 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In the present study, weed plants viz., Calotropis procera, Ricinus communis, Lantana camara, Achyranthes aspera, Wedelia chinensis, and Colocasia esculenta were evaluated against Meloidogyne incognita affecting spinach. For in vitro egg masses hatching experiment, four different concentrations of aqueous extracts of weed leaves, i.e. 100%, 50%, 10%, and 1%, were analyzed. All the extracts showed varied but significant results over control (Distilled water). Furthermore, C. procera and R. communis treatment significantly inhibited J2s hatching (100% inhibition) at the 4th and 7th days of incubation in 100% concentration, while C. esculenta inhibited the least. In pot study, it was also found that the pots treated with 50 and 100 g dry leaf powder of C. procera has efficiently suppressed the root-knot disease in spinach and significantly increased growth in terms of length, fresh and dry weights, number of leaves and biochemical parameters such as chlorophyll, carotenoid content and nitrate reductase activity followed by A. aspera, L. camara, R. communis, W. chinensis while C. esculenta showed the least effect amongst the treatments. Thus, the results provide considerable data for the use of weed plants to manage the root-gall disease in spinach.
Introduction
Vegetables are a rich source of carbohydrates, vitamins, and minerals contributing to the essential requirements of our daily diet. Spinach (Spinacia oleracea L.) plants belonging to the family Amaranthaceae is widely cultivated in India for their nutritious leaves. It is unique among vegetable crops because of its tremendously high yield in a comparatively short period. In addition to a significant source of Vitamin K, spinach contains a great number of minerals, Vitamin B complex, ascorbic acid, and carotene. However, spinach is often attacked by bacteria, viruses, and nematodes resulting in a decline in the quality and quantity of leaves. Among the plant-parasitic nematodes, the root-knot nematode (Meloidogyne spp.) is a specified plant pathogen that infects spinach root (Murungi et al. Citation2018). About 2,000 plants worldwide are susceptible to root-knot infection and globally responsible for losing about $157 billion every year (Abad et al. Citation2008; Science Daily Citation2008). From this viewpoint, the endoparasite root-knot nematode Meloidogyne incognita has concerned significant attention.
In India, the root-knot nematode, M. incognita (Kofoid &White) Chitwood is the most common species, responsible for approximately 70% of the yield losses (Khan et al. Citation2014), and is economically the major crop-damaging plant pest in the Western parts of the Uttar Pradesh (Ahmad Citation2009). M. incognita also interacts with other plant-parasitic nematodes and pathogens, forming complex associations (Divon and Fluhr Citation2007) and thus increases the infection level. Hence, preventive measures are needed to control the root-knot disease in the field crops. The most commonly used approaches for managing plant-parasitic nematode include cultural practices, synthetic nematicides, and resistant cultivars (Curto et al. Citation2006). Synthetic nematicides are the most popular option for nematode prevention in most countries; however, these methods cause severe health and environmental threats. Therefore, their application is not justifiable (Ahmad et al. Citation2003).
Hence, there is a need to focus on non-toxic, harmless, and environment-friendly options to control these plant pests (Li-ping et al. Citation2018). Plants-based products that are a rich source of nematicidal compounds that may serve as safer alternatives for synthetic nematicides have caught many researchers’ attention (Ahmad et al. Citation2010a, Citation2010b; Zaidat et al. Citation2020). Organic amendments are one of the eco-friendly approaches identified to enhance soil texture, fertility and maintaining the crops yield (Muller and Gooch Citation1982; Ansari et al. Citation2016). Therefore, keeping in mind the importance of organic amendments, a in vitro and pot study were conducted.
In this study, we investigated whether weed plants are killed the infective juveniles (J2s) of M. incognita and reduced the root-galls of spinach plants. We first characterised the nematicidal properties of several weed plants, which are unwanted growing plants everywhere. The nematicidal activity of extract concentrations of weed plants was tested in in vitro experiment of J2s hatching from egg masses. We then accessed whether weed plants used as organic amendments affect the root-galls disease. Weed plants were found to reduce the root-galls infection in roots. Moreover, the treatment of weed plants enhances the plant growth parameters in J2s inoculated spinach.
Materials and methods
Roots sample collection and confirmation of the root-knot disease
A population of the root-knot nematode was originally collected a year ago from the infected agriculture field of the brinjal plant. After collection, cultured the J2s and maintained from a single egg mass on brinjal seedlings at the experimental site. For this experiment, brinjal roots were carefully uprooted and washed gently with tap water to remove the adhering soil particles and kept on the blotting sheet for 10 min to soak the excess moisture. The infected roots were associated with severe root galls and egg masses, indicating root-knot infection. The stereomicroscope was used to check for any visible contamination in egg masses.
Meloidogyne species identification
The female perineal pattern was used as the basis for the identification of the root-knot nematode species. About ten females were teased out from the root-knot galls and placed on a glass slide. The posterior part was sliced with a thin blade under the stereomicroscope, and the perineal pattern was trimmed and observed. The species was identified as Meloidogyne incognita, as characterised and described by Eisenback et al. (Citation1980).
Plant material, extraction and preparation of aqueous solution
Fresh leaves of Calotropis procera (Asclepiadaceae), Ricinus communis (Euphorbiaceae), Lantana camara (Verbenaceae), Achyranthes aspera (Amaranthaceae), Wedelia chinensis (Asteraceae), Colocasia esculenta (Araceae) were collected and kept at room temperature for three weeks for air drying. Dried leaves were ground in powder form with a Cyclone sample mill before use. Five grams of each powdered leaf of the above-selected plants was suspended in 50 ml of distilled water (DW). The extracts were then passed through a four-ply muslin cloth to remove the plant debris, and the crude aqueous leaf extracts were then centrifuged at 10,000 rpm for 15 min at 4°C. Centrifuged supernatants were collected and filtered through Whatman No.1 filter paper. The collected filtrates were termed as 100% concentration and were further diluted to 50%, 10%, and 1% by adding the requisite amount of distilled water (DW).
Nematicidal effect of leaf powder extract on second-stage juvenile hatching
A hatching inhibition test was performed in vitro using five healthy egg masses gently isolated from infected roots using forceps. The egg masses were transferred to Petri dishes containing 5 ml of aqueous leaf powders extract at concentrations 100%, 50%, 10%, and 1%. Petri dishes containing 5 ml of DW without plant extract were served as control. The plates were covered with glass coverlid and kept at room temperature. After the 4th and 7th days of the incubation period, hatched second-staged juveniles (J2s) of M. incognita were counted under the stereomicroscope. Each treatment consisted of five replicates and was repeated twice. Per cent inhibition in egg hatching was calculated as follows:
Where, Co=J2s that emerged from egg masses in control. Tα=J2s that emerged from egg masses in each concentration of extracts.
Pot study with doses and treatment
The pot experiment was carried out under glasshouse conditions in the Department of Botany, AMU, Aligarh (UP) in the earthen clay pots (15 cm diameter) filled with 1 kg soil (7 clay:2 loam:1farmyard manure), pH 7.2, autoclaved at 20 psi at 121°C for 20 min. Each pot containing autoclaved soil were mixed individually with two different doses (50 and 100 g) of dry leaf powders of six weed plants viz., Calotropis procera, Ricinus communis, Lantana camara, Achyranthes aspera, Wedelia chinensis and Colocasia esculenta. All the treated pots were watered immediately and kept for 15 days to ensure proper decomposition of organic additives into the soil. The spinach seeds were sterilised adequately with 0.5% sodium hypochlorite for 10–15 min and, after that, rinsed properly using DW. Five seeds were sown in each treated pot. After three weeks of germination, thinning of the plants was done, and only one plant per pot was considered for further study. At this stage, each spinach seedlings were inoculated with approximately 2000 J2s of M. incognita by making 3.5 cm deep holes around the plant. The study was performed with five replicates per treatment, and the experiment was repeated twice. The untreated inoculated and untreated uninoculated pots served as control.
Observation and data collection
Six-week post J2s inoculation, plants were gently uprooted and washed under running tap water to remove the loosely adhered soil. The data were evaluated for the plant physiological, biochemical (total length (cm), fresh and dry weight (g) of shoot, root, number of leaves per plant, chlorophyll content (mg/g), carotenoid content (mg/g), nitrate reductase activity (μmh−1g−1) of leaves) and pathological parameters (number of egg masses and nematode population/250 g soil, and root-knot index). The roots were stained for 15 min in an aqueous solution of Phloxine B stain (0.15 g/L water), then washed with running tap water to remove the residual stain, and the egg mass was determined (Holbrook et al. Citation1983). The population of the root-knot was assessed by Cobb's sieving and decanting technique (Cobb Citation1918), followed by modified Baermann's funnel technique (Southey Citation1986). Nematode suspension was obtained after 72 h, and five aliquots of 1 mL from each sample were counted using a stereomicroscope for the number of nematodes. The means of five counts were used to calculate the population of nematodes/250 g soil. Roots were carefully washed under flowing tap water for the root gall evaluation, and the nematode-induced galls were counted in the infected roots. The root-knot index was expressed on a 0-5 scale (where, 0 = No galls; 1 = 1–2 galls; 2 = 3–10 galls; 3 = 11–30 galls; 4 = 31–100 galls and 5 = more than 100 galls per root system) according to Taylor and Sasser (Citation1978).
Chlorophyll and carotenoid estimation
The chlorophyll and carotenoid content in fresh leaves of each treatment was determined using the method given by MacKinney (Citation1941). In 20 mL of 80% acetone, one g of freshly cut leaves from each treatment was crushed using a mortar and pestle. The mixture was allowed to be centrifuged for 5 min at 5000 rpm, and the supernatant was collected. The residue was washed thrice using 80% acetone. A spectrophotometer (Shimadzu UV-1700, Tokyo, Japan) was employed to analyze absorbance at 645 and 663 nm for chlorophyll and 480 and 510 nm for carotenoid beside the blank (80% acetone).
Nitrate reductase activity (NRA) estimation
The nitrate reductase activity in fresh leaves was calculated by the protocol given by Jaworski (Citation1971). 200 mg of finely chopped leaves were moved to plastic vials. 2.5 mL phosphate buffer (pH 7.5) and 0.2 mL potassium nitrate solution was pipetted to each vial accompanied by 2.5 mL of 5% isopropanol. Thereafter, vials were allowed to incubate for 2 h at 30+2°C in the dark. The 0.4 ml of the incubated mix was transferred in a test tube into which 0.3 ml of sulphanilamide solution and NED-HCL were introduced. For maximum colour development, the test tubes were allowed to rest for 20 min. Afterward, the sample was diluted to 5 ml using double distilled water. A spectrophotometer was used to interpret the absorbance (OD) at 540 nm. Concurrently, a blank was run for each sample. A standard curve was plotted by using a known graded concentration of Sodium nitrite solution. OD of each sample was correlated to that of the calibration curve, and NRA was measured at µmh−1g−1.
Data analysis
Data were statistically analyzed using SPSS software version 17 for window (SPSS, Chicago, IL, USA) to determine the significance at p < 0.05. The significance of differences among treatments was determined by Duncan's multiple range test (DMRT).
Results
Effect of aqueous leaf extract on egg hatching
In this bioassay, inhibition in egg hatch was analyzed, and a significant difference was reported among the concentrations (). All the botanical extracts significantly reduced egg hatching at different concentrations (100%, 50%, 10%, and 1%) compared to control (DW) at the 4th and 7th day of the exposure period. Results showed that inhibition in the egg hatching was increased along with the increase in strength of concentrations. At 100% aqueous extract concentration, C. procera and R. communis caused the highest inhibition (100%), followed by L. camara with 98.79% and 97.86% and A. aspera with 98.55% and 96.68%, while C. esculenta was found to be least effective with 97.63% and 94.59% hatching inhibition after the 4th and 7th day, respectively. At 50% aqueous extract concentration, C. procera caused the highest inhibition with 95.97% and 94.54%, followed by R. communis with 94.07% and 92.65%, L. camara with 92.28% and 90.04%, while C. esculenta was found to be least effective with 86.49% and 82.22% hatching inhibition after the 4th and 7th day, respectively. At 10% aqueous extract concentration, C. procera caused the highest inhibition with 65.63% and 63.74%, followed by R. communis with 62.32% and 60.42%, L. camara with 60.18% and 56.63%, while C. esculenta was found to be least effective with 54.26% and 48.10% hatching inhibition after the 4th and 7th day, respectively. At 1% aqueous extract concentration, C. procera caused the highest inhibition with 51.42% and 48.81%, followed by R. communis with 47.15% and 43.36%, L. camara with 44.07% and 40.08%, while C. esculenta was found to be least effective with 35.54% and 28.90% hatching inhibition after the 4th and 7th day, respectively.
Table 1. Effect of aqueous solution of dried leaf powder of weed plant species on the hatching of Meloidogyne incognita juveniles in vitro.
Effect on plant growth parameters
Under pots conditions, the experimental results revealed the weed plant parts have a potential role as organic amendments with two strategic doses of 50 and 100 g respectively against M. incognita under greenhouse conditions. All the treatments were effective in increasing plant growth compared to the untreated inoculated ones. Main taproots of spinach were found thick and healthy in almost all treated pots. In contrast, the taproots of untreated inoculated pots were thin, small, and stunted. The results showed that higher growth was found in C. procera treated plants than nematode infested plants (). A significant difference (P ≤ 0.05) in plant growth parameters was found in all the treatments. Results shows that among the treated pots, highest plant height (183.75 cm @ 50 g and 191.50 cm @ 100 g) was recorded in C. procera followed by A. aspera (173.25 cm @ 50 g and 181.50 cm @ 100 g) and L. camara (158.75 cm @50 g and 180.25 cm @ 100 g), while the least plant height (119.75 cm) was recorded in untreated inoculated control. Maximum fresh weight was recorded in C. procera (149.75 g @100 g) followed by A. aspera (134.75 g @ 50 and 137.25 g @ 100 g) and L. camara (122.25 g @50 and 130.75 g @ 100 g), while the least plant fresh weight (86.50 g) was recorded in untreated inoculated control. The maximum dry weight of plant was recorded in the application of C. procera (25.25 g @50 and 23.82 g @100 g) followed by A.aspera (22.63 g @50 and 23.30 g @100 g) and L. camara (21.92 g @50 and 22.82 g @ 100 g) while the least dry weight (12.42 g) was observed in the untreated inoculated pots.
Table 2. Potential of plant materials treated as organic amendment at two different doses to enhance the plant parameters of M. incognita infected spinach.
The number of leaves per plant was also found to be significantly (P ≤ 0.05) higher in C. procera treated pots (34.0 @50 g and 39.0 @100 g), followed by A. aspera (31.0 @50 g and 32.0 @100 g), and L. camara (27.0 @50 g and 30.0 @100 g) while the least number of leaves (17) was recorded in the untreated inoculated pots.
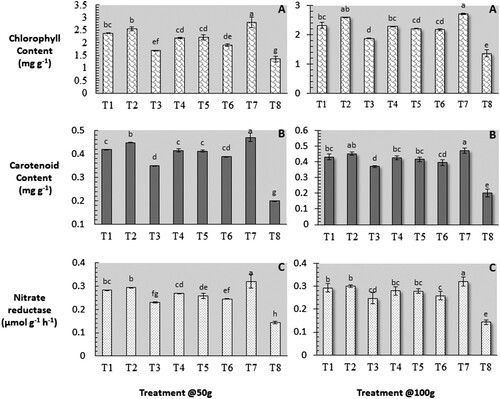
Effects on the biochemical parameters
Biochemical parameters were also determined to confirm the influence of pathological events on the yield of chlorophyll, carotenoids, and nitrate reductase, and comparisons were made with control. Results in signify that the chlorophyll and carotenoid content and NRA were significantly (P ≤ 0.05) improved by various treatments compared to the untreated inoculated control. The highest chlorophyll content (2.55 mg/g @ 50 g and 2.59 mg/g @ 100 g) was recorded in C. procera, followed by A. aspera (2.38 mg/g @ 50 g and 2.32 mg/g @ 100 g), and L. camara (2.18 mg/g @ 50 g and 2.32 mg/g @ 100 g) while the least chlorophyll content (1.36 mg/g) was found in untreated inoculated control. Carotenoid content was found highest (0.448 mg/g @ 50 g and 0.451 mg/g @ 100 g) in C. procera, followed by A. aspera (0.418 mg/g @ 50 g and 0.430 mg/g @ 100 g), and L. camara (0.414 mg/g @ 50 g and 0.425 mg/g @ 100 g) while the least carotenoid content (0.2 mg/g) was found in untreated inoculated control. NRA was found to be highest (0.295 μmol g−1 h−1 @ 50 g and 0.300 μmol g−1 h−1 @ 100 g) in C. procera, followed by A. aspera (0.289 μmol g−1 h−1 @ 50 g and 0.293 μmol g−1 h−1 @ 100 g), and L. camara (0.270 μmol g−1 h−1 @ 50 g and 0.280 μmol g−1 h−1 @ 100 g) while the least carotenoid content (0.143 μmol g−1 h−1) was recorded in untreated inoculated control.
Figure 1. Role of the organic amendments in physiological activities of M. incognita inoculated spinach plant. (A) Chlorophyll, (B) Carotenoid and (C) Nitrate reductase. Data presented as means ± SE (n = 5). According to Duncan's multiple range tests, bars (means ± SE) with the same letters are not significantly different (Treatment: T1-Achyranthes aspera; T2-Calotropis procera; T3-Colocasia esculenta; T4-Lantana camara; T5-Ricinus communis; T6-Wedelia chinensis; T7-Untreated Uninoculated Control; T8- Untreated inoculated control).
Effect on egg masses, nematode population and root-knot index (RKI)
The number of root galls was significantly reduced in treated plants than untreated inoculated control. However, C. procera treatment was significant in controlling the root-knot infection. Branched roots of spinach were associated with few root-knot galls in C. procera treated pot.
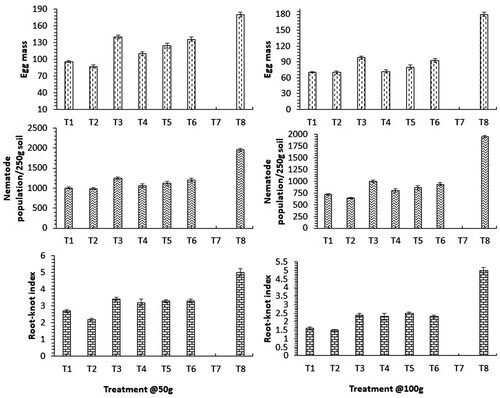
Results from show that a significant (P ≤ 0.05) reduction in nematode population, egg masses, and RKI were found in all the treated pots. When estimating the nematode population in the soil, a significant reduction (P ≤ 0.05) was found among all treatments tested, compared to nematode infected plants. The least population of J2s in 250 g soil was reported in pots treated with C. procera (987 @50 g and 642 @100 g), followed by A. aspera (1002 @50 g and 720 @100 g) and L. camara (1060 @50 g and 802 @ 100 g) compared to the 1953 J2s found in untreated inoculated control. The number of egg masses on the roots was also found to be significantly reduced in all treatments. The least number of egg masses was reported in C. procera (87 @50 g and 70 @100 g), followed by A. aspera (96 @50 g and 70 @100 g) and L. camara (110 @50 g and 72 @ 100 g) compared to the 180 egg masses found in untreated inoculated control. However, C. procera treated plants was also found highly effective in suppressing the disease with the lowest RKI (2.2 @ 50 g and 1.5@ 100 g), followed by A. aspera (2.7 @ 50 g and 1.6 @ 100 g), and L. camara (3.2 @50 g and 2.3 @ 100 g) as compared to nematode infected plants (5.0 ± 0).
Figure 2. Effect of organic amendments on the nematode population per 250 g soil and root parasitism. Correspondence analysis showing reduction in nematode population in soil and root-disease in treated spinach plant with organic amendments per pot. A significant reduction is observed in the nematode population, egg masses of Meloidogyne incognita, and root knot index compared to control. Data are the mean of five replicates (n = 5). (Treatment: T1-Achyranthes aspera; T2-Calotropis procera; T3-Colocasia esculenta; T4-Lantana camara; T5-Ricinus communis; T6-Wedelia chinensis; T7-Untreated Uninoculated Control; T8-Untreated inoculated control).
Discussion
The present study showed the positive performances of organic amendments in plants. During the in vitro studies, aqueous extract of all botanicals viz., C. procera, R. communis, L. camara, A. aspera, W. chinensis, and C. esculenta were found significantly useful in decreasing egg hatching of M. incognita (). The nematicidal potential was directly proportional to concentrations of aqueous extract samples. The exposure time also functioned as a significant factor. The highest concentration (100%) of each extract resulted in a significantly lower hatching percent of M. incognita compared to distilled water control (DW). Among the treatments, C. procera at all the concentrations was highly effective in reducing the egg hatching of M. incognita, contrary to reports published by Elbadri et al. (Citation2008). They reported that leaf extracts of C. procera are not much effective against M. incognita. However, in this study, C. procera caused 100% hatching inhibition after the 4th and 7th day of incubation period in 100% concentration. These results are in agreement with Ahamad and Siddiqui (Citation2018) and Chedekal (Citation2013), who also reported that C. procera caused 100% and 99.83% hatching inhibition after five days of the incubation period, respectively. Plant extracts’ mechanism of action is not well understood, but there is no doubt that an organic amendment possesses critical secondary metabolites. The secondary metabolites released in soil by organic amendments may have resistance properties against plant-pathogen (Kafle and Wurst Citation2019). Asif et al. (Citation2017) reported that many secondary metabolites of plant origin act as nematicidal compounds, including alkaloids, flavonoids, saponins, phenol, tannins, diterpenes, glucosinolates, isothiocyanates, phenols, polyacetylenes, sesquiterpenes, and thienyls. Also, studies revealed that Lantana camara contains 11-oxo triterpenic acid (lantanolic acid, lantoic acid, pomolic acid, and ursolic acid), and this compound was effective against M. incognita with a mortality rate of 85–90% (Srivastava et al. Citation2006). The effect of volatile organic compounds has also been reported against root-knot nematodes in recent years (Pedroso et al. Citation2019). In this study, these secondary metabolites might be present in the extracts sample therefore egg hatching inhibition of juveniles was significantly observed.
In pot experiment, soil treatment with botanicals resulted in more remarkable improvement in spinach plants growth than untreated inoculated control. Treatment of soil with dry leave powder of C. procera shows significant nematicidal potential to varying degrees. C. procera at 100 g dose show the highest reduction in egg masses and nematode population of M. incognita/250 g soil () hence improved the growth and biochemical parameters of spinach plant ( and ). These results are in agreement with the previous findings of Chimbekujwo and Bukar (Citation2013) and Hussain et al. (Citation2018) who reported that application of C. procera as soil amendment cause significant reduction in root-knot nematode infestation in cowpea and eggplant respectively which consequently led to increasing the growth of different plants.
Earlier studies on phytochemical analysis reported that the leaves of C. procera possess various cardiac glycosides viz., calotropin, calactin, calotoxin, usharin, usharidin and voruscharin are found in the latex of the plant (Rastogi and Mehrotra Citation1993). Yadav et al. (Citation2010) reported the presence of alkaloids, flavonoids, and tannins in the methanolic extract of Calotropis procera leaves. It is also essential to expand our knowledge to find an appropriate dose of organic amendments that significantly reduce plant nematode infection and improved plant growth parameters. In this study, among all the botanicals, the least impact in enhancing plant growth parameters was observed in plants treated with C. esculenta (). It might be due to the delay in the decomposition of the used organic amendment or secondary metabolites were not present in enough concentration to suppress the harmful activity of root-knot nematode. The treatment of pots with C. procera leaves powder @ 100 g dose showed enhancement in biochemical parameters compared to the untreated inoculated control (). It may be due to enough availability of assimilates in plants after given treatments of organic amendments. It is also known that Nitrate reductase (NR) is the crucial enzyme for nitrogen assimilation in plant cells, and also works as an essential enzymatic source of nitric oxide (NO). It regulates plant growth and resistance to biotic and abiotic stresses (Fu et al. Citation2018). These findings were similar as reported by Berger et al. (Citation2007) that the photosynthesis rate decreased as plants contact pathogens. It was also observed that soil treatment with botanicals results in a significant reduction of nematode population, and the results were similar to the findings of Oluwatayo et al. (Citation2019) and Abubakar et al. (Citation2004). This study confirms that weed plants brought a significant increase in plant growth and physiological parameter to reduce root-knot development and nematode population. Thus, it can be concluded that using these weed plants as an organic amendment is a need for sustainable agricultural production and minimises the use of toxic and hazardous chemical nematicides in the environment. Thus, this study paves the way for identifying natural biopesticides from eco-friendly plant-based materials for plant growth.
Acknowledgement
The corresponding author thankful to the University Grants Commission for providing financial support (Grant number F30-409/2018).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Mudassara Hasan
Mudassara Hasan, is doing her Ph.D. in Botany from Aligarh Muslim University, Aligarh. She is working on the management of plant nematode through organic soil amendments and biocontrol agents.
Faheem Ahmad
Faheem Ahmad, is an Assistant Professor at the Department of Botany, Aligarh Muslim University. He has done his Ph.D. from the AMU, Aligarh in 2009. Earlier, he has worked as a postdoctoral fellow at the Ehime University (Japan), North-West University (South Africa) and National Sun Yat-Sen University (Taiwan). His research interests include plant-associated microbe identification, biological control of plant-parasitic nematodes, and plant disease studies of economic crops. He published more than 30 research articles in peer-reviewed journals and supervises master students. He was the Principal Investigator of UGC-BSR Start -up Grant from 2018 to 2020.
Pieter Malan
Pieter Willem Malan, is an Associate Professor at the North West University, Mafikeng Campus. He published more than 20 research articles in peer reviewed journals. His resrarch interest is terrestrial ecology and more specifically bush encroachment and alian plant invasion in rangelands. He supervised several Masters and Ph.D students to completion of their studies.
Hera Nadeem
Hera Nadeem, is a research scholar in the Department of Botany, Aligarh Muslim University, Aligarh. Presently, she is working on managing root-knot nematode disease in vegetables through microbial-based compounds.
Mohd Asif
Mohd Asif, completed his Ph.D. in Botany from Plant Pathology and Nematology, Department of Botany, Aligarh Muslim University, Aligarh. He is actively engaged in the characterisation and identification of novel and natural bio-pesticides for nematode disease management and the promotion of organic farming.
Amir Khan
Amir Khan, completed his Ph.D. in Botany specialising in Plant Pathology and Nematology from the Department of Botany, Aligarh Muslim University, Aligarh. He has done his B.Sc. and M.Sc. from the AMU, Aligarh, in 2012 and 2014, respectively. He is working on the management of root-knot nematodes through eco-friendly approaches, i.e. organic material and biocontrol agents.
Mansoor A. Siddiqui
Mansoor Ahmad Siddiqui, is a professor at the Department of Botany, Aligarh Muslim University. His research area is the studies on the effect of organic soil amendments and biocontrol agents on plant-parasitic nematodes. He attended about 32 National/International conferences including XVIII IPPC in July 2015 at Berlin, Germany. He received Young Scientist Award (1994) by BRS, Allahabad, for outstanding contribution in the field of Plant Nematology.
References
- Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC, et al. 2008. Genome sequence of the metazoan plant parasitic nematode Meloidogyne incognita. Nat Biotechnol. 26:909–915.
- Abubakar U, Adamu T, Manga SB. 2004. Control of Meloidogyne incognita (Koffoid and white) Chitwood (root-knot nematode) of Lycopersicon esculentum (tomato) using Cowdung and urine. Afri J Biotecnol. 3:379–381.
- Ahamad L, Siddiqui MA. 2018. Efficacy of botanicals and carbofuran for the control of Meloidogyne incognita affecting Solanum lycopersicum L. Int J Phytopathol. 7:69–75.
- Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan M, Kumar IR, Sastry M. 2003. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B. 28:313–318.
- Ahmad F. 2009. Studies on the management of root-knot nematode (Meloidogyne incognita) with organic soil amendments [Ph.D. Thesis]. Aligarh: Aligarh Muslim University; p. 262.
- Ahmad F, Rather MA, Siddiqui MA. 2010a. Nematicidal activity of leaf extracts from Lantana camara L. against Meloidogyne incognita (kofoid and white) chitwood and its use to manage roots infection of Solanum melongena L. Braz Arch Biol Technol. 53:543–548.
- Ahmad F, Rather MA, Siddiqui MA. 2010b. Influence of organic additives on the incidence of root-knot nematode, Meloidogyne javanica in roots of tomato plants. Arch Phytopathol Plant Protect. 43:168–173.
- Ansari T, Asif M, Siddiqui MA. 2016. Potential of botanicals for root knot nematode management of tomato. Chisinau: Lambert Academic Publishing; Republic of Moldova; p. 1–121. ISBN No. 9783659910920.
- Asif M, Tariq M, Khan A, Siddiqui MA. 2017. Biocidal and antinemic properties of aqueous extracts of Ageratum and Coccinia against root-knot nematode, Meloidogyne incognita in vitro. J Agric Sci. 12:108–122.
- Berger S, Sinha AK, Roitsch T. 2007. Plant physiology meets phytopathology: plant primary metabolism and plant–pathogen interactions. J Exp Bot. 58:4019–4026.
- Chedekal AN. 2013. Effect of four leaf extracts on egg hatching and juvenile mortality of root knot nematode Meloidogyne incognita. Int J Adv Life Sci. 6:68–74.
- Chimbekujwo IB, Bukar AM. 2013. Evaluation of organic soil amendments on the growth and yield of Meloidogyne incognita on cowpea plants. J Biol Agri Healthc. 3:9–17.
- Cobb NA. 1918. Estimating the Nema populations of the soil. Agriculture Technical Circulation Bulletin of Plant Industries, Department of Agriculture, US; p. 48.
- Curto G, Lazzeri L, Dallavalle E, Santi R, Malaguti L. 2006. Effectiveness of crop rotation with Brassicaceae species for the management of the southern root-knot nematode Meloidogyne incognita. Abstracts 2nd International Biofumigation Symposium; June 25–29; Moscow, Russia. p. 51.
- Divon HH, Fluhr R. 2007. Nutrition acquisition strategies during fungal infection of plants. FEMS Microbiol Lett. 266:65–74.
- Eisenback JD, Hirschmann H, Triantaphyllou AC. 1980. Morphological comparison of Meloidogyne female head structures, perineal patterns, and stylets. J Nematol. 12:300.
- Elbadri GM, Lee DW, Park JC, Yu HB, Choo HY. 2008. Evaluation of various plant extracts for their nematicidal efficacies against juveniles of Meloidogyne incognita. J Asia-Pacific Entomol. 11:99–102.
- Fu YF, Zhang ZW, Yuan S. 2018. Putative connections between nitrate reductase S-nitrosylation and NO synthesis under pathogen attacks and abiotic stresses. Front Plant Sci. 9:474.
- Holbrook CC, Knauft DA, Dikson DW. 1983. A technique for screening peanut for resistance to Meloidogyne arenaria. Plant Dis. 67:957–958.
- Hussain MA, Ahmad S, Anum W, Khanum M, Raza H, Aslam MN. 2018. Microenvironmental alteration by the use of some plants for the effective control of root-knot nematode (Meloidogyne incognita) on brinjal. Plant Prot. 02:93–99.
- Jaworski EG. 1971. Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun. 43:1274–1279.
- Kafle D, Wurst S. 2019. Legacy effects of herbivory enhance the performance and resistance of progeny plants. J Ecol. 107:58–68.
- Khan MR, Jain RK, Ghule TM, Pal S. 2014. Root-knot Nematodes in India-a comprehensive monograph. All India Coordinated Research Project on Plant Parasitic nematodes with Integrated approach for their Control, Indian Agricultural Research Institute, New Delhi, India. http://iari.res.in/ files/Divisions/Root-Knot-Nematode-Corrected_10032014.pdf.
- Li-ping Z, Zhong D, De-liang P, Huan P, Ling-an K, Shi-ming L, Ying L, Zhong-cai L, Wen-kun H. 2018. Evaluation of Chinese rice varieties resistant to the root-knot nematode Meloidogyne graminicola. J Integr Agric. 17:621–630.
- MacKinney G. 1941. Absorption of light by chlorophyll solutions. J Biol Chem. 140:315–322.
- Muller R, Gooch PS. 1982. Organic amendments in nematode control: an examination of the literature. Nematropica. 12:319–326.
- Murungi LK, Kirwa H, Coyne D, Teal PEA, Beck JJ, Torto B. 2018. Identification of key root volatiles signaling preference of tomato over spinach by the root knot nematode Meloidogyne incognita. J Agric Food Chem. 66:7328–7336.
- Oluwatayo JI, Jidere CI, Nwankiti A. 2019. Nematicidal effect of some botanical extracts for the management of Meloidogyne incognita and on growth of tomato. Asian J Agricul Horticul Res. 4:1–8.
- Pedroso LA, Campos VP, Pedroso MP, Barros AF, Freire ES, Resende FM. 2019. Volatile organic compounds produced by castor bean cake incorporated into the soil exhibit toxic activity against Meloidogyne incognita. Pest Manag Sci. 75:476–483.
- Rastogi RP, Mehrotra BN. 1993. Compendium of Indian Medicinal Plants. Vol. 1. Central Drugs Research Institute, Lucknow and Publication & Information Directorate, New Delhi; p. 71–72.
- Science Daily. 2008. Plant parasitic nematode genome sequenced. http://www.sciencedaily.com/releases/2008/09/080904215901.htm.
- Southey JF. 1986. Laboratory methods for work with plant and soil nematodes. London: HMSO.
- Srivastava M, Kapoor A, Sharma S, Siddiqui NU, Aslam M. 2006. Microbial active triterpene from Lantana camara. Biosci Biotechnol Res Asia. 3:505–507.
- Taylor AL, Sasser JN. 1978. Biology, identification and control of root-knot nematodes Meoidoglyne spp. Department of Plant Pathology, Raleigh; p. 1–111.
- Yadav P, Kumar A, Mahour K, Vihan VS. 2010. Phytochemical analysis of some indigenous plants potent against endoparasite. J Adv Lab Res Biol. 1:72–77.
- Zaidat SAE, Mouhouche F, Babaali D, Abdessemed N, De Cara M, Hammache M. 2020. Nematicidal activity of aqueous and organic extracts of local plants against Meloidogyne incognita (Kofoid and White) Chitwood in Algeria under laboratory and greenhouse conditions. Egypt J Biol Pest Co. 30:46.
