strategies. African Crop Sci J. 26:73–87.
- Nuijten E, Van Treuren R, Struik PC, Mokuwa A, Okry F, Teeken B, Richards P. 2009. Evidence for the emergence of new rice types of interspecific hybrid origin in West African farmers’ fields. PLoS ONE. 4(10):e7335.
- Palanga KK, Traore K, Bimpong K, Jamshed M, Mkulama MAP. 2016. Genetic diversity studies on selected rice varieties grown in Africa based on aroma, cooking and eating quality. Afr J Biotechnol. 15:1136–1146.
- Panaud O, Chenayd X, McCouch SR. 1996. Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet. 252(5):597–607.
- Peakall R, Smouse PE. 2007. GENALEX6: genetic analysis in excel. population genetic software for teaching and research. Mol Ecol. 6:288–295.
- Perrier X, Jacquemoud-Collet JP. 2006. DARwin software http://darwin.cirad.fr/darwin.
- Pervaiz ZH, Rabbani MA, Khaliq I, Pearce SR, Malik SA. 2010. Genetic diversity associated with agronomic traits using microsatellite markers in Pakistani rice landraces. Electron J Biotechnol. 13(3):1–12.
- Prathepha P. 2012. Genetic diversity and population structure of wild rice (Oryza rufipogon) from North-eastern Thailand and Laos. Australian J Crop Sci. 6:717–723.
- Pritchard J, Stephens M, Rosenberg N, Donnelly P. 2000. Association mapping in structured populations. Am J Hum Genet. 67:170–180.
- Rahman MM, Rasaul MG, Hossain MA, Iftekharuddaula KM, Hasegawa H. 2012. Molecular characterization and genetic diversity analysis of rice (Oryza sativa L.) using SSR markers. J Crop Improvement. 26(2):244–257.
- Rao VR, Hodgkin T. 2002. Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell Tissue Organ Cult. 68:1–9.
- Sorkheh K, Masaeli M, Chaleshtori MH, Adugna A, Ercisli S. 2016. AFLP-based analysis of genetic diversity, population structure, and relationships with agronomic traits in rice germplasm from North region of Iran and world core germplasm set. Biochem Genet. 54(2):177–193.
- Sun CQ, Wang XK, Yoshimura A, Iwata N. 2000. A study of the genetic diversity of common wild rice (Oryza rufipogon Griff.) and cultivated rice (Oryza sativa L.) by RFLP analysis. Acta Genet Sin. 27:227–234.
- Sun XY, Kang S, Zhang YJ, Tan XQ, Yu YF, He HY, Zhang XY, Liu YF, Wang S, Sun WX, et al. 2013. Genetic diversity and population structure of rice pathogen Ustilaginoidea virens in China. PLoS ONE. 8:e76879.
- Temnykh S, Park WD, Ayes N, Cartinhour S, Hauck N, Lipovich L, Cho YG, Ishii T, McCouch SR. 2000. Mapping and genome organization of microsatellite sequences in rice (Oryza sativa L. Theor Appl Genet. 100(5):697–712.
- Wang J, Jiang T, Zou D, Zhao H, Li Q, Liu H, Zhou C. 2014. Genetic diversity and genetic relationships of japonica rice varieties in Northeast Asia based on SSR markers. Biotechnol Biotechnol Equip. 28(2):230–237.
- Wang X, Zhu J, Mansueto L, Bruskiewich R. 2005. Identification of candidate genes for drought stress tolerance in rice by the integration of a genetic (QTL) map with the rice genome physical map. J Zhejiang University Sci. 6B(5):382–388.
- Wright S. 1978. Evolution and the genetics of populations: variability within and among natural populations. Chicago: University of Chicago Press.
- Xiao XY, Wang YP, Zhang JY, Li SG, Rong TZ. 2016. SSR marker-based genetic diversity fingerprinting of hybrid rice in Sichuan, China. China J Rice Sci. 20:1–7.
- Yan AO, Yong X, Xiao-fen C, An W, Fei T, Li-qun S, Qiao-quan L. 2016. A genetic diversity assessment of starch quality traits in rice landraces from the Taihu basin, China. J Integrative Agri. 15(3):493–501.
- Yelome OI, Audenaert K, Landschoot S, Vanhove ADW, Silue D, Van Damme P, Haesaert G. 2018. Analysis of population structure and genetic diversity reveals gene flow and geographic patterns in cultivated rice (O. sativa and O. glaberrima) in West Africa. Euphytica. 214–215. doi:10.1007/s10681-018-2285-1.
- Zeng Y, Zhang H, Li Z, Shen S, Sun J, Wang M, Liao D, Liu X, Wang X, Xiao F, Wen G. 2007. Evaluation of genetic diversity of rice landraces (Oryza sativa L.) in Yunnan, China. Breed Sci. 57:91–99.
- Zhang H, Wei L, Miao H, Zhang T, Wang C. 2012. Development and validation of genetic SSR markers in sesame by RNA-seq. Bio Medical Central Genomics. 13:316–317.