ABSTRACT
Nowadays, in sustainable agriculture growing winter cover crops and using organic manure have been widely implemented to improve soil quality which leads to increase of microbial activity. Six-year study was performed to evaluate the effect of cropping system on soil microbial hydrolytic activity, content of soil organic carbon (SOC) and total nitrogen (Ntot) under potato cultivation by considering various management systems, while comparing the soil parameters after previous crop. The experiment consisted of five-field crop rotation with two different organic (Org 0 as control and Org II with winter cover crops plus added composted manure) and conventional (Conv 0 as control and Conv II with mineral N, 150 kg ha–1) farming systems. The results showed that hydrolytic activity of soil microbes decreased in every farming system under potato cultivation. Interestingly, after potato cultivation, the lowest and highest decrease in the soil microbial hydrolytic activity were seen in Org II and Conv II systems, respectively. The highest soil microbial hydrolytic activity was measured in system Org II where incorporation of biomass from winter cover crops and cattle manure was used. Finally, SOC and Ntot were higher in organic farming systems and there no significant changes after potato cultivation.
Introduction
Soil microbial activity that reflects microbiological processes of soil microorganisms is the potential indicator of soil quality, as plants rely on soil microorganisms to mineralise organic nutrients for growth and development. Soil microorganisms also process plant litter and residues into soil organic matter, a direct and stable reservoir of carbon and nitrogen that consists of living and dead organic materials subject to rapid biological decomposition. In natural systems, the action of soil microorganisms is a major determinant of efficient nutrient cycling (Chen et al. Citation2003). Soil microbial activity is significantly more sensitive against changes in the soil than physico-chemical parameters (e.g. soil organic carbon (SOC) and Ntot content) (Oldare et al. Citation2008, Citation2011; Tejada et al. Citation2008). The microbial activity in the soil can be indicated by hydrolytic activity and the soil microbial respiration, which is measured through CO2 production (Tejada et al. Citation2008).
The soil microbial communities and their activity depending on the cultivated crops and the farming practices (Chirinda et al. Citation2008; Wallis et al. Citation2010; Šteinberga et al. Citation2012). In organic production where only organic fertilisers are used, amount and quality of fertilisers affect the soil physico-chemical properties, microbial biomass and its activity in the soil (Gomez et al. Citation2006; Gomiero et al. Citation2011; Bonilla et al. Citation2012). Organic amendments increase crop yields by improving soil fertility (Baldwin Citation2006; Luo et al. Citation2018), and fertile soils rich in microbes suppress plant pathogens (Agrios Citation2005).
It has been reported that the higher rates of organic material in organic production have increased the mass and activity of microbes (Watson et al. Citation2002; Larkin et al. Citation2010). One of the most important indicators of soil fertility and quality is the SOC content (Plaza-Bonilla et al. Citation2014), that influences the chemical, physical and biological properties of the soil (Lal et al. Citation2007). The soils with higher SOC content are more fertile and increase the crop yields (Plaza-Bonilla et al. Citation2014). Therefore it is important to monitor SOC content in the soils in order to avoid any decrease in SOC content. It is also necessary to know which crop management methods may increase the SOC. Presence of nitrogen in soil organic matter is also an important source of soil fertility and it is released during the decomposition process of organic matter (Sincik et al. Citation2008).
Potato needs a lot of nutrients for root and stem growth and tuber development. The use of organic fertilisers such as manure, various mulches and green manure crops increase the organic carbon content and the microbial activity in the soil (van Vliet et al. Citation2006; Larkin Citation2008; Larkin et al. Citation2010; Mandic et al. Citation2011; Bhagat et al. Citation2016). In potato cultivation, activity of bacterial populations and soil microbes have been increased by using the green manure cover crops which have also had a positive effect on the tuber yield (Larkin et al. Citation2017). In conventional potato farming system, soil is intensively managed during the growing period (e.g. planting and soil management methods as well as using chemicals). It has been found that soil microbial activity decreased after potato cultivation (Chirinda et al. Citation2008; Šteinberga et al. Citation2012). The reduction in the activity of soil microbes has been shown in conventional farming practices where herbicides and fungicides had been used for weed and late blight control (Niemi et al. Citation2009; Järvan et al. Citation2014). The herbicides and fungicides may either be directly toxic to soil microbes or interfere with the relations between species that may have a negative impact on plant growth (Vukicevich et al. Citation2016). The insecticide usage increased the occurrence of Rhizoctonia damage in potato because of reduction of soil bacteria, which act as growth stimulators to reduce the occurrence of infection, resulting in an increase in the resistance to diseases (Thornton et al. Citation2010).
Considering above mentioned issues, it is possible to hypothise that organic farming without synthetical agrochemicals and higher amount organic materials in rotation could promote soil microbial activity, which is important in processing materials to organic matter – stable reservoir of carbon and nitrogen. The aim of the present study was to investigate the effect of potato cultivation under two conventional and two organic systems on the soil microbial hydrolytic activity (FDA), change in SOC content and total nitrogen content (Ntot) in comparison to soil parameters after previous crop.
Materials and methods
Experimental setup
Long-term field experiment comparing organic and conventional farming systems was set up in 2008 at Estonian University of Life Sciences (Chair of Crop Science and Plant Biology) (58°22ʹN, 26°40ʹE). 5-field crop rotation is used in the experiment. The potato was part of a crop rotation experiment where red clover (Trifolium pratense L.), winter wheat (Triticum aestivum L.), peas (Pisum sativum L.), potato and barley (Hordeum vulgare L.) undersown with red clover followed each other in that sequence every year. The period between the end of first crop rotation and the end of second crop rotation (2012–2017) has been investigated in this article.
The crops in rotation are fertilised with organic and mineral fertilisers in organic system and conventional system, respectively. Four farming systems including two organic and two conventional systems were investigated in this study. Organic systems consisted of system without winter cover crops (Org 0) that only followed the rotation and system with winter cover crops and composted cattle manure (for spring barley and winter wheat 10 t ha–1 and 20 t ha–1 for potato in the spring) (Org II). In Org II system after harvesting of winter wheat, a mixture of winter rye (Secale cereale L.) and winter rapeseed (Brassica napus L. var. oleifera L.) was sown as winter cover crops. The nutrient amounts applied in 2012–2017 (every year) with manure were 41–54 kg N ha–1, 12–19 kg P ha–1 and 27–43 kg K ha–1. Furthermore, in organic systems neither mineral fertilisers nor synthetic pesticides were used.
In our experiment, the conventional system included two different systems; – Conv 0 as a control (N0P0K0) and Conv II (N150 P25 K95) for potato. In autumn herbicide Roundup Gold (4.0 lha–1) and in spring Titus (50 g ha–1) were used for weed control. Against Colorado beetle Fastac 50 (0.3 l ha–1) and Decis 2.5 EC (0.2 l ha–1) were used. Late blight control was performed twice: Ridomil Gold MZ 68 WG (2.5 kg ha–1) and Ranman (0.2 kg ha–1) together with Ranman activator (0.15 l ha–1). Each year the field was levelled before potato planting (beginning of May), deep ploughed and twice cultivated. The furrows were formed before planting and potatoes were planted by 2-row potato planter Juku. After planting the field was harrowed once and ridged three times.
Tubers of cultivar ‘Maret’ were planted in the furrow with a distance of 25 cm between them and the width of furrows was 70 cm. For planting, seed tubers with 35–55 cm diameter were chosen.
The experiment was set up in a systematic block design with four replicates and the size of the test plot was 60 m2 (Alaru et al. Citation2014). The soil was Stagnic Luvisol (WRB Citation2014), sandy silt loam with humus depth of 20–30 cm (Reintam and Köster Citation2006).
Chemical analyses
Once a year in mid-April before starting of field operations, soil samples were taken from the depth of 0–25 cm. Eight samples were taken from each plot to obtain the average for each plot. Air-dried soil samples were sieved through a 2 mm sieve. The content of SOC was measured using the Tjurin method (Soil Survey Laboratory Staff Citation1996) and Ntot content was measured using the Kjeldahl method (Procedures for Soil Analysis Citation2002).
The spectrophotometric determination of soil microbial hydrolytic activity is a simple and fast method for evaluation of the microbial activity in the soil (Schnürer and Rosswall Citation1982; Nannipieri et al. Citation2003). For determination of soil microbial hydrolytic activity, the samples of 500 g were taken from the depth of 5–10 cm according to ISO 10381–Citation6 (Citation1993) method and these were sieved through the 2 mm sieve (Procedures for Soil Analysis Citation2002). The preparation of reagents for following soil microbial hydrolytic activity analysis was performed according to the method described by Adam and Duncan (Citation2001).
Statistical analysis
The statistical analysis of collected data was performed with the software Statistica 13 (Quest Software Inc, Aliso Viejo, CA, USA). Full-factorial analysis of variance (ANOVA) was used to test the statistical significance of year, farming system and their interaction effects on soil properties (soil microbial hydrolytic activity, SOC and Ntot). The significance of the effects on the soil microbial hydrolytic activity, content of SOC and Ntot in the soil was assessed with one-way ANOVA. In the comparison of the differences between plots Tukey HSD (honest significant difference) post-hoc test (P < 0.05) was used. Correlation analysis was used as linear correlation coefficients between variables and the significance of coefficients were taken as P < 0.001, P < 0.01, P < 0.05 or ns (no significant).
In this paper, all the experimental data are presented on the average of 2012–2017 years.
Results and discussion
Results of factorial analysis showed that the significant (P < 0.05) effect of the climatic conditions of the experimental years and the combined effect of years and farming systems were noticed only for Ntot content in soil () The significant effects of the farming systems on most parameters under investigation were apparent.
Table 1. Impact of trial factors on soil microbial hydrolytic activity (FDA, μg of fluorescein g–1 soil dry weight h–1), SOC (%) and total nitrogen (%) content.
Soil microbial hydrolytic activity
As an average of six years crop rotation (2012–2017), the highest soil microbial hydrolytic activity was measured in Org II before potato cultivation where winter cover crops and cattle manure were used (). Composted cattle manure increases not only the fertility of the soil by adding nutrients but also the microbial diversity in the soil. Edesi et al. (Citation2012) found that long-term crop rotations and organic fertilisers have a positive impact on the soil microbial populations and their activity.
Table 2. Soil microbial hydrolytic activity (FDA, μg of fluorescein g–1 soil dry weight h–1), organic carbon (%) and total nitrogen content (%) before and after the potato cultivation as an average of 2012–2017.
As an average of experimental years of potato cultivation, microbial hydrolytic activity decreased in each farming system (). After harvesting the potato the lowest decrease of soil microbial hydrolytic activity (by 3.9%) was observed in Org II system (F(1,46) = 2.17, P = 0.148) and the highest decrease was related to Conv II system, where soil microbial hydrolytic activity decreased after the cultivation of potato by 8.6% (F(1,46) = 16.23, P < 0.001). The decrease in soil microbial hydrolytic activity of both control plots was between Conv II and Org II respective values: Conv 0 by 6.2% (F(1,46) = 5.82, P < 0.001) and Org 0 by 7.6% (F(1,46) = 12.40, P < 0.001) ().
Every year the lowest soil microbial hydrolytic activity was measured in control plot (Conv 0) without fertilisers, but where chemical herbicides had been used (before F(3,92) = 35.95, P < 0.001 and after potato F(3,92) = 46.94, P < 0.001). Madsen et al. (Citation2016) reported that the soil microbial hydrolytic activity was the lowest in test areas without any fertiliser where chemical herbicides were used. This is confirmed by organic system control plots (Org 0) where the soil microbial hydrolytic activity was by 17.7% (before potato) and 15.4% (after potato) higher than in Conv 0 plots. Our results are in accordance with previous findings that show the inhibition effect of pesticide residues on the soil biota and activity of microorganisms (Makaw et al. Citation1979; Angelini et al. Citation2013). Based on the literature, glyphosate affects adversely the free-living bacteria populations by reducing the amount of soil bacteria as well as the ratios of various bacteria groups and thereby decreasing the soil fertility (Newman et al. Citation2016; Aristilde et al. Citation2017).
The experiment carried out on the same test area showed that as an average of crop rotation the highest soil microbial hydrolytic activity was measured in organic system plots, where the amount of organic material (plant residues, cover crops and manure) ploughed into the soil were the highest (Madsen et al. Citation2016). The undersowing of legumes into cereals increases the soil biological activity and their roots create suitable conditions for bacteria which produce polysaccharides responsible for the physical improvement of the soil parameters (Russell Citation1971; Tejada et al. Citation2008). The soil physical fractions comprising of several organic compounds create structural and functional properties of the soil carbon (Christensen Citation1996). Sánchez de Cima et al. (Citation2016) observed that the winter cover crops and manure had a significant positive impact on the enzymatic activity of microorganisms.
Soil organic carbon content
In our experiment, SOC in Org II systems was significantly higher than in conventional systems before and after potato cultivation ().
Compared to conventional systems (Conv 0 and Conv II), SOC of samples collected from organic plots (Org 0 and Org II) before and after potato cultivation was higher by the ratio of 13.6% (F(1,94) = 44.99, P < 0.001) and 14.0% (F(1,94) = 33.81, P < 0.001), respectively (). There were no significant differences in SOC between soil samples collected before and after the potato cultivation. Moreover, there was a small decline of SOC by 3.2% (statistically not significant) in Conv 0 after potato cultivation as an average of experimental years (F(1,46) = 1.96, P = 0.168) compared to SOC before potato cultivation (). Kauer et al. Citation2015 found that in well-developed crop rotations high rates of N fertilisers might be useful for stabilisation of SOC. This could have been the reason why the changes in soil SOC content in potato cultivation showed an increasing tendency. Higher amount of SOC in Org II is due to the bigger input of organic matter from sources such as winter cover crops and manure (Org II) and also due to greater number of earthworms (Kahu et al. Citation2017) which play an important role in the humification of organic matter.
Statistically significant relationship between SOC and microbial activity was observed in samples which were collected before and after the potato cultivation in conventional systems ((a,b)) – correlation between SOC and soil microbial hydrolytic activity before potato cultivation was R = 0.29, P = 0.046 and after potato cultivation R = 0.61, P < 0.001. Therefore, after potato cultivation SOC influenced the soil microbial activity in the conventional system more than organic system. In experiments by Kuht et al. (Citation2019a) soil SOC was significantly affected by microbial hydrolytic activity of the soil in cultivation of barley undersown with red clover.
Figure 1. The relationship between hydrolytic activity of soil microorganisms (FDA, μg fluorescsein g–1 dry soil h–1) and the content of SOC (%) in conventional before (a), and after the potato (b), organic system before (c) and after the potato (d) cultivation as an average of 2012–2017 (N = 24 farming system).
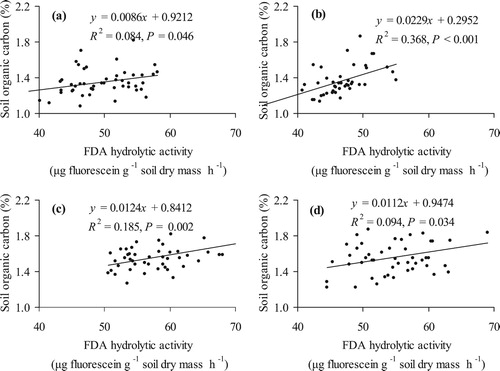
In organic systems before potato cultivation the correlation between SOC and soil microbial hydrolytic activity was (R = 0.43, P = 0.002) significantly higher than in conventional systems and also after potato cultivation it was R = 0.31, P = 0.034, slightly less than in samples collected before potato cultivation ((c,d)).
Soil total nitrogen content
Our results showed that organic farming system had a positive effect on the soil Ntot content. The Ntot content of samples collected from organic systems before potato cultivation was higher than conventional systems by 19.6% as an average of experimental years (F(1,94) = 44.06, P < 0.001) (). Interestingly, after potato cultivation, Ntot content of soil in organic systems (Org 0 and Org II) was higher than in conventional systems (Conv 0 and Conv II) by 20.0% (F(1,94) = 40.19, P < 0.001).
Before and after potato cultivation Ntot contents on average of investigated years were higher under organic farming systems (). Our results showed that on average potato cultivation had some decreasing effect on Ntot content. In terms of soil Ntot content, the reduction has been shown only in the conventional farming system where no mineral fertilisers were used. However, in this study Ntot values were higher in organic farming systems. Suppressing the soil microbiota by use of pesticides and more acidity of the soil may be the reasons for lower Ntot content in conventional system (Talgre et al. Citation2018). It should also be taken into account that if soil biological processes are inhibited, the decomposition of organic matter and then the release of nutrients are reduced (Thornton et al. Citation2010).
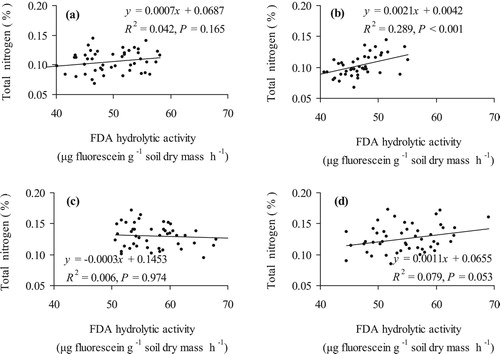
A significant relationship between Ntot and soil microbial hydrolytic activity content was seen only after potato cultivation where correlation in conventional systems was strong, R = 0.54, P < 0.001 ((a,b)). In organic systems before potato cultivation the correlation between Ntot and soil microbial hydrolytic activity was negative, R = 0.05, P = 0.974 ((c)) although relationship was stronger after the potato cultivation, R = 0.28, P = 0.053 ((d)). Therefore, the Ntot content had a significant impact on the soil microbial hydrolytic activity only in conventional system after potato cultivation. But in the same experiment in the soil after the cultivation of barley, that followed potato, undersown with red clover the effect of FDA on Ntot content decreased (Kuht et al. Citation2019b). Therefore, the cultivation of potato with the use of organic fertilisers in crop rotation is highly appreciated concerning the Ntot content of the soil.
Figure 2. The relationship between hydrolytic activity of soil microorganisms (FDA, μg fluorescsein g–1 dry soil h–1) and the content of total nitrogen (Ntot, %) in conventional before (a) and after the potato (b), organic system before (c) and after the potato (d) cultivation as an average of 2012–2017 (N = 24 farming system).
In organic systems, higher activity of soil microbes and their relationship with SOC was seen in Org II (with winter cover crops and composted cattle manure). Our previous results have proved that cover crops and composted cattle manure have significantly improved soil physical parameters such as water holding capacity and infiltration in organic systems (Talgre et al. Citation2015). The improvement in infiltration properties decreases the accumulation of excess water on the field and also improves resistance to drought. Although most of soil-borne diseases are naturally suppressed, sometimes foliar diseases can be problematic (van Bruggen et al. Citation2016).
For good crop health and high yields, good soil status is the main indicator that has been frequently addressed. If soil chemical composition and structure are optimal, microbial activity will be high and if soil carbon levels are stable, the conditions for crop production will be ideal. Besides soil physico-chemical parameters, soil biological characteristics (incl microbial and enzymatic activity) are mentioned (Wallis et al. Citation2010; Šteinberga et al. Citation2012) as the most important parameters for soil quality.
We concluded that the cultivation of potato decreased the hydrolytic activity of soil microbes in each farming system. After potato cultivation, the lowest and highest decrease in soil microbial hydrolytic activity was shown in Org II and Conv II systems, respectively. The SOC and Ntot content before and after potato cultivation were significantly higher in organic systems compared to conventional systems. However, significantly higher activity of soil microbes and their relationship with SOC content was seen in organic systems, especially in Org II, where winter cover crops and composted cattle manure were used.
Acknowledgements
The technical assistance of Rõhu experimental station from the Estonian University of Life Sciences is gratefully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Viacheslav Eremeev, is a research fellow at the Chair of Crop Science and Plant Biology, Estonian University of Life Sciences (EULS). His area of expertise include field crop husbandry, potato, crop rotation, regulation humus status of soil in conventional and organic farming.
Liina Talgre, is a research fellow at the Chair of Crop Science and Plant Biology, EULS. Her area of expertise include elaboration of the practical means and the theoretical basis of weed control system, regulation humus status of soil in conventional and organic farming, the circulation of carbon and nitrogen during the growth of different cultures of green manure.
Jaan Kuht, is a research fellow at the Chair of Crop Science and Plant Biology, EULS. He is a Professor Emeritus of Agricultural Sciences. His area of experise include soil physical propertis.
Erkki Mäeorg, is a research fellow at the Chair of Crop Science and Plant Biology, EULS. His area of expertise include quality of oil crops, cultivation of bioenergy crops, principles of yield formation.
Keyvan Esmaeilzadeh-Salestani, student, is a research fellow at the Chair of Crop Science and Plant Biology, EULS. His area of research include comparison of conventional and organic farming systems and technologies in terms of sustainability.
Maarika Alaru, is a research fellow at the Chair of Crop Science and Plant Biology, EULS. Her area of expertise include winter crops, winter triticale, physiological maturity, energy plants.
Evelin Loit, is a research fellow at the Chair of Crop Science and Plant Biology, EULS. Her area of expertise include crop husbandry and genetics.
Eve Runno-Paurson, is a research fellow at the Chair of Crop Science and Plant Biology, Estonian EULS. Her area of expertise include crop husbandry, horticulture, crop protection, phytopathology.
Anne Luik , is a research fellow at the Chair of Plant Health, EULS. Her area of expertise include insects eco-physiology, insect-plant interactions, environmentally friendly plant protection methods, organic plant production.
ORCID
Viacheslav Eremeev http://orcid.org/0000-0003-3409-8918
Liina Talgre http://orcid.org/0000-0003-0949-6973
Jaan Kuht http://orcid.org/0000-0002-9810-6035
Erkki Mäeorg http://orcid.org/0000-0002-0293-8647
Keyvan Esmaeilzadeh-Salestani http://orcid.org/0000-0002-6882-7616
Maarika Alaru http://orcid.org/0000-0001-7642-4270
Evelin Loit http://orcid.org/0000-0001-6635-8740
Eve Runno-Paurson http://orcid.org/0000-0001-7231-1703
Anne Luik http://orcid.org/0000-0002-2830-9574
Additional information
Funding
References
- Adam G, Duncan H. 2001. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem. 33:943–951. doi:10.1016/S0038-0717(00)00244-3.
- Agrios GN. 2005. Environmental effects on the development of infectious plant disease. In: Plant pathology. 5th edn. Amsterdam: Academic Press; p. 251–265.
- Alaru M, Talgre L, Eremeev V, Tein B, Luik A, Nemvalts A, Loit E. 2014. Crop yields and supply of nitrogen compared in conventional and organic systems. Agr Food Sci. 23:317–326. doi:10.23986/afsci.46422.
- Angelini J, Silvina G, Taurian T, Ibanez F, Tonelli ML, Valetti L, Anzuay MS, Luduena L, Munoz V, Fabra A. 2013. The effect of pesticides on bacterial nitrogen fixers in peanut-growing area. Arch Microbiol. 195:683–692. doi:10.1007/s00203-013-0919-1.
- Aristilde L, Reed ML, Wilkes RA, Youngster T, Kukurugya MA, Katz V, Sasaki CRS. 2017. Glyphosate-induced specific and widespread perturbations in the metabolome of soil Pseudomonas species. Front Environ Sci. 5:34. doi:10.3389/fenvs.2017.00034.
- Baldwin KR. 2006. Soil fertility on organic farms. Organic Production. Soil Fertility on Organic Farms. [accessed 2019 Mar 15] https://content.ces.ncsu.edu/soil-fertility-on-organic-farms/.
- Bhagat P, Gosal SK, Singh CB. 2016. Effect of mulching on soil environment, microbial flora and growth of potato under field conditions. Indian J Agric Res. 50:542–548. doi:10.18805/ijare.v50i6.6671.
- Bonilla N, Cazorla FM, Martínez-Alonso M, Hermoso JM, González-Fernández J, Gaju N, Landa BB, de Vicente A. 2012. Organic amendments and land management affect bacterial community composition, diversity, and biomass in avocado crop soils. Plant Soil. 357:215–226. doi:10.1007/s11104-012-1155-1.
- Chen G, Zhu H, Zhang Y. 2003. Soil microbial activities and carbon and nitrogen fixation. Res Microbiol. 154:393–398. doi:10.1016/S0923-2508(03)00082-2.
- Chirinda N, Olesen JE, Porter JR. 2008. Effects of organic matter input on soil microbial properties and crop yields in conventional and organic cropping systems. 16th IFOAM Organic World Congress; Modena, Italy. [accessed 2019 Mar 23] http://orgprints.org/14656/1/Paper_ISOFAR.pdf.
- Christensen BT. 1996. Matching measurable soil organic matter fractions with conceptual pools in simulation models of carbon turnover. In: Powlson DS, Smith P, Smith JU, editors. Evaluation of soil organic matter models. Berlin: Springer; p. 143–159.
- Edesi L, Järvan M, Noormets M, Lauringson E, Adamson A, Akk E. 2012. The importance of solid cattle manure application on soil microorganisms in organic and conventional cultivation. Acta Agric Scand Sect B - Soil Plant Sci. 62:1–12. doi:10.1080/09064710.2012.678380.
- Gomez E, Ferreras L, Toresani S. 2006. Soil bacterial functional diversity as influenced by organic amendment application. Bioresource Technol. 97:1484–1489. doi:10.1016/j.biortech.2005.06.021.
- Gomiero T, Pimentel D, Paoletti MG. 2011. Environmental impact of different agricultural management practices: conventional versus organic agriculture. Crit Rev Plant Sci. 30:95–124. doi:10.1080/07352689.2011.554355.
- ISO 10381-6. 1993. Soil quality-sampling. Guidance on the collection, handling and storage of soil for the assessment of aerobic microbial processes in laboratory. Geneva: International Organization for Standardization.
- Järvan M, Edesi L, Adamson A, Võsa T. 2014. Soil microbial communities and dehydrogenase activity depending on farming systems. Plant Soil Environ. 60:459–463. doi:10.17221/410/2014-PSE.
- Kahu G, Talgre L, Reintam E, Eremeev V, Luik A. 2017. Mullaharimise ja väetamise mõju vihmaussikooslusele. [Impact of soil cultivating and fertilization on earthworms]. In: Metspalu L, Luik A, Peetsmann E, editors. Teaduselt mahepõllumajandusele, Eesti Loodusfoto. Tartu: SA Eesti Maaülikooli Mahekeskus; p. 55–58. Estonian.
- Kauer K, Tein B, Sanches de Cima D, Talgre T, Eremeev V, Loit E, Luik A. 2015. Soil carbon dynamics estimation and dependence on farming system in a temperate climate. Soil Till Res. 154:53–63. doi:10.1016/j.still.2015.06.010.
- Kuht J, Eremeev V, Alaru M, Talgre L, Loit E, Luik A. 2019b. Changes in microbial hydrolytic activity and the total nitrogen content in soil by undersowing red clover with barley. Agronomy 2019, p. 22–28, Estonian.
- Kuht J, Eremeev V, Talgre L, Alaru M, Loit E, Mäeorg E, Esmaeilzadeh-Salestani K, Luik A. 2019a. Changes in the soil microbial hydrolytic activity and the content of organic carbon and total nitrogen by growing spring barley undersown with red clover in different farming systems. Agriculture. 146:1–9. doi:10.3390/agriculture9070146.
- Lal R, Follet RF, Stewart BA, Kimble JM. 2007. Soil carbon sequestration to mitigate climate change and advance food security. Soil Sci. 172:943–956. doi:10.1097/ss.0b013e31815cc498.
- Larkin RP. 2008. Relative effects of biological amendments and crop rotations on soil microbial communities and diseases of potato. Soil Biol Biochem. 40:1341–1351. doi:10.1016/j.soilbio.2007.03.005.
- Larkin RP, Griffin TS, Honeycutt CW. 2010. Rotation and cover crop effects on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis. 94:1491–1502. doi:10.1094/PDIS-03-10-0172.
- Larkin RP, Honeycutt CW, Griffin TS, Olanya OM, He Z, Halloran JM. 2017. Cumulative and residual effects of different potato cropping system management strategies on soilborne diseases and soil microbial communities over time. Plant Pathol. 66:437–449. doi:10.1111/ppa.12584.
- Luo G, Li L, Friman VP, Guo J, Guo S, Shen Q, Ling N. 2018. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: a meta-analysis. Soil Biol Biochem. 124:105–115. doi:10.1016/j.soilbio.2018.06.002.
- Madsen H, Talgre L, Eremeev V, Luik A. 2016. Pestitsiidid suruvad alla mulla mikroobide hüdrolüütilist aktiivsust [Pesticides decreased soil microbial hydrolytic activity]. Est Plant Prot. 95:79–82. Estonian.
- Makaw AA, Abdel-Nasser M, Abdel-Moneim AA. 1979. Effect some pesticides on certain micro-organisms contributing to soil fertility. Zentralbl Bakteriol Naturwiss. 134:5–12.
- Mandic L, Djukić D, Beatovic I, Jovovic Z, Pesakovic M, Stevovic V. 2011. Effect of different fertilizers on the microbial activity and productivity of soil under potato cultivation. Afr J Biotechnol. 10:6954–6960. doi:10.5897/AJB11.947.
- Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G. 2003. Microbial diversity and soil functions. Eur J Soil Sci. 54:655–670. doi:10.1046/j.1351-0754.2003.0556.x.
- Newman MM, Hoilett N, Lorenz N, Dick RP, Liles M, Ramsier C, Kloepper J. 2016. Glyphosate effects on soil rhizosphere-associated bacterial communities. Sci Total Environ. 543:155–160. doi:10.1016/j.scitotenv.2015.11.008.
- Niemi RM, Heiskanen I, Ahtiainen J, Rahkonen A, Mäntykoski K, Welling L, Laitinen P, Ruuttunen P. 2009. Microbial toxicity and impacts on soil enzyme activities of pesticides used in potato cultivation. Appl Soil Ecol. 41:293–304. doi:10.1016/j.apsoil.2008.12.002.
- Oldare M, Arthurson V, Pell M, Svensson K, Nehrenheim E, Abubaker J. 2011. Land application of organic waste – effects on the soil ecosystem. Appl Energy. 88:2210–2218. doi:10.1016/j.apenergy.2010.12.043.
- Oldare M, Pell M, Svensson K. 2008. Changes in soil chemical and microbiological properties, during 4 years of application of various organic residues. Waste Manag. 28:1246–1253. doi:10.1016/j.wasman.2007.06.005.
- Plaza-Bonilla D, Alvaro-Fuentes J, Cantero-Martınez C. 2014. Identifying soil organic carbon fractions sensitive to agricultural management practices. Soil Till Res. 139:19–22. doi:10.1016/j.still.2014.01.006.
- Procedures for Soil Analysis. 2002. In LP van Reeuwijk, (Ed.), 6th edn. Wagenengen, The Netherlands, 119 pp.
- Reintam E, Köster T. 2006. The role of chemical indicators to correlate some Estonian soils with WRB and soil taxonomy criteria. Geoderma. 136:199–209. doi:10.1016/j.geoderma.2006.03.028.
- Russell EW. 1971. Soil structure: its maintenance and improvement. Eur J Soil Sci. 22:137–150. doi:10.1111/j.1365-2389.1971.tb01601.x.
- Sánchez de Cima D, Tein B, Eremeev V, Luik A, Kauer K, Reintam E, Kahu G. 2016. Winter cover crop effects on soil structural stability and microbiological activity in organic farming. Biol Agric Hortic: An Intern J Sustain Prod Syst. 32:170–181. doi:10.1080/01448765.2015.1130646.
- Schnürer J, Rosswall T. 1982. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol. 43:1256–1261. doi:0099-2240/82/061256-06$02.00/0.
- Soil Survey Laboratory Staff. 1996. Soil survey laboratory methods manual. Soil Survey Investigations Rep. No. 42, Version 3.0 Lincoln, NE, USA: National Soil Survey Center.
- Sincik M, Turan ZM, Göksoy AT. 2008. Responses of potato (Solanum tuberosum L.) to green manure cover crops and nitrogen fertilization rates. Am J Potato Res. 85:150–158. doi:10.1007/s12230-008-9043-1 doi: 10.1007/s12230-008-9011-9
- Šteinberga V, Mutere O, Jansone I, Alsiòa I, Dubova L. 2012. Effect of buckwheat and potato as forecrops on soil microbial properties in crop rotation. Proc Latvian Academy Sci Section B Natural Exact Appl Sci. 66:185–191.
- Talgre L, Eremeev V, Reintam E, Tein B, Sanches de Cima D, Madsen H, Alaru M, Luik A. 2015. Talvised vahekultuurid parandavad mulda ja kultuuride saagikust. [Winter cover crops improve soil and crop yield]. In: Alaru M, Astover A, Karp K, Viiralt R, Must A, editors. Agronomy 2015, Tartu: Ecoprint; p. 40–44. in Estonian.
- Talgre L, Madsen H, Eremeev V, Kuht J, Alaru M, Peetsmann E, Luik A. 2018. Impact of winter cover crops on soil quality and weeds in organic cropping systems. Dynamic developments in organic research. Strengthening partnerships across. Europe and beyond. 6th International Conference on Organic Agriculture Sciences; 7–9 November 2018; Eisenstadt, Austria. Eisenstadt, Austria: FiBL Austria, 88.
- Tejada M, Gonzalez JL, Garcia-Martinez AM, Parrado J. 2008. Effects of different green manures on soil biological properties and maize yield. Bioresource Technol. 99:1758–1767. doi:10.1016/j.biortech.2007.03.052.
- Thornton M, Miller J, Hutchinson P, Alvarez JM. 2010. Response of potatoes to soil-applied insecticides, fungicides, and herbicides. Potato Res. 53:351–358. doi:10.1007/s11540-010-9166-x.
- Vukicevich E, Lowery T, Bowen P, Úrbez-Torres JR, Hart M. 2016. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A Review. Agron Sustain Dev. 36:48. doi:10.1007/s13593-016-0385-7.
- Wallis PD, Haynes RJ, Hunter CH, Morris CD. 2010. Effect of land use and management on soil bacterial biodiversity as measured by PCR-DGGE. Appl Soil Ecology. 46:147–150. doi:10.1016/j.apsoil.2010.06.006.
- Watson CA, Atkinson D, Gosling P, Jackson LR, Rayns FW. 2002. Managing soil fertility in organic farming systems. Soil Use Manage. 18:239–247. doi:10.1111/j.1475-2743.2002.tb00265.x doi: 10.1079/SUM2002131
- van Bruggen AH, Gamliel A, Finckh MR. 2016. Plant disease management in organic farming systems. Pest Manage Sci. 72:30–44. doi:10.1002/ps.4145.
- van Vliet PCJ, Bloem J, de Goede RGM. 2006. Microbial diversity, nitrogen loss and grass production after addition of effective micro-organisms (EM) to slurry manure. Appl Soil Ecol. 32:188–198. doi:10.1016/j.apsoil.2005.07.001.
- WRB. 2014. IUSS Working Group WRB.2014. World reference base for soil resources, 2014. International soil classification system for naming soils and creating legends for soil maps. Rome: FAO. World Soil Resources Reports No. 106. ISBN978-92-5-108369-7.
