ABSTRACT
Purpose: Due to environmental concerns, there is a demand to reduce the use of peat as a growing medium for horticultural crops. Simultaneously, there is an interest to recycle organic waste materials in the form of compost. This study aimed to document effects on growth, yield, and fruit quality of tomato plants when cultivated in a sewage digestate-based compost in a subirrigation container system. Materials and methods: The compost used in this experiment consisted of 30% hygienised sewage digestate from biogas extraction and 70% garden waste. The treatments were 100% compost, a peat mix and mixtures of the two in 25/75, 50/50 and 75/25 ratios. Results and conclusion: Considering the contrast in chemical and physical properties of the treatments, variations in growth, yield and quality were expected. The plants differed in leaf area and number of leaves, but there were no differences in yield or quality of the tomato fruits. It is assumed that this is in great part due to the remediating effects of subirrigation with an ideal nutrient solution, and the use of pre-established plants. Further research should focus on benefits of this cultivation system for use in sustainable horticulture in combination with recycled organic waste.
Introduction
The EU Horizon 2020 project ‘Sino-European innovative green and smart cities – SiEUGreen explores a sustainable circular system for food production in urban and semi-urban areas. Food production in the urban environment is often container-grown, which requires reliable growing media. Currently, an important component of soilless but soil-like growing media both in professional and hobby cultivation practices is peat. Peat is an organic soil harvested from wetlands and has been important as a growth medium for decades due to its large availability in the northern parts of the world, and its suitable physical and chemical properties (Schmilewski Citation2008). It is low in nutrients and is therefore easily fertilised for each specific plant culture, the cation exchange capacity is high, and the physical properties ensure good water retention (Michel Citation2010; Rippy and Nelson Citation2007). Although the use of peat has these advantages horticulturally, previous studies indicate strongly that the extraction of this natural resource has a negative impact on the environment. Peatlands are large carbon sinks that emit substantial CO2 after disturbance by harvesting and serves as important habitats for wetland wildlife (Maltby and Immirzi Citation1993; Cleary et al. Citation2005; Mitra et al. Citation2005; Alexander et al. Citation2008; Boldrin et al. Citation2010; Atzori et al. Citation2021)
Thus, there is a demand to reduce the use of peat as a growing medium for use in sustainable food production. Simultaneously, there is an interest and potential to recycle waste materials based on organic nutrient-rich content (Gajdos Citation1989; Blok et al. Citation2014). This is one of the aims in the SiEuGreen project, particularly the use of biogas-processed sewage residue. This residue, called digestate, has been thoroughly hygienised prior to the anaerobic processing (Olsson et al. Citation2014). Although digestates show some promise as a growing medium (Zanin et al. Citation2010) and is recognised as essential in sustainable urban food production (Battista et al. Citation2020), it lacks the suitable physical and chemical properties for versatile use in horticulture. The premise for better utility is to co-compost it further aerobically in combination with a less dense structure component such as garden waste that ensure more optimal physical properties for plant growth and break down of harmful substances produced under anaerobic conditions (Verdonck Citation1988; Bustamante et al. Citation2013; Zeng et al. Citation2016; Nesse et al. Citation2019). More specifically, studies on the use of composed digestates as growth media have positive results on yield with no effects on phytotoxicity (Chang et al. Citation2021; Perez-Murcia et al. Citation2006). In combination with digestate, the material providing structure should preferably also be based on waste, such as lignin-rich garden waste (Abad et al. Citation2019; Bustamante et al. Citation2012). Ideally, a sustainable growth media for urban horticulture is both fully based on such waste material and has ideal physical and chemical properties for plant growth. However, previous studies have shown that acquiring ideal chemical and physical properties from such material affordably is challenging (Atzori et al. Citation2021).
When making a compost-based growing media, the physical properties are particularly important as chemical properties are more easily manipulated. Physical properties in growing media have been thoroughly studied previously (Wallach Citation2008), and there are valuable guidelines for ranges of values for properties that are essential for efficient plant growth (De Boodt and Verdonck Citation1971; Yeager et al. Citation1997; Fernandes and Corá Citation2004). These ranges were meant to ensure effortless uptake of water and sufficient aeration specifically for plants growing in limited space in containers with various watering systems. However, it is evident that specific cultivation systems that can provide sufficient water and nutrients continually to the plant roots, as in the case of a hydroponic or semi-hydroponic system, can support high-quality plant growth considerably regardless of the properties in the growing media (García-Santiago et al. Citation2019). In a system like this, a compost based on sustainable principles rather than quality-driven principles could lower the use, or even replace peat with minimal detrimental effects on food crops. Few studies have been done on growth, yield and quality of crops grown in this way, and the results in previous studies on cultivation in compost are often unclear and varied (Roberts et al. Citation2007; Hargreaves et al. Citation2009; Aminifard et al. Citation2012; Santos et al. Citation2016), or have shown good results in a 25–50% peat-based growing media mixed with compost (Perez-Murcia et al. Citation2006; Restrepo et al. Citation2013; Urlic et al. Citation2015; Jara-Samaniego et al. Citation2017). Therefore, with the aims of sustainable food production in the urban environment and the challenges of growth media properties in mind, this study aimed to document the effect on growth, yield performance and fruit quality of tomato plants using a sewage digestate-based compost in a subirrigation container system. The hypothesis to be tested was whether sewage digestate compost could perform as optimally as peat-based growing media when cultivating tomatoes in a subirrigation container system.
Materials and methods
Growing media and treatments
A detailed description and source of the different growing media mixtures provided by Lindum AS (Drammen, Norway) is given in . The compost-based growing medium consisted of a mature sewage digestate compost (SDC) based on 30% sewage-digestate and 70% finely chopped (<2 mm) garden waste by volume. The control growing medium was a standard commercial peat-based medium (PBM) (NORGRO AS, Hamar, Norway). The physical and chemical properties are given in and . Five mixes were prepared for the experiment: 100% SDC, three mixes of SDC with a standard peat product (75%, 50% and 25%), and 100% standard peat product. These are respectively henceforth referred to as 100C, 75C, 50C, 25C and 0C, where C = compost.
Table 1. Description of materials used as growing media and composition of treatments.
Table 2. Selected physical properties of treatments 100C, 75C, 50C 25C and 0C.
Table 3. Selected chemical properties for 100C and 0C – EC, Loss of Ignition, Carbon/Nitrogen ratio (C/N), essential plant nutrients and heavy metals of concern.
Chemical properties of the growing media
Selected chemical properties () were analysed for 100C and 0C by Eurofins Environmental Testing Norway AS using their standard methods. Total Cu, chromium (Cr), Zn, aluminium (Al), boron (B), phosphorus (P), Fe, potassium (K), calcium (Ca), magnesium (Mg), manganese (Mn) and sulphur (S) were determined by use of ICP-OES (NS-EN ISO 11885 2009) after extraction with 7M nitric acid. Total cadmium (Cd) was determined by the use of an ICP-MS (NS-EN ISO 17294–2 2016). Total nitrogen (N) content was determined by a modified Kjeldahl method (EN 13654–1 2001). For determination of ammonium-N and nitrate-nitrite-N, samples were extracted with 2M potassium chloride (KCl), while sodium (Na) was extracted by ammonium lactate, according to standard methods of Eurofins. Electrical conductivity (EC) and pH were determined according to the standards NS-EN 12176 (1998) and NS-EN ISO 7888 (1993) respectively. Additionally, total nutrient content is shown in , and chemical content in the fertigation water in .
Table 4. Total supply of each element in each 18 L pot in all treatments. In this table, content in 18 L (FW) of the growth media from start is added to the total content in the fertigation water (97.5L) from the beginning to the end of the experiment. Due to the subirrigation container system, there was no runoff.
Table 5. Content of elements in the fertigation water compared to what is considered optimal content in mg/l for tomatoes on average throughout all growing stages.
Main physical properties: water retention and pore size distribution of the growing media
Physical properties in the form of bulk density, total pore space, air content and moisture content at different suctions were measured by determining water release curves as described by De Boodt et al. (Citation1973). Six samples of each of the five different growth media were sampled after each was thoroughly mixed. The samples were packed into 100 cm3 steel cylinders. These cylinders were then subjected to a range of suctions (5, 10, 20 and 50 hPa) in a sand box (Eijkelkamp Agrisearch Equipment, Giesbeek, The Netherlands). For suctions at 100 and 1000 hPa, water retention was determined by using pressure plates (Soil moisture Equipment, Santa Barbara, California, USA) inside pressure chambers. Pore size distribution was estimated based on the soil water retention. After analysis, the moisture content by percentage was divided into five practical categories: air content at 0–10 hPa; easily available water (EAW) at 10–50 hPa; water buffer capacity (WBC) at 50–100 hPa; a range of 100–1000 hPa that approach plant-unavailable water, and plant-unavailable water (UW) at >1000 hPa. These categories are meant for use specifically in the limited volume of containers in horticultural production, coined by De Boodt and Verdonck (Citation1971) and revisited by Arguedas et al. (Citation2007).
Plant growth experiment
The experimental set up was performed under greenhouse conditions at the Norwegian University of Life Sciences during the months of January-March 2020. The relative humidity and air temperature were maintained by a Priva-system (Groscale; Priva BV, De Lier, The Netherlands) that ensured 85% humidity and air temperatures at 22°C during the day and 18°C during the night (± 1°C). A light intensity of 125 µmol quanta m−2 s−1 was added to the rooms by high-pressure metal halide lamps (400W Philips HPI-T) automatically during the light period whenever the photosynthetic photon flux (PPF) in the compartments fell below 150 µmol quanta m−2 s−1 (as commonly occurs largely during short winters days in Norway). Tomato plants Solanum lycopersicum cv. ‘Tastery’ were pre-cultivated from seed (Norgro, Norway) for 55 days in 500 ml pots in a standardised sphagnum mix before they were transplanted into containers with the five treatments 100C, 75C, 50C, 25C, and 0C. The containers were 18 L in volume with additional 9 L water reservoirs below. Each treatment had eight containers as replicates. At the time of transplanting, the tomato plants selected for the experiment were approximately one meter tall, and each had two trusses of flowers. The containers were equally filled by volume with the treatment media and moistened with water as the plants were transplanted. The container reservoirs were then filled with fertigation water, which contained a complete fertiliser solution consisting of a 25:25 (w:w) mixture of KristalonTM (9-11-30% NPK + micronutrients) and YaralivaTM (N 15.5% and Ca 19%) both from Yara International (Oslo, Norway). The fertiliser solution was mixed with water to an electric conductivity (EC) of 1.3–1.5 mS cm−1. The reservoirs were refilled continually throughout the experiment. The plants showed signs of excess nitrogen (curled leaves at the top of the plants) during the second week and were thus watered with tap water for the following two weeks. After that, the reservoirs were refilled with the fertigation solution 1–2 times a week, and the total volume of supplied fertigation water was logged. Side-shoots were removed continually. After eight weeks, the top shoots were pruned at the same point of height on each plant, and all flower trusses above the first five trusses on each plant were removed to promote ripening of the first five trusses before the termination date was due. The plants were cultivated in total for 85 days after transplanting before they were terminated.
Vegetative plant growth
Plant height and number of leaves were registered weekly for eight weeks. Plant height was measured with a tape measure from the surface of the soil up to the apex of the plant. Leaves were counted to the last unfurled leaf near the apex, with a minimum size limit of 2 cm in size for counting. The top shoot of a plant in the 25C treatment was damaged late in the experiment due to growing into the [light source], this resulted in the necessity to impute a mean value for number of leaves and height from this treatment to replace this plant. For leaf area, the terminal leaflet () on leaves from the middle of each plant (leaf no. 26, 28, 30, 32 and 34 counted from the bottom) was measured with a leaf area meter (model LICOR-3100, Licor Inc., Lincoln, Nebraska, USA). Terminal leaflets were chosen because the full leaves were too large for the area meter, and it was decided that the terminal leaflet would represent the surface area well enough. All terminal leaflets were cut at the exact same point on each leaf.
Generative plant growth
A truss was considered ready for harvest when nearly all tomatoes were at a light red stage, and the terminal (lowest) tomato on each truss was at a turning stage according to the method of Zhang et al. (Citation2020). When removed from the plant, the pedicel and leaflets were removed and not included in the weighing. Tomatoes were then counted and further weighed together as a total weight of each truss. If the truss had unpollinated fruits, they were removed and not included in the data. From each truss harvested, 125–150 grams of whole tomatoes were put in cotton bags and then in a styrofoam box filled with liquid nitrogen and kept for two minutes until cracked into frozen solid pieces. The frozen samples were then wrapped in a sheet of aluminium foil, put in 2 L ziplock plastic bags and stored at −50°C for three months before fruit quality analyses.
Tomato fruit quality
l-ascorbic acid
The content of l-ascorbic acid was determined in accordance with the method previously described by Aaby et al. (Citation2007) with some modifications. A frozen sample of 50 g was weighed up to 150 g by adding 100 g 1% oxalic acid. The fruit material was homogenised with a handmixer (Braun 450 Watt) and filtered through a Whatman TM filter (Whatman filters, 125 mm, Schleicher & Schuell, Dassel, Germany). The samples were analysed in duplicates.
Antioxidant activity, total phenolic compounds and total dry matter
For analyses of antioxidant capacity (AOC, determined as Ferric Reducing Ability of Plasma, the FRAP assay) and total phenolic compounds (TP), 70 g of material was homogenised with a blender (Philips 650 W). 3 g of homogenate was extracted with 1 mM HCl (37%) in methanol (30 mL). The samples (30 mL) were capped and vortexed (Vortex-T Genie 2), followed by sonication at 0°C for 15 min in an ultrasonic bath (Bandelin SONOREX RK 100). The 30 mL samples were stored at –20°C until analysed. Prior to analysis, the samples were poured into a 2 mL micro tube and centrifuged at 13200 rpm for three minutes at 4°C (Eppendorf 5415 R, Hamburg, Germany). For analyses of AOC, TMA and TP a KoneLab 30i (Thermo Electron Corp, Waltham, Massachusetts, USA) analyser was used. The AOC was determined by the FRAP assay as described by (Benzie and Strain Citation1996), and reported as µmol Fe2+ per g of fresh weight. Total phenolic compounds (TP) was determined using the Folin–Ciocalteu method (Singleton et al. Citation1999) and are reported as g gallic acid equivalents (GAE) per kg of fresh weight. Dry matter was determined by drying homogenate (6–7 g) at 100°C for 24 h in a drying oven (Termaks, Bergen, Norway) and stabilised in a desiccator before weighing.
Ph, soluble solids and titrable acids
Tomatoes (70 g) thawed overnight at 20°C were homogenised using a food processor (CombiMax 700,) prior to analysis and prepared by filtering with Whatman TM filters. The pH was measured with a pH meter, (Methrom 691 pH Meter, Herisau, Switzerland). Soluble solid (SS) concentration was determined by a digital refractometer (Atago refractometer model PR-1 CO, LTD, Tokyo, Japan) and expressed as %. Titratable acids (TA) were determined by a radiometer endpoint titrator (Methrom 716 DMS Titrino and 730 Sample Changer, Herisau, Switzerland) that calculated citric acid expressed as a percentage.
Statistical analysis
All statistical analysis was conducted using R studio v1.4. To test for differences between treatments, analysis of variance (ANOVA) was used by conducting the aov function for continual data, and glm with Poisson distribution in combination with anova functions for count data, except number of leaves which was approximately normally distributed. Pairwise post hoc comparison between treatments was conducted with TukeyHSD for continual data, and a Tukey option in glht in the multcomp package for count data. The assumptions of normality and equality of error variances were checked using the Shapiro Wilks test and the Bartlett test, respectively.
Results
Growth media physical properties were dissimilar between all treatments (). Bulk density (p = 0.00), total pore space (p = 0.00), air capacity (p = 0.004), easily available water (p = 0.00) and unavailable water (p = 0.004) showed significant differences in all treatments, with the largest contrast found between pure compost (100C) and peat (0C). Plant available water (Easily available water (EAW) + water buffer capacity (WBC)) decreased, and air content increased with increasing content of compost. The plant available water in 100C was at a much lower 7.1% out of total pore volume in contrast to 21.2% in 0C. The air content for 100C was 31.7%, while it was 27.8% in 0C.
Several chemical properties also showed dissimilar values (). The pH differed greatly between peat (0C) and 100% compost (100C) with a starting pH of 6.1 for 0C and 7.6 for 100C. Although the content of the macronutrients nitrogen (N), phosphorus (P) and potassium (K) differ greatly in the dry weight analysis of peat and compost as shown in , the fertigation ensured that the total supply of these nutrients was within close range in all treatments (). The content of trace minerals (called heavy metals in an environmental context), is also given in this table, showing that the content of several heavy metals (zink (Zn), nickel (Ni), cadmium (Cd) and chromium (Cr)) were above the maximum values recommended by the Norwegian Ministry of Agriculture and Food (Citation2003) for growth media intended for cultivating edible plants.
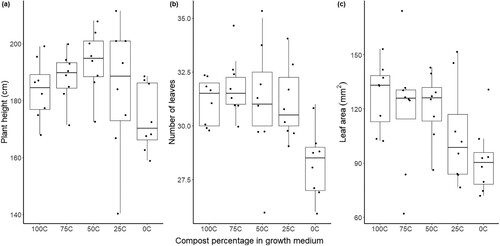
Despite the differences in physical properties and in pH, there were no differences in total yield weight, average individual tomato weight or number of tomatoes (p > 0.05) (). Trend wise, however, the largest yield could be found in neither of the pure media, but in 50C and 25C. In addition, regarding the quality of the fruits, there were no differences in any of the five quality parameters vitamin c, soluble solids, acidity, antioxidants, and phenols (p > 0.05). As for the vegetative parameters, a few differences were found in leaves and leaf area, but not in height (). All plants that grew in media containing compost (25C, 50C, 75C and 100C) had more leaves compared to 0C (p < 0.05). The largest difference in number of leaves was found in 75C compared to 0C (diff = 3.623, p = 0.003). Leaf area also showed one difference, where the leaves were larger in 100C compared to 0C (diff = 35.710, p = 0.044). Also worth mentioning is that manganese deficiency symptoms were observed in the first month of the experiment in all plants except the ones growing in 0C. These symptoms disappeared and were not a further issue. The vegetative growth of the plant where thereby affected to some extent by the presence of compost in the media, while the total yield, average weight of each tomato, number of tomatoes and quality of the fruits were not affected.
Figure 2. Weight of total yield (a), average weight of each fruit (b) and number of fruits (c) on tomato plants ‘Tastery’ cultivated in 100% sewage digestate compost (100C), 100% peat (0C) and a 25, 50 and 75% mixture of the two. The distribution is characterised by box and whisker plots, where the boxes show the 25th and 75th percentile and the whiskers the 10th and the 90th percentile (N = 8). The median is represented by the line in the box.
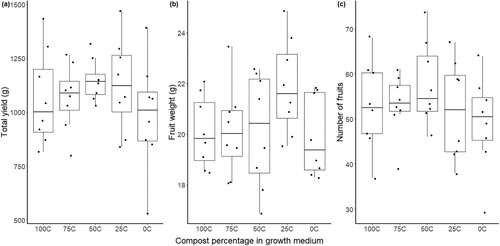
Figure 3. Plant height (a), number of leaves (b) and leaf area (c) on tomato plants ‘Tastery’ cultivated in 100% sewage digestate compost (100C), 100% peat (0C) and a 25, 50 and 75% mixture of the two over 8 weeks of growth in each treatment. The distribution is characterised by box and whisker plots, where the boxes show the 25th and 75th percentile and the whiskers the 10th and the 90th percentile (N = 8). The median is represented by the line in the box.
Discussion
It is widely accepted that compost alone does not completely match the common expectations for growing media (Atzori et al. Citation2021). The challenges in using compost as a growing medium are commonly due to immaturity of the compost, poor water holding capacity, unbalanced salinity and pH (Rogers Citation2017), and conclusions of how well compost can substitute peat are varying. Apparently, plant species and cultivation practises largely influence the outcome, which makes previous research challenging to compare. According to findings by (Pronk Citation1994), composted material could substitute peat at a level of only 15% before the pH levels compromised plant growth. Other findings (Prasad and Carlile Citation2007) show good growth of several plant species, including tomatoes, in growing media amended with compost up to 40%. However, (Farrell and Jones Citation2010) suggested that concerns of replacing more than 50% of peat with compost were unfounded in their case, which can be supported by the results in the present study. Although there are large differences in physical and chemical properties of the different compositions of growing media in the present study, the yield and quality of the tomatoes did not reflect this. In our case this is most likely due to the positive effects of the subirrigation that remediate the negative impact of suboptimal chemical and physical properties in compost. It is also important to note that the plants in this experiment were well established from pre-cultivation in peat at the point of transplantation. Well-established plants have more energy stored in the roots and larger leaf surface area to supply vigorous root expansion in a growing media, which would be advantageous compared to plants sown directly in the same media.
Physical properties of the growing media in the treatments with high content of compost were far from ideal. The 100C treatment had physical properties that were inconsistent with ranges that are considered optimal (De Boodt and Verdonck Citation1971; Yeager et al. Citation1997; Fernandes and Corá Citation2004), such as a total pore space of 72.8% opposed to the ideal 85%, easily available water of 6.2% opposed to the ideal 20–30%, and a water buffering capacity of 1.0% as opposed to the ideal 3.6% (). Our results thus showed that the compost had a low ability to hold plant available water and would therefore dry out faster. Regardless of this, the subirrigation system ensured an equal supply of water to the roots for tomato plants in all treatments within the first few weeks of establishment in the containers. Root growth in all treatments penetrated the growth media in the containers rapidly after transplantation and established contact with the water reservoir within seven days, which ensured enough water and nutrients for optimal growth conditions. Thus, a lower content of easily available water in the compost (100C) had little impact when the roots quickly gained access to the subirrigation supply of fertigation water early in the experiment.
The differences in chemical properties of the growing media seemed also to have had minimal effects on plant growth, yield and quality in the present study. The 100C treatment had a higher pH of 7.6 () than what is considered optimal for plant growth (pH 5.5-6.5). Several plant species are sensitive to high pH, as it compromises the availability of essential nutrients as phosphorous and micro nutrients (Peterson Citation1982). The brief manganese deficiency symptoms visible on plants in all treatments containing compost are most likely due to the high pH. Manganese is known to oxidise into plant-unavailable Mn4+ under conditions of high air porosity/low moisture content in combination with high pH (Miransari Citation2012). Likely, there was a shortage of manganese availability in the beginning of the experiment due to these factors, but the symptoms disappeared as the plants established roots into the water reservoir with nutrient solution provided from below within three weeks and were not a further issue. A concern regarding the chemical properties is, however, the high content of metals deriving from the sewage digestate. Iron (Fe), zink (Zn), nickel (Ni), cadmium (Cd) and chromium (Cr) were found in excessive quantities in all treatments containing compost ( and ). Although these levels did not cause any detectable signs of threat to plant growth in our experiment, the values for Zn, No, Cd and Cr exceeded what is recommended by the Norwegian Ministry of Agriculture and Food (Citation2003) for crop cultivation. However, the high pH in the compost is advantageous in this context, as these metals, much like the manganese, are less plant available in high pH conditions (da Conceicao Gomes et al. Citation2017). Additionally, plants may possess strategies to limit uptake in the roots (da Conceicao Gomes et al. Citation2017). For example, Murtić et al. (Citation2018) found that tomato plants accumulate unwanted heavy metals mainly in the roots and not in the fruit. Thus, the content of heavy metals in growing should be of concern, but in this context, i.e. high pH, removes possible negative impacts on plant growth and quality.
Furthermore, the differences in the growth of tomato plants suggest that the plants had somewhat unequal access to nutrients. The fertigation in addition to the nutrient content in the growing media ensured that none of the plants would in theory suffer from nutrient deficiencies unless other factors influence nutrient availability (). There would rather likely be an overfertilisation and consequently, a high electrical conductivity (EC) since the growing media initially had a higher content of nutrients, particularly in the mixture with 100% compost. In this treatment, there was particularly a high content of total nitrogen, although not immediately in plant available form (shown in ). As shown in , plant available nitrogen is lower in the 100C treatment, yet more of the higher total nitrogen content found in the compost could have mineralised during the growth period. This surplus of nitrogen could have contributed to the larger leaf tip area in 100C and more leaves in the treatments containing compost. Overfertilisation with nitrogen is known to increase N-rich tissue in vegetative organs in many plant species, including tomato (Elia and Conversa Citation2012). Conversely, it has been demonstrated before that vegetative growth in tomato plants does not necessarily lead to higher yield (Heuvelink Citation1999; Massa et al. Citation2019).
The general lack of difference in yield in the compost and peat treatments in the present study are in contrasts with other recent studies such as (Ghoreishy et al. Citation2018; Subramani et al. Citation2020; Adamczewska-Sowińska et al. Citation2021; Zawadzińska et al. Citation2021). Some studies that emphasise large differences in yield in plants cultivated in peat compared to compost must be used with consideration, however, as they did not balance or supply the difference in nutrient content in peat and compost with fertiliser (Perez-Murcia et al. Citation2006; Zhang et al. Citation2013; Luo et al. Citation2015). There are studies more in alignment with the present results in which the yield and quality of fruits on tomato plants show few differences, particularly in soilless systems similar to the present study where the water supply is sustained hydroponically or by thorough drip-irrigation (Massa et al. Citation2019; Nerlich et al. Citation2022).
The similar values in quality parameters indicate that tomato fruits cultivated in this system do not get any qualitative advantage nor disadvantage. Other studies show varying results in the fruit quality when comparing different growing media, where some found differences in antioxidant activity, total phenolic, total flavonoid (Aminifard et al. Citation2012; Verma et al. Citation2015) soluble solids and ascorbic acid (Subramani et al. Citation2020) and others find few to no differences in quality parameters (Roberts et al. Citation2007; Hargreaves et al. Citation2009; Elias et al. Citation2018). According to Massa et al. (Citation2019), the main driving variable for fruit quality parameters is EC. In the present study, the EC was much higher in compost compared to peat before fertigation (), yet this was not sufficient to lead to differences.
The results thus point to few differences in yield and quality parameters despite strong differences in treatments, with the explanation that subirrigation with fertigation water equalised the conditions for plants in all treatments. This supports the hypothesis that the sewage digestate compost can perform as optimally as peat-based growing media when cultivated in this manner. Furthermore, these results can argue for less use of peat as growing media to achieve a better circular horticulture, particularly aimed at hobby cultivation where mature but suboptimal compost products are a viable resource. Further research should focus on the benefits of this cultivation system for use in sustainable agriculture in combination with recycled organic wastes as growing media.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Acknowledgements
The authors wish to thank Øyvind Vartdal, Kari Grønnerød, Signe Hansen for their help with conducting the analysis of the substrate and fruit material. We greatly appreciate the help of Ronny Steen, Ngan Bao Huynh and Hilde Vinje for help with analysing the data.
Additional information
Funding
Notes on contributors
Siv M. Aurdal
Siv Mari Aurdal is a Ph.D. candidate in Plant Science at the Norwegian University of Life Sciences. She is a PhD-student in the project SiEUGreen: Sino-European innovative green and smart cities. Her areas of research are growing media for urban agriculture, use of compost in agriculture and soil fertility.
Bente Foereid
Bente Foereid is a researcher at the Norwegian Institute of Bioeconomy Research. Her fields of interest are nutrient and carbon cycling, plant and soil interactions, and use of organic residues as fertilisers and soil improvers.
Trine Sogn
Trine Sogn is a professor at the Norwegian University of Life Sciences. She received her PhD in 1992 at the Norwegian University of Life Sciences. Her areas of research are bio-geo-chemical processes in soils, mechanistic modelling, soil fertility and plant nutrition.
Trond Børresen
Trond Børresen is a professor at the Norwegian University of Life Sciences. He received his PhD in 1987 at the Norwegian University of Life Sciences. His areas of research are soil physics, soil structure, soil compaction, soil tillage and soil erosion
Trine Hvoslef-Eide
Trine Hvoslef-Eide is a professor at the Norwegian University of Life Sciences. She received her PhD in 1989 at the Norwegian University of Life Sciences. Her areas of research are applied biotechnology (including, but not only, nutrient media composition), urban horticulture, greenhouse production physiology.
Siv Fagertun Remberg
Siv Fagertun Remberg is an Associate professor at the Norwegian University of Life Sciences. She received her PhD in 2006 at the Norwegian University of Life Sciences. Her fields of research are within plant sciences (plant- and fruit quality) in different production systems and post-harvest physiology, with special emphasis on fruits and berries in addition to plants in urban agriculture environments.
References
- Aaby K, Wrolstad RE, Ekeberg D, Skrede G. 2007. Polyphenol composition and antioxidant activity in strawberry purees; impact of achene level and storage. J Agric Food Chem. 55(13):5156–5166.
- Abad V, Avila R, Vicent T, Font X. 2019. Promoting circular economy in the surroundings of an organic fraction of municipal solid waste anaerobic digestion treatment plant: biogas production impact and economic factors. Bioresour Technol. 283:10–17.
- Adamczewska-Sowińska K, Sowiński J, Jamroz E, Bekier J. 2021. Combining willow compost and peat as media for juvenile tomato transplant production. Agronomy. 11(10):2089.
- Alexander P, Bragg N, Meade R, Padelopoulos G, Watts O. 2008. Peat in horticulture and conservation: the UK response to a changing world. Mires & Peat. 3. Article 8.
- Aminifard MH, Aroiee H, Azizi M, Nemati H, Jaafar HZ. 2012. The influence of compost on antioxidant activities and quality of Hot pepper (Capsicum annuum L.). Caspian J Appl Sci Res. 1:9.
- Arguedas FR, Lea-Cox JD, Ristvey AG. 2007. Revisiting the measurement of plant available water in soilless substrates. Proc. Southern Nursery Assoc. Res. Conf.
- Atzori G, Pane C, Zaccardelli M, Cacini S, Massa D. 2021. The role of peat-free organic substrates in the sustainable management of soilless cultivations. Agronomy. 11(6):1236.
- Battista F, Frison N, Pavan P, Cavinato C, Gottardo M, Fatone F, Eusebi AL, Majone M, Zeppilli M, Valentino F. 2020. Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J Chem Technol Biotechnol. 95(2):328–338.
- Benzie IF, Strain JJ. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 239(1):70–76.
- Blok C, Rijpsma E, Ketelaars J. 2014. New growing media and value added organic waste processing. XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014). p. 1112.
- Boldrin A, Hartling KR, Laugen M, Christensen TH. 2010. Environmental inventory modelling of the use of compost and peat in growth media preparation. Resour Conserv Recycl. 54(12):1250–1260.
- Bustamante M, Alburquerque J, Restrepo A, De la Fuente C, Paredes C, Moral R, Bernal M. 2012. Co-composting of the solid fraction of anaerobic digestates, to obtain added-value materials for use in agriculture. Biomass Bioenergy. 43:26–35.
- Bustamante M, Restrepo A, Alburquerque J, Pérez-Murcia M, Paredes C, Moral R, Bernal M. 2013. Recycling of anaerobic digestates by composting: effect of the bulking agent used. J Cleaner Prod. 47:61–69.
- Bævre OA. 1999. Plantedyrking i regulert klima. Landbruksforlaget Valdres, Norway (in Norwegian).
- Chang R, Guo Q, Pandey P, Li Y, Chen Q, Sun Y. 2021. Pretreatment by composting increased the utilization proportion of pig manure biogas digestate and improved the seedling substrate quality. Waste Manage. 129:47–53.
- Cleary J, Roulet NT, Moore TR. 2005. Greenhouse gas emissions from Canadian peat extraction, 1990–2000: a life-cycle analysis. AMBIO: J Human Environ. 34(6):456–461.
- da Conceicao Gomes MA, Hauser-Davis RA, Suzuki MS, Vitoria AP. 2017. Plant chromium uptake and transport, physiological effects and recent advances in molecular investigations. Ecotoxicol Environ Saf. 140:55–64.
- De Boodt M, Verdonck O. 1971. The physical properties of the substrates in horticulture. III Symposium on Peat in Horticulture 26.
- De Boodt M, Verdonck O, Cappaert I. 1973. Method for measuring the water release curve of organic substrates I Symposium on Artificial Media in Horticulture 37.
- Elia A, Conversa G. 2012. Agronomic and physiological responses of a tomato crop to nitrogen input. Eur J Agron. 40:64–74.
- Elias A, Mutalib SA, Mustapha WAW, Shahimi S, Mohamed N, Repin RAM. 2018. Antioxidant content of tomatoes (lycopersicon esculentum cv mt1) treated by different type of pesticide, fertilizer and growth medium in compost. Int J ChemTech Res. 11(5):387–393.
- Farrell M, Jones D. 2010. Food waste composting: Its use as a peat replacement. Waste Manage. 30(8–9):1495–1501.
- Fernandes C, Corá JE. 2004. Bulk density and relationship air/water of horticultural substrate. Scientia Agricola. 61:446–450.
- Gajdos R. 1989. The use of organic waste materials as organic fertilizers-recycling of plant nutrients. International Symposium on Compost Recycling of Wastes 302.
- García-Santiago JC, Valdez-Aguilar LA, Cartmill DL, Cartmill AD, Juárez-López P, Alvarado-Camarillo D. 2019. Subirrigation of container-grown tomato II: physical and chemical properties of the growing medium. Water (Basel). 11(11):2211.
- Ghoreishy F, Ghehsareh AM, Fallahzade J. 2018. Using composted wheat residue as a growth medium in culture of tomato. J Plant Nutr. 41(6):766–773.
- Hargreaves J, Adl MS, Warman P. 2009. The effects of municipal solid waste compost and compost tea on mineral element uptake and fruit quality of strawberries. Compost Sci Util. 17(2):85–94.
- Heuvelink E. 1999. Evaluation of a dynamic simulation model for tomato crop growth and development. Ann Bot. 83(4):413–422.
- Jara-Samaniego J, Pérez-Murcia M, Bustamante M, Pérez-Espinosa A, Paredes C, López M, López-Lluch D, Gavilanes-Terán I, Moral R. 2017. Composting as sustainable strategy for municipal solid waste management in the Chimborazo region, Ecuador: suitability of the obtained composts for seedling production. J Cleaner Prod. 141:1349–1358.
- Luo J, Fan R, Wang T, Gao Y, Liu L, Yan S, Zhang Z. 2015. Evaluation of spent pig litter compost as a peat substitute in soilless growth media. Biol Agric Hortic. 31(4):219–229.
- Maltby E, Immirzi P. 1993. Carbon dynamics in peatlands and other wetland soils regional and global perspectives. Chemosphere. 27(6):999–1023.
- Massa D, Bonetti A, Cacini S, Faraloni C, Prisa D, Tuccio L, Petruccelli R. 2019. Soilless tomato grown under nutritional stress increases green biomass but not yield or quality in presence of biochar as growing medium. Horticult Environ Biotechnol. 60(6):871–881.
- Michel J-C. 2010. The physical properties of peat: a key factor for modern growing media. Mires and Peat. 6(2):non paginé.
- Miransari M. 2012. Soil nutrients. Hauppauge, New York: Nova Science Publishers.
- Mitra S, Wassmann R, Vlek PL. 2005. An appraisal of global wetland area and its organic carbon stock. Curr Sci. 88(1):25–35.
- Murtić S, Zahirović Ć, Čivić H, Karić L, Jurković J. 2018. Uptake of heavy metals by tomato plants (lycopersicum esculentum mill.) and their distribution inside the plant. Agricul Forest/Poljoprivreda i Sumarstvo. 64(4):251–261.
- Nerlich A, Karlowsky S, Schwarz D, Förster N, Dannehl D. 2022. Soilless tomato production: effects of hemp fiber and rock wool growing media on yield, secondary metabolites, substrate characteristics and greenhouse Gas emissions. Horticulturae. 8(3):272.
- Nesse AS, Sogn T, Børresen T, Foereid B. 2019. Peat replacement in horticultural growth media: the adequacy of coir, paper sludge and biogas digestate as growth medium constituents for tomato (Solanum lycopersicum L.) and lettuce (Lactuca sativa L.). Acta Agric Scand, B – Soil & Plant Sci. 69(4):287–294.
- Norwegian Ministry of Agriculture and Food. 2003. Forskrift om gjødselvarer mv. Av organisk opphav (in Norwegian). https://lovdata.no/dokument/SF/forskrift/2003-07-04-951.
- Olsson J, Philipson M, Holmström H, Cato E, Nehrenheim E, Thorin E. 2014. Energy efficient combination of sewage sludge treatment and hygenization after mesophilic digestion–pilot study. Energy Procedia. 61:587–590.
- Perez-Murcia M, Moral R, Moreno-Caselles J, Perez-Espinosa A, Paredes C. 2006. Use of composted sewage sludge in growth media for broccoli. Bioresour Technol. 97(1):123–130.
- Peterson JC. 1982. Effects of pH upon nutrient availability in a commercial soilless root medium utilized for floral crop production. Res Cir. 268:16–19.
- Prasad M, Carlile W. 2007. Practical experiences and background research on the use of composted materials in growing media for the UK market. International Symposium on Growing Media. p. 819.
- Pronk A. 1994. Composted vegetable, fruit and garden waste as a substitute for peat in container-grown nursery stock. International Symposium on Growing Media & Plant Nutrition in Horticulture. p. 401.
- Restrepo A, Medina E, Pérez-Espinosa A, Agulló E, Bustamante M, Mininni C, Bernal M, Moral R. 2013. Substitution of peat in horticultural seedlings: suitability of digestate-derived compost from cattle manure and maize silage codigestion. Commun Soil Sci Plant Anal. 44(1–4):668–677.
- Rippy JF, Nelson PV. 2007. Cation exchange capacity and base saturation variation among Alberta, Canada, moss peats. HortScience. 42(2):349–352.
- Roberts P, Jones DL, Edwards-Jones G. 2007. Yield and vitamin C content of tomatoes grown in vermicomposted wastes. J Sci Food Agric. 87(10):1957–1963.
- Rogers MA. 2017. Organic vegetable crop production in controlled environments using soilless media. HortTechnology. 27(2):166–170.
- Santos FT, Goufo P, Santos C, Botelho D, Fonseca J, Queirós A, Costa MS, Trindade H. 2016. Comparison of five agro-industrial waste-based composts as growing media for lettuce: effect on yield, phenolic compounds and vitamin C. Food Chem. 209:293–301.
- Schmilewski G. 2008. The role of peat in assuring the quality of growing media. Mires & Peat. 3, 1–8.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299:152–178. Elsevier.
- Subramani T, Gangaiah B, Baskaran V, Swain S. 2020. Effect of soilless growing media on yield and quality of tomato (Solanum lycopersicum L.) under tropical island condition. Int J Curr Microbiol Appl Sci. 9(5):2084–2090.
- Urlic B, Runjic M, Dumicic G. 2015. Olive-mill waste compost as a peat substitute in leafy vegetables transplants production. Poljoprivreda i Sumarstvo. 61(3):35.
- Verdonck O. 1988. Composts from organic waste materials as substitutes for the usual horticultural substrates. Biological Wastes. 26(4):325–330.
- Verma S, Sharma A, Kumar R, Kaur C, Arora A, Shah R, Nain L. 2015. Improvement of antioxidant and defense properties of tomato (var. Pusa rohini) by application of bioaugmented compost. Saudi J Biol Sci. 22(3):256–264.
- Wallach R. 2008. Physical characteristics of soilless media. Soilless Cult Theory Practice. 3:41–116. . Raviv, M & JH Leith.
- Yeager T, Gilliam C, Bilderback T, Fare D, Niemiera A, Tilt K. 1997. Best management practices: guide for producing container-grown plants. Marietta, GA: Southern Nursery Association.
- Zanin G, Evans M, Bassan A, Sambo P. 2010. Use of fresh rice hulls and anaerobic digestion residues as substrates alternative to peat. XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 927.
- Zawadzińska A, Salachna P, Nowak JS, Kowalczyk W, Piechocki R, Łopusiewicz Ł, Pietrak A. 2021. Compost based on pulp and paper mill sludge, fruit-vegetable waste, mushroom spent substrate and rye straw improves yield and nutritional value of tomato. Agronomy. 12(1):13.
- Zeng Y, De Guardia A, Dabert P. 2016. Improving composting as a post-treatment of anaerobic digestate. Bioresour Technol. 201:293–303.
- Zhang L, Sun X, Tian Y, Gong X. 2013. Composted green waste as a substitute for peat in growth media: effects on growth and nutrition of Calathea insignis. PLoS One. 8(10):e78121.
- Zhang Z, Bian B, Jiang Y. 2020. A joint decision-making approach for tomato picking and distribution considering postharvest maturity. Agronomy. 10(9):1330.
Appendix
Pairwise post hoc comparison between treatments conducted in RStudio v1.4 with TukeyHSD for continual data, and a Tukey option in glht in the multcomp package for count data. Only the parameters that showed significant differences (leaves and surface area) are included.

