ABSTRACT
Almost 10,000 of apple cultivars are described worldwide but only a few of them are dominating in commercial apple orchards. The decline in genetic diversity could lead to negative consequences in terms of adaptability, resistance and even consumption. Apple genetic resources in Norway are conserved in several local clonal archives. The aim of this study was to evaluate Norwegian heritage apple cultivars from a pomological, agronomical, and chemical point of view, identify the most important quality parameters, and select cultivars with desirable traits for modern markets and breeding purposes. In total 75 heritage and 4 standard apple cultivars were evaluated at the Norwegian Institute of Bioeconomy Research – NIBIO Ullensvang, during 2018–2020. Based on individual investigations of various fruit quality characters, cultivar groups with special properties were selected for the industry, for making concentrate and fresh juice, and for medical properties. According to the soluble solid content, sweetness index, fruit size, acid, dry matter, and phenolic content, several groups of cultivars have high potential value for modern breeding programmes. Based on overall fruit quality, heritage cultivars Løeeple, Raud Gravenstein, and Rondestveit were selected for fresh consumption.
Introduction
Approximately 10,000 of apple cultivars are described in the literature but only a few of them are dominating in the commercial apple orchards, where Golden Delicious and Red Delicious take the leading positions in the world market. Even more, one of these two cultivars is the parent of other worldwide popular cultivars as Gala, Jonagold and Fuji (af Sätra et al. Citation2020). Such decline in genetic diversity could lead to negative consequences in terms on loosing interesting and well-adapted traditional and local varieties, thus loss of adaptability, resistance and even consumption (Benzie and Strain Citation1996; Anastasiadi et al. Citation2017). Therefore, great efforts were taken in various countries around the globe to establish apple cultivar collections with as much as possible heterogeneous material. Many of these collections are focused on preserving unique apple genotypes and enlarging genetic diversity and also to sustain cultural heritage.
In order to minimise genetic erosion and avoid loss of special genotypes, a number of local clonal archives were established across northern Europe with the goal of retaining old and local cultivars (Garkava-Gustavsson et al. Citation2013; Larsen et al. Citation2017; af Sätra et al. Citation2020). During the last few decades genetic evaluation has been very extensively used and many apple collections worldwide were screened (Benzie and Strain Citation1996; Blažek and Pištěková Citation2017; Bolos et al. Citation2021; Brown Citation2015; Butkevičiūtė et al. Citation2020; Chmielewski Citation2003; Currie et al. Citation2000; Denver and Jensen Citation2014; Duralija et al. Citation2021; Dzhuvinov and Gandev Citation2016; Eccher Zerbini et al. Citation2003; Endrizzi et al. Citation2015; Espino-Díaz et al. Citation2016; Fazio et al. Citation2018. Apple genetic resources in Norway are conserved in several local clonal archives (Meland et al. Citation2022; Sæther et al. Citation2021). This is how the current gene pool was created, which has been adapted to local climatic conditions and now represents a valuable and available resource of genes of interest for future breeding programmes. Genetic characterisation in some of these apple accessions was done as well in order to assess the genetic diversity. Out of 181 analysed accessions, from 6 ex situ collections located in western and southeastern Norway, 158 displayed a unique SSR profile (Fotirić Akšić et al. Citation2022). Most of these ancient apple cultivars are either of Norwegian origin or were introduced to Norway centuries ago and are named as heritage cultivars. However, pomological and chemical contents of the fruit from these ex situ collections are still not documented as is usually not done in previously mentioned genetic studies either. Besides, genetic characterisation research of heritage or local apple cultivars is also focused on fruit bioactive compounds, and extensively phenolic profiles have been evaluated in different studies performed in various countries (Garkava-Gustavsson et al. Citation2013; Gasi et al. Citation2016; Frøynes et al. Citation2020; Göksen and Keles Citation2020; Frøynes et al. Citation2021; Fotirić Akšić et al. Citation2022).
The importance of research and conservation of local, traditional cultivars is evident as this genetically heterogeneous material represents a potential source of positive pomological traits and resistance to biotic (including pests and diseases) and abiotic stresses (Harker et al. Citation2008; Heinonen and Bitz Citation2019). Therefore, heritage apple cultivars should be included and used in the breeding programmes (Hokanson et al. Citation2001; Holdershaw and Konopka Citation2018) or having specific features where high disease resistance local apple cultivars can be directly cultivated in orchards (Iacopini et al. Citation2010; Iaccarino et al. Citation2019; Jakobek et al. Citation2020).
The commercial orchards in Norway are modern and maintained at an international standard with high-density plantings (3–4000 trees per ha), with cultivars grafted on dwarfing rootstocks (M9) and trickle-irrigated with fertigation. This apple production in Norway is located in areas having the most favourable climate in the country. It is limited to small regions along the fjord areas in the southwest part of the country and around lakes or near the sea in the southeast part. Western part has a marinate climate with relatively cool summers and mild winters. Western winds from the Atlantic Ocean bring clouds, rain and wind throughout the year. The majority of the rainfall appears during the wintertime, mostly as rain, but there is some snow from time to time. The growth period from May to September can be relatively dry and all orchards have trickle irrigation to supply the trees with enough water during the summer. The temperature in Ullensvang during the growing season (May–September) is 14.3°C (30 years average) and July is the warmest month (16.1°C). The annual accumulated precipitation is 1870 mm and the average for the growing season is 541 mm (Vimic et al. Citation2023). The soil factors vary in Norwegian orchards, from sandy soils with high rainfall influenced by the mild climate in the fjord area on the west coast of Norway to soils with finer texture and lower rainfall in the southeastern part of the country (Krogstad et al. Citation2023).
Old traditional varieties are almost not grown in commercial orchards. Meanwhile, there is growing interest from consumers for old varieties and increased demand for health benefits. Such changes in consumer’s demand give new tasks for horticulture and can be partly solved by comprehensive evaluation and re-selection of heritage cultivars. Preservation of unique cultivars should focus on keeping genetic diversity and, not less important, to sustain socio-cultural heritage.
The aim of this study was to evaluate Norwegian heritage apple cultivars and select cultivars with desirable traits for modern markets and breeding purposes.
Materials and methods
Plant material
The study included 75 heritage and 4 standard apple cultivars. Fruit quality (pomological features and biochemical characteristics) was evaluated during the project period in 2018–2020, meanwhile tree growth characteristics, phenology and bearing pattern were established after long-term observations. All heritage trees were propagated on M.26 rootstock and planted at Hjeltnes (60.5695794, 6.9120983) during 1995–1998. All of them have a unique SSR profile which was proved by Gasi et al. (Citation2016). Commercial cultivars Aroma, Elstar, Rubinstep and Summerred were propagated on M.9 rootstock and planted during 2010–2012 at Lofthus (60.3267400, 6.6571653) and used as reference cultivars. Planting distances in the row were 3 m for apple trees on M.26 and 1 m for trees on M.9 rootstock. Each cultivar was represented by three trees.
Phenological aspects of Norwegian apple cultivars
The phenological stages of flowering (1 – start of bloom period; 2 – 20% of flowers open; 3 – full bloom, 80% of flowers open) and harvesting (1 – extremely early; 2 – very early; 3 – early; 4 – early/mid-season; 5 – mid-season; 6 – mid-season/late; 7 – late; 8 – very late; 9 – extremely late) were assessed each year according to UPOV descriptor (Janick et al. Citation1996).
Following apple tree characters were assessed according to UPOV descriptor (Janick et al. Citation1996): vigour (very weak, weak, medium, strong), growth habit (upright, spreading, dropping, weeping), type of bearing (on spurs only, on spurs and long, on long shoots only).
Pomological features
Average fruit weight (g) was calculated based on all fruits per every tree and total yield. Fruit quality characteristics were determined on samples of 10 randomly collected fruits per tree. Fruit cover colour (blush) was evaluated in 1–9 point scale, where 1–0% of blush, 9–100% of fruit surface is covered by red blush. Fruit firmness (kg cm−2) was measured using an FTA penetrometer (FT-327, TR Turoni, Forli, Italy) equipped with an 11-mm plunger on two opposite sides of each fruit after removing the disk of the skin. The two readings were averaged for each fruit. Soluble solids content (%) was measured by Atago® Pallete Digital refractometer PR-101 (Atago®, Tokyo, Japan). Titratable acidity was estimated by titrating filtered juice with 0.1 N NaOH solution to the end point of pH 8.2 w was expressed as a percentage of malic acid equivalent. Fruit dry matter content (DM) was measured by drying samples of fruit at 70°C in an oven to a constant weight.
Fruit taste (0–6 points, where 0 – uneatable, 6 – excellent taste), attractiveness (sell appearance) (0–6 points, where 0 – very poor appearance, 6 – very attractive), and aroma (0–4 points, where 0 – no aroma, 4 – very aromatic) was evaluated by 4–6 trained panellists during the harvest of early ripening cultivars and after 2 months storage of late ripening cultivars. The different evaluation dates for early and late ripening cultivars were chosen according to their consumption period. Overall fruit quality (0–16 points, where 0 – very poor, 16 – the highest possible overall quality) was calculated as a combination of all sensory analyses.
Other fruit characters were recorded according to UPOV descriptor (Janick et al. Citation1996): shape (cylindrical waisted, conic, ovoid, cylindrical, ellipsoid, globose, obolid), ground colour (not visible, whitish yellow, yellow, whitish green, yellow green, green), hue of over colour – with wax bloom removed (orange red, pink red, red, purple red, brown red), area of russet (absent or small, medium, large), colour of flesh (white, cream, yellowish, greenish, pinkish, reddish).
Biochemical characteristics
The total phenol content (TPC) in apple fruits was evaluated by using the Folin–Ciocalteu method (Kellerhals et al. Citation2004). The TPC was expressed as gallic acid equivalents (GAE) in mg/100 g. Antioxidant activity (FRAP-method) was analysed according to Benzie and Strain (Citation1996) and expressed in µmol/g (Kiprijanovski et al. Citation2020).
Statistical analysis
Data were analysed by general analysis of variance (ANOVA) using the statistical program Minitab® 16 statistical software (Minitab Ltd., UK). Standard deviation was calculated for the main fruit characters. All main tree and fruit characters were used to perform the clustering of cultivars into similarity groups using the method of an un-weighed pair group method with arithmetic mean (UPGMA). Principal component analysis (PCA) was carried out employing the program PLS_Tool Box software package for MATLAB (Version 7.12.0) and all data were group-scaled prior to PCA (Király et al. Citation2015). All phenological data together with yield and fruit quality parameters are presented as an average of two years.
Results and discussion
Phenology of flowering and fruit ripening
Flowering time
Plant flowering is under the strong influence of genotype, location exposure, geographic position, availability of water, age, vigour soil type, rootstocks and others. Flowering window is important not only for optimal pollination and subsequent fruit set but also to avoid risks of late spring frost damage in the regions where they occur. Besides, flowering is affecting the timing of pesticide applications, fertilisation, irrigation and harvesting (Krogstad et al. Citation2023).
In general, over the last 50-year period, apple tree flowering in Norway was advanced by 16 days in response to the temperature rises (Vimic et al. Citation2023). But apple flowering time in a particular season can greatly depend on the weather conditions and could vary 3–4 weeks as reported in previous reports (Kviklys and Robinson Citation2010; Lacis et al. Citation2011).
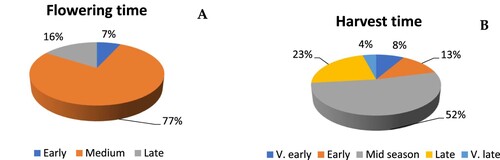
In two years of this study, the variation of full flowering time between tested cultivars was 10 days: 15–25 May in year 1 and 10–20 May in year 2 (Table S1). Early flowering was recorded for cvs. Franskar, Raud Sävstaholm and Rosvolleple in both years and was similar to flowering time of the standard cultivar Summerred. Flowering time of Vintergul, Signe Tillish, Ingrid Marie, Strutar, Lord Lambourne, Sitroneple, Norfolk Royal, Bananeple, Haugeeple, Ribston, Cox’s Orange and Cox’s Orange cultivars (12 out of 75, 16%) was late or medium late and was comparable to standard cultivars Aroma and Elstar. All other 58 tested cultivars (77.33%) belong to medium flowering cultivar group ((A)).
Harvest time
The start time of fruit harvest maturity is a primary characteristic of each cultivar and is closely connected with the climatic conditions of the location (Larsen et al. Citation2017). The fruits must be picked at the right moment, when they reach genotype chracteristic features. If they are picked too early, apples have inferior consumption quality, have less attractive colouration and taste, but if picked too late fruits are more often have physiological disorders and shorter shelf life (Lauri and Lespinnasse Citation1993).
Fruit maturation and harvest time is very important at Norwegian climatic conditions. Cultivars which ripen after the mid-October cannot reach proper fruit quality every year due to temperature drop (Lacis et al. Citation2011). It could be presumed that all heritage cultivars grown during the centuries should be well adapted to the local growing conditions. However, in our study three tested cultivars (Belle de Boskoop, Bramley Seedling and Cox’s Orange) had very late harvest time and their cultivation could be risky in Norway. All these cultivars are not native genotypes but have been introduced to Norway during the last two centuries.
Based on two years of observations, heritage cultivars were grouped according to their harvest time (Table S1). Six cultivars belong to very early ripening group (8%), nine cultivars (12%) fell into early ripening group, while 50% of tested cultivars formed the largest mid-season ripening group ((B)). Since harvest time is controlled by additive genes, and progeny from certain crosses are within a parental set (Lespinasse Citation1977), it can be presumed that apple crossings during long cultivation created the largest number of mid-season apple genotypes.
Apple tree growth and productivity
Tree vigour
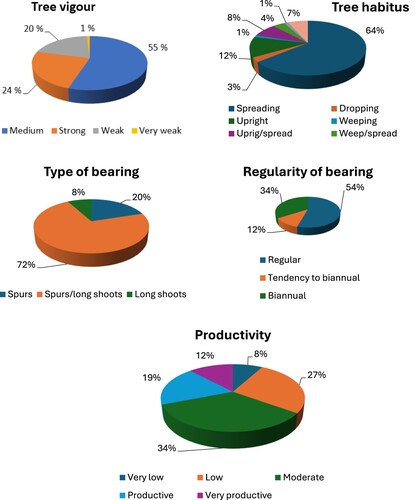
Apple tree cultivars, due to their genetics, exhibit different growth vigour. Cultivation of weak or strong-growing apple cultivars requires additional attention therefore optimal rootstock/scion combinations should be chosen, combining strong-growing cultivars with more dwarfing apple rootstocks and weak-growing cultivars with more vigourous ones (Magby et al. Citation2019; Lončarić et al. Citation2020). In our study cv. Marta-Moster was the only very weak growing cultivar (Table S1). According to the assessed tree vigour 20% of heritage cultivars were weak growing and 24% of cultivars belong to the strong growing cultivar group. Finally, 55% of tested cultivars exhibited medium vigourous growth ((A)). No matter that tree vigour is a polygenic trait, where dominance and epistasis are involved; the progeny mean is related to the parental mean (Marconi et al. Citation2018), which can explain the vast majority of cultivars with medium vigour.
Tree habitus
Tworkoski and Miller (Citation2007) indicated that cultivars with spreading growth habits grew faster in early spring than cultivars with upright growth habits. Also, they found that the effects of dwarfing rootstock on shoot growth vary within apple tree growth habitus. Based on these findings, optimal rootstock/scion combinations could be suggested for orchard establishment.
Our study revealed that tree canopy in 64% of tested cultivars had spreading growth habitus. But there were cultivars with another pattern of canopy growth ( and (B)): upright (9 of 75, 12%), upright – spreading (6/75, 8%), weeping (only one, 1.3%), weeping – spreading (3/75, 4%), weeping – dropping (only one), dropping (2/75, 2.7%) and dropping – spreading: (5/75, 6.7%).
Table 1. Fruit firmness, chemical content, total phenolics content, and antioxidant activity (FRAP), average 2018–2019.
Type of bearing
Lespinasse (Citation1977) proposed to divide apple cultivars into groups concerning the age of the fruiting wood: Type I (Starkrimson) – fruiting is predominantly on 2- and 3-year-old wood, Type II (Reine des Reinettes) – fruiting is mainly on 3- and 4-year-old wood, Type III (Golden Delicious) – fruiting predominantly on 1-, 2- and 3-year wood and Type IV (Granny Smith) – fruiting mainly on 1- and 2-year-old wood. UPOV guidelines (Janick et al. Citation1996) grouped apple cultivars into three groups according to type of bearing: (a) on spurs only (reference cultivar Starkrimson Delicious); (b) on spurs and long shoots (reference cultivar Jonagold) and (c) on long shoots only (reference cultivars Cortland and Rome Beauty). Both classifications are important for the establishment of the right pruning strategy.
In our study, along with commercial cultivars Aroma, Summered and Rubinstep, 15 (20%) heritage cultivars (Borsdorfer, Cox’s Orange, Gullspir, Furuholm, Franskar, Husmoreple, Ingrid Marie, Laxton’s Exquisite, Løeeple, Øskaug, Raud Gravenstein, Søteple, Stor Granat, Sureple grøn and Vanleg Torstein) set fruits only on spurs ( and (C)). The study conducted in Bulgaria (Meike et al. Citation2022) showed that apple cultivars produced the highest percentage of fruits on spurs. It is known that the spur-type cultivars are characterised by a biennial-bearing habit, but on long-branching cultivars leaf-bearing shoots appeared to be able to balance vegetative and reproductive growth (Meland et al. Citation2022). Only six cultivars (8.0%) were typical tip bearers (Cox’s Pomoma, Fristeren, Herrasaleple, Langballeeple, Paradiseple and Transparente Blanche) which set fruits only on long shoots, meanwhile, the largest part of cultivars (72.0%) were fruiting both on spurs and long shoots.
Productivity
For acceptable productivity in some apple genotypes the presence of high adaptability to local environmental conditions is extremely important. Nine cultivars, or 12% (Cox’s Pomona, Løeeple, Håkonseple, Raud Gravenstein, Ribston, Silkeeple, Strutar, Tormodseple and Transparente Blanche) were very productive and gave the highest average yield (16–18 kg/tree) among all tested cultivars. Most of these cultivars have biennial-bearing habit or tendency to biennial bearing ((D)), but Cox’s Pomona, Raud Gravenstein and Ribston gave a stable yield. Six cultivars (8.0%) Knuteple, Norfolk Royal, Høyneseple, Sureple grøn, Ölands Kungsäpple and Vinterrosenstrips were very low productive and gave an average yield of less than 5 kg/tree. The majority of the cultivars had moderate productivity (34%, (E)), which is expected due to the polygenic control of this trait where the progeny mean is always related to the parental mean (Mignard et al. Citation2022).
Fruit quality
Fruit weight and diameter
Fruit weight directly depends on crop load, planting distances and orchard management practices (Mratinic and Fotric Akšic Citation2011; Mignard et al. Citation2021). In our study, the average fruit weight of tested cultivars depended on the crop load in a particular year, but mostly was determined by genotype and varied from 36 g till 222 g (Table S2). Cultivars Bramley Seedling, Husmoreple, Leiknes, Rosvolleple, Signe Tillish, Sysekavil, Storesteineple and commercial cultivar Aroma had the biggest fruit weight around or over 200 g. The average fruit weight of 14 cultivars was lower than 100 g. Cultivars Sureple grøn and Paradiseple had especially small fruits with an average fruit weight of 38 and 36 g, respectively.
Fruit yield and fruit number of most cultivars were higher in 2019. Therefore, in general, the average fruit weight was lower in that year. The highest variation of fruit weight (31–45%) due to differences in crop load between years was recorded for cultivars Early Red Bird, Garborg, Fristeren, Husmoreple, Marta-Moster, Norfolk Royal and Oster. On the other hand, almost the half of tested cultivars had similar fruit weight during the years of observation and the variation in their fruit weight was only 0–15%.
It is obvious that testing of a large number of cultivars reveals significant variation in fruit size. Fruit size (diameter) directly correlated with the fruit weight. Cvs. Bramley seedling, Cox’s Pomona, Husmoreple, Leiknes, Rossvolleple, Sysekavil and Storesteinseple had the largest fruits with the average diameter over 80 mm (data is not presented). Only one of the commercial cultivars, Aroma, reached such size. On the other hand, there were 11 cultivars which average fruit diameter was less than 65 mm, while fruit diameter of Paradiseple and Sureple Grønn even did not overcame 50 mm.
Fruit background and over colour
Apple skin colour is determined by the contents of anthocyanins, carotenoids, and chlorophyll, as well as their distribution over the skin surface. Scientifically described, genes involved in the flavonoid biosynthetic pathway and epigenetics are linked with apple skin colour. Besides, rootstocks are also linked with apple skin colour (Natić et al. Citation2015).
Heritage cultivars Early Red Bird, Eldraud pigeon, Garborg, Ingrid Marie, Ölands Kungsäpple, Raud Sävstaholm, Raudt Laupsaeple, Raud Gravenstein, and Rondestveit the same as commercial Summered and Elstar had extensively coloured fruits (Table S2). Their blush (over colour) covered 60–80% of the fruit surface. Over colour of these cultivars varied from red to pink/violet – red and the blush pattern was mainly solid blush or solid blush with strongly defined stripes. Several cultivars as Bananeple, Belle de Boskoop, Fristeren, Grønt Laupsaeple, Gul Granat, Gullspir, Hjartneseple, Rossvolleple, Sitroneple and Transparente Blanche had fruits without any blush and their ground colour was mostly white – green or green, and only Hjartneseple had yellow green fruits. Very little or no blush was found on fruits of cvs. Håkonseple, Haugeeple, Husmoreple, Kavil, Knuteple, Kviteple, Langballeeple, Signe Tillish, Silkeeple, Søteple and Tokheimseple.
Skin colour in apples is one of the most important factors determining the acceptance and economic value of apples. Colour preferences depend on the uniformity, repeatability, intensity of the colour, the size of high colour area, and brightness–darkness contrast (Nybom and Garkava-Gustavsson Citation2009).
Fruit flesh colour
Flesh colour of 75 heritage cultivars was white (32%), white cream (23%) or white greenish (16%) (Table S2). Cultivar Early Red Bird was the only one with pinkish flesh, and the rest of the cultivars had various modifications of pinkish colour – green pinkish, white pinkish or pinkish cream.
The majority of worldwide growing apples are having white to off-white flesh colours. Coloured apple flesh, besides being a fashion lately, it is also a source of potentially health-benefiting compounds, in most cases polyphenols (Oszmiański et al. Citation2020).
Fruit shape
This trait is quantitatively inherited with a low genotype × environment interaction, where fruit shape in the progeny is approximately equal to the mid-parent value (Palmer et al. Citation2010). In our study predominant fruit shape of over seventy percent of tested cultivars was obloid with some variations (globose obloid, conic obloid) (Table S2). The progeny mean for fruit shape was found to be approximately equal to the mid-parent value. Cultivars Strutar and Worcester Permain fruits had conic shape, fruits of Borsdorfer, Laxton’s Exquisite, Lord Lambourne and Sureple Grønn were globose, and four cultivars had cylindrical waisted fruit shape.
Fruit chemical content
Soluble solids (SSC)
Different sugars mostly determine fruit soluble solid content (SSC) in fruits e.g. more sugars higher SSC. The average of SSC in this study varied within cultivars from 9.85% (cv. Øskaug) to 13.72% (cv. Ribston) [from 9.6% (cv. Øskaug) to 14.2% (cv. Ribston) in 2018 and from 9.4% (cv. Garborg) to 15% (cv. Raud Sommerkavil) in 2019]. A higher SSC than 13% was found in cvs. Raud Sommerkavil, Ribston, Rubinstep and Paradiseple fruits (). Very low SSC (10% or less) was recorded for cvs. Franskar, Garborg, Haugeeple and Øskaug. Endrizzi et al. (Citation2015) stated that overall liking of apple fruits was positively influenced by high levels of sweetness. In our study, it was true in the case of cv. Rubinstep and Raud Sommerkavill, which high SSC correlated with high taste scores. On the other hand, cultivar Ribston got a low taste score and Paradiseple was rated as uneatable. Low SSC of cvs. Franskar Garborg and Haugeeple also correlated with low taste scores, only Øskaug got higher than average scores.
Titratable acidity (TA)
Acidity in all cultivars is influenced by the season, by crop load and by cultural methods (Pérez-Romero et al. Citation2015). Fruits with pH lower than 3.1 and TA higher than 10.0 mg/ml are considered too sour while those with pH higher than 3.8 and TA lower than 3.0 mg/ml will taste flat or flavourless, i.e. too low in acidity (Racskó et al. Citation2009).
Very distinctive differences among tested cultivars in our study were recorded for the acid content – from 0.24% to 1.98%. Titratable acidity of cvs. Early Red Bird, Fristeren, Gullspir, Haugeeple, Knuteple, Sureple Grønn and Tokheimseple fruits was over 1.5% (). Only one commercial cultivar Geneva Early was on the same range. Cultivars with high TA could be used for processing purposes (e.g. concentrated juice or ciders) where high acidity is a preference (Jakobek et al. Citation2020). A low average of fruit acidity (0.52% and less) was determined in cultivars Marte-Moster, Herrasaleple, Hjartneseple, Raudt Laupsaeple, Strutar and Søteple.
SSC/TA ratio
Based on sugar and acid content (Reig et al. Citation2015) heritage apple cultivars were separated into three groups: cultivars for ‘fresh consumption’, for ‘industry use’ and for ‘contract industry use’. In our study 25 tested cultivars had very acid fruits. Their SSC/TA ratio was less than 10 and equalled to a ratio of sour wild apples (Rivero et al. Citation2017). Interesting that two commercial cultivars Quinte and Geneva Early were also in this group. Due to the very low TA level, extremely high SSC/TA ratio (over 40) was recorded in cvs. Hjartneseple, Herrasaleple and Søteple. The fruit flavour of cvs. Marte-Moster, Raudt Laupsaeple, Strutar and Worcester Permain had also prevailing sweet character, though their SSC/TA ratio was twice lower than previously mentioned cultivars (). Despite everything, it should be stated that the most of the Norwegian heritage apple cultivars had mild sour or sour fruits.
Dry matter (DM)
The average dry matter content of tested cultivars felt in a broad range and varied between low 10.68 up to high 16.01 (). Palmer et al. (Citation2010) proposed fruit dry matter as a new quality parameter for apple fruits, providing a positive relationship between it and consumer preference. But it should be noticed that it is true on the cultivar level but likely cannot be used for the comparison of different cultivars due to genetically determined differences of fruit chemical content. In our study, the highest dry matter content was recorded for cultivars Ulgenes and Elstar Boerekamp which also got the highest scores for the fruit taste and at the same time for cultivar Søteple which taste was rated as not acceptable (Table S3). The lowest dry matter content among tested cultivars was in Strutar, and the same as Haugeeple or Langballeeple fruits corresponded to the low taste score of fruits, but the taste of other cultivars with low dry matter content (e.g. Sävstaholm) was rated relatively high.
Fruit firmness
Fruit firmness is one of the main factors determining overall eating and liking of apple fruits (Rufato et al. Citation2021). In our study most of the heritage cultivars had fruits with very high fruit firmness. Cultivars Bananeple, Belle de Boskoop, Borsdorfer, Eldraud Pigeon, Gul Granat, Raud Granat Ribston and Silkeeple had fruit firmness of over 11 kg/cm2 (). Fruit firmness of the commercial cultivar Rubinstep was 9.6 kg/cm2 but all other commercial cultivars had low fruit firmness. Only few of heritage cultivars had firmness comparable to Aroma or lower, including cultivars Rossvolleple and Sävstaholm which were rated as the tastiest one.
Total phenol content and antioxidant activity
Extremely high total phenol content was recorded in fruits of cvs. Grønt Laupsaeple, Sureple Grønn, Paradiseple (more than 500 mg GAE/100 g) (). These cultivars are distinguished significantly from all other studied cultivars. Cultivars with the highest phenol content showed the highest antioxidant-reducing capacity, which was already proved in other studies too (Wicklund et al. Citation2021). It is interesting that all previously mentioned cultivars were small-fruited (especially Paradiseple and Sureple Grønn which had fruits with weight less than 40 g.) and had fruits with relatively high acid content and low SSC/TA ratio. Anastasiadi et al. (Citation2017) found a relation between fruit size and phenol content. Since higher content of phenolic compounds is mostly found in the apple peel compared to the flesh (Kschonsek et al. Citation2018), in large-fruited dessert apples TPC is lower due to the smaller skin share in fruit weight, while in small-fruited cider and ornamental apple cultivars the peel share in fruit is much higher.
Relatively high total phenol content (330–360 mg GAE/100 g) was also found in fruits of cvs. Bramley Seedling, Høyneseple, Signe Tillish, Sitroneple andVintergul. If cvs. Sitroneple and Vintergul were again small-fruited cultivars, so cv. Bramley Seedling and cv. Signe Tillish had one of the biggest fruits among the tested group. It could be stated that not only fruit size but also genotype have a crucial influence on total phenol content.
Studies conducted in the UK showed that dessert cultivars had a lower phenolic content than cider or culinary apples (Shewfelt Citation1999; Samuolienė et al. Citation2016). Studies performed in Austria, Italy, Spain, Croatia and Hungary also support evidence that old apple cultivars have higher levels of polyphenolic compounds and antioxidant capacity as compared to commercial cultivars (Tancred et al. Citation1995; Singleton et al. Citation1999; Sikorskaite et al. Citation2012; Garkava-Gustavsson et al. Citation2013; Gasi et al. Citation2016; Göksen and Keles Citation2020). In our trial, all tested commercial cultivars Aroma, Elstar, Rubinstep and Summered had ∼2.5 time less total phenols and 3–4 times less antioxidant activity than the top three heritage apples (Grønt Laupsaeple, Paradiseple, Sureple Grønn). Along with them heritage cultivars Bananeple, Early Red Bird, Maria Moster, Oster, Raud Gravenstein, Stor Granat, Tveiteple and some others had the same low total phenol content.
Fruit organoleptic properties
Fruit taste
Test panel evaluation indicated cultivars with the best-tasting fruits (Table S3). Along with the commercial cultivars as Aroma, Elstar, Quinte, Rubinstep and Summered, on the average for both years, the highest taste scores were given to Gul Granat, Laxton’s Exquisite, Løeeple, Maglemer, Raud Gravenstein, Raud Sommerkavill, Rondestveit, Rossvolleple, Sävstaholm and Ulgenes (5–5.5 points in 6-point scale).
The taste of 32 cultivars was evaluated lower than 3 points and was rated as poor. Cultivars Borsdorfer, Bramley seedling, Garborg, Grønt Laupsaeple, Herrasaleple, Knuteple, Søteple, Storesteinseple, Strutar, Sureple Grønn and Paradiseple received extremely low scores for fruit taste (0.5–1.5).
Fruit sell appearance
The major attributes influencing the choice of apple are fruit size and colour (Testolin et al. Citation2019). According to several studies, country of origin and sustainable fruit growing is another interest of consumers (Tóth et al. Citation2004; UPOV Citation2005; Tworkoski and Miller Citation2007). In this study, we did not perform market research, and panellists were not informed about the origin of cultivars. Visual evaluation by test panel distinguished cultivars with the best sell appearance. Along with the commercial cultivars as Elstar, Geneva Early, Quinte, Rubinstep, and Summered, on the average of both years, the highest scores were given to Fuhr, Løeeple, Raud Gravenstein, Raudt Laupsaeple, Raud Sävstaholm, Rondestveit, Sävstaholm, Transparente Blanche and Ölands Kungsäpple. Thirteen cultivars were rated as not suitable for sale and among them there were cultivars Belle de Boskoop, Knuteple, Marte-Moster, Sureple Grønn and Vinterrosenstrips that received scores lower than 1.
Fruit aroma
Biosynthesis of volatile aroma compounds in apples varies due to cultivar genetics, orchards management practices and degree of fruit maturity. The main products of biosynthesis are aldehydes, alcohols, and esters. Such enzymes as lipoxygenase, alcohol dehydrogenase, and alcohol acyltransferase play the most important role in the synthesis of volatile compounds (Vimic et al. Citation2023).
The most aromatic fruits were harvested from cvs. Fuhr, Gyllenkroks Astrakan and Rondestveit trees (Table S3). Their flesh aroma was rated as high as cv. Aroma. Fruits of cvs. Fristeren, Furuholm, Øskaug, Quinte and Stor Granat also received high scores. Studies performed in Denmark distinguished a couple of heritage cultivars which juices were characterised by very peculiar odours and flavours such as apricot and peach (Volz et al. Citation2009). That gives an idea to try some selected aromatic cultivars for single juice production.
On the other hand, cvs. Belle de Boskoop, Brureple, Herrasaleple, Husmoreple, Kaupanger, Paradiseple, Prins, Sureple grøn, Sysekavil and Vanleg Prins fruits had no or very little aroma.
Overall fruit quality
Several authors (Iacopini et al. Citation2010; Holdershaw and Konopka Citation2018; Iaccarino et al. Citation2019) indicated that heritage apple cultivars can be re-selected and directly cultivated in orchards. Our study also distinguished cultivars that could be successfully grown for fresh fruit production.
Bolos et al. (Citation2021) noted that visual appearance has a significant impact on how food is experienced. Apple fruit colour, size and shape are the main perquisites for consumers choice. However, after apple fruit tasting, actual liking could differ from expected liking. In our study, combing both taste and sell appearance characters following best heritage cultivars were Løeeple, Maglemer, Raud Gravenstein, Raud Sävstaholm, Sävstaholm, Rondestveit and (Table S3 and ). The overall quality of these heritage cultivars was rated as high as a fruit quality of commercial cultivars Aroma, Elstar Boerekamp Rubinstep and Summered. Cultivars Early Red Bird, Gyllenkroks Astrakan and Worcester Permain were rated as high as previously mentioned heritage cultivars, but the taste of their fruits and/or sell appearance of cv. Worcester Permain was not consistent in different years.
Adding aroma component to the taste and sell appearance characters cultivars Gyllenkroks Astrakan, Løeeple, Raud Gravenstein, Rondestveit and Sävstaholm would be rated as the best.
Cluster analysis
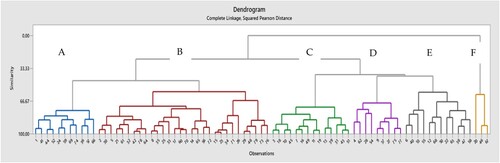
Cluster analysis was used to divide apple cultivars into groups of increasing dissimilarity. Six clusters were identified () corresponding to the tested fruit internal and external quality parameters. Almost all commercial cultivars – Aroma, Rubinstep and Summerred – belong to the A cluster. Cluster B was the largest, where were 40% of studied cultivars with average tree growth and fruit quality characteristics. Cultivars Raud Sävstaholm, Beauty of Bath, Garborg along with 11 other cultivars formed cluster C. Cultivars which belong to this cluster had good fruit colouring, very early or early harvest time with some exceptions, low or moderate fruit firmness, relatively high fruit acidity and relatively low SSC/TA ratio. Cultivars Bele de Boskoop and Ribston distinguished by lower overal fruit quality, later harvest and flowering time and high SSC along with the other seven cultivars formed cluster D. All heritage cultivars selected for the fresh consumption Løeeple, Maglemer, Raud Gravenstein, Rondestveit and Sävstaholmexcept cv. Raud Sävstaholm formed cluster E along with commercial cv. Elstar. Cultivars within E cluster distinguished by very good or good taste, high-sell appearance and overall fruit quality. Three cultivars distinguishing by very high phenol content Grønt Laupsaeple, Paradiseple and Sureple Grønn felt in the most distant F cluster.
Figure 4. Cluster analysis corresponds to the main fruit quality parameters. Numbers on the figure correspond to those in .
A molecular study that aimed to genotype 171 apple accessions from the Ullensvang apple heritage cultivar collection included these apple heritage cultivars has been conducted. In this study, 171 apple accessions were genotyped using a set of 20 different SSR markers. Based on the obtained molecular data, the apple heritage cultivar collection was determined to hold a key part of the overall genetic diversity of the Norwegian apple germplasm. Furthermore, the SSR markers were able to differentiate several accession groups originally thought to be synonyms, as well as to provide a more detailed insight into the genetic structure of this germplasm (Meland et al. Citation2022).
Principal component analysis (PCA)
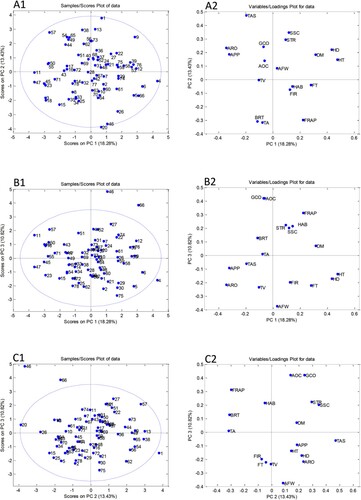
PCA was done to improve the understanding of the results obtained for a large number of apple samples and to establish the existence of similarities/differences among samples based on morphological, pomological, phenological and chemical properties. The initial matrices 75 (the number of apple samples × 18 morphological, pomological, phenological and chemical parameters) was processed using the covariance matrix with autoscaling. The cumulative variation explained by the first three components was only 42.5%, where the first component accounted for 18.3%, second 13.4%, and third 10.8% of total variability. The PCA score and loading plots for the first three principal components are shown in . Generally, all apple samples were distributed within the Hotelling T2 ellipse, with no clear separation into groups. However, some samples stood out from the other apple samples. Apple samples Grønt Laupsaeple (20) and Paradiseple (46) were distinguished along to PC 2 axis according to the higher FRAP values ((A1,A2)). Higher values of harvest time; harvest date, and dry matter were the most important factors responsible for the separation sample Cox’s Orange (9) from the other apples ((A1,A2)). The PC1/PC3 score plot ((B1)) showed three samples lying outside the Hotelling T2 ellipse [Belle de Boskoop (4), Paradiseple (46), and Summered (66)], suggesting that they were outliers. As already pointed out, sample Paradiseple (46) was separated due to higher value of the antioxidant activity ((B2)), while apple sample Belle de Boskoop (4) was distinguished according to higher harvest time and harvest date values ((B2)) and cultivar Summered due to the lowest level of phenols. Based on (C1), it was clear that sample Aroma (1) was also outlier and that the parameter was responsible for its separation was taste ((C2)).
Figure 5. Principal component analysis (PCA) performed on all studied parameters obtained for apple heritage apple cultivars: Scores plots of the first three principal components (A1, B1, C1) and loadings plots (A2, B2, C2). AFW – average fruit weight; HT – harvest time; HD – harvest date; FT – flowering time; TV – tree vigour; BRT – type of bearing; HAB – habitus; GCO – ground colour; AOC – area of overcolour; SSC – percentage of soluble solids, STR – total starch; FIR – Firmness, TA – total acidity; DM – % dry matter; FRAP – antioxidative activity; TAS – taste; APP – sell appearance; ARO – aroma.
Conclusions
To the best of our knowledge, this is the first comprehensive analysis of the phenological, pomological, agronomical, and chemical traits in 75 heritage apple cultivars grown in Norway. From the obtained results, many of the investigated heritage apple cultivars had significantly higher pomological traits and content of biologically active compounds compared to the tested commercial cultivars. Based on overall fruit quality, and productivity level, heritage cultivars Løeeple, Raud Gravenstein and Rondestveit could be recommended for fresh consumption. Cultivars Early Red Bird, Fristeren, Gullspir, Haugeeple, Knuteple, Sureple Grønn and Tokheimseple had very high acid content and could be valuable for processing into concentrated juice or as additives to products where higher acidity is required. Cultivars Grønt Laupsaeple, Paradiseple, Sureple Grønn had extremely high phenol content and could be used to produce health-promoting compounds and thus be a functional food.
Proper characterisation of germplasm collections is the backbone for more effective management and utilisation of genetic resources in research and breeding. In light of this, this ex situ collection is expected to contain considerable variation that can aim the goals of future breeding efforts. Since this collection of heritage apples exhibits a large genetic structure many of the studied cultivars could be used for the following breeding programmes. In that way cultivars Cox’s Orange, Leiknes, Lord Lambourne, Paradiseple, Raud Sommerkavill, Worcester Permain, could be used to develop new genotypes with high soluble solid content. Enlargement of fruit size can be done with cultivars Bramley Seedling, Husmoreple, Leiknes, Signe Tillish and Storesteineple, while aroma can be improved if cultivars Fuhr, Gyllenkroks Astrakan and Rondestveit are used as parentals in some crossings.
Supplemental Material
Download MS Word (64.4 KB)Acknowledgements
We thank Marianne Hotle, NIBIO Ullensvang, Norwegian Institute of Bioeconomy Research, Lofthus, Norway, and Signe Hansen and Kari Grønnerød, The University of Life Science, Norway for technical support analysing the fruit samples. Conception and design (M.M., M.F.A.), analysis and interpretation of the data (M.M., O.F., D.D.Z, M.F.A.); the drafting of the paper (M.M., M.F.A., D.K.) revising it critically for intellectual content (M.M., M.F.A., D.K.); and the final approval of the version to be published (M.M., M.F.A., D.K.); and that all authors agree to be accountable for all aspects of the work (M.M., M.F.A., D.K., O.F., D.D.Z.).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Mekjell Meland
Dr. Mekjell Meland, a research professor working at the Department of Horticulture, Norwegian Institute of Bioeconomy Research and located at NIBIO Ullensvang. His expertise is in orchard management, working with fruit cultivars and genetic resources, nutrition, pollination, fruit physiology, crop load management, cherry tunnel production and fruit quality.
Oddmund Frøynes
Oddmund Frøynes is an adviser, working at the Department of Horticulture, Norwegian Institute of Bioeconomy Research and located at NIBIO Ullensvang. His expertise is in fruit cultivar evaluations.
Darius Kviklys
Dr. Darius Kviklys is a researcher working at the Department of Horticulture, Norwegian Institute of Bioeconomy Research and located at NIBIO Ullensvang. His expertise is fruit research.
Dragana Dabić Zagorac
Dr. Dragana Dabić Zagorac, a senior research associate at the Innovation Center of the Faculty of Chemistry, University of Belgrade, Serbia. Her field of research includes the chemical characterisation of natural materials and plant food using modern analytical techniques for the determination of polyphenolics, sugar and organic acid profiles.
Milica Fotirić Akšić
Dr. Milica Fotirić Akšić, an associate professor works at the Department of Pomology, Faculty of Agriculture, University of Belgrade, Serbia. Her expertise is in fruit breeding, genetic resources, fruit physiology, fruit quality and organic production.
References
- af Sätra JS, Troggio M, Odilbekov F, Sehic J, Mattisson H, Hjalmarsson I, Ingvarsson PK, Garkava-Gustavsson L. 2020. Genetic status of the Swedish Central collection of heirloom apple cultivars. Sci Hort. 272:109599. doi:10.1016/j.scienta.2020.109599.
- Anastasiadi M, Mohareb F, Redfern S, Berry M, Simmonds M, Terry L. 2017. Biochemical profile of heritage and modern apple cultivars and application of machine learning methods to predict usage, age, and harvest season. J Agri Food Chem. 65(26):5339–5356. doi:10.1021/acs.jafc.7b00500.
- Benzie FF, Strain JJ. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. doi:10.1006/abio.1996.0292.
- Blažek J, Pištěková I. 2017. Prediction of the harvesting time for four apple cultivars on the basis of beginning of flowering and attaining of T-stage of fruitlets and dependence of diameter of fruitlets at T-stage and fruits at ripening stage. J Hort Res. 25(1):55–59.
- Bolos LA, Lagerkvist CJ, Normann A, Wendin K. 2021. In the eye of the beholder: expected and actual liking for apples with visual imperfections. Food Qual Prefer. 87:1–9. doi:10.1016/j.foodqual.2020.104065.
- Brown GS. 2015. Heritage apples revisited. Acta Hortic 1091:29–36. doi:10.17660/ActaHortic.2015.1091.2.
- Butkevičiūtė A, Liaudanskas M, Kviklys D, Gelvonauskienė D, Janulis V. 2020. The qualitative and quantitative compositions of phenolic compounds in fruits of Lithuanian heirloom apple cultivars. Molecules. 25(22):5263. doi:10.3390/molecules25225263.
- Chmielewski FM. 2003. Phenology and agriculture. In: Schwartz MD, editor. Phenology: an integrative environmental science. Dordrecht: Kluwer Acadamic; p. 505–522. doi:10.1007/978-94-007-0632-3_31.
- Currie AJ, Ganeshanandam S, Noiton DA, Garrick D, Shelbourne CJ, Oraguzie N. 2000. Quantitative evaluation of apple (Malus × domestica Borkh.) fruit shape by principal component analysis of Fourier descriptors. Euphytica. 111:221–227. doi:10.1023/A:1003862525814.
- Denver S, Jensen JD. 2014. Consumer preferences for organically and locally produced apples. Food Qual Prefer. 31:129–134. doi:10.1016/j.foodqual.2013.08.014.
- Duralija B, Putnik P, Brdar D, Bebek Markovinović A, Zavadlav S, Pateiro M, Domínguez R, Lorenzo JM, Bursać Kovačević D. 2021. The perspective of Croatian old apple cultivars in extensive farming for the production of functional foods. Foods. 10:708. doi:10.3390/foods10040708.
- Dzhuvinov V, Gandev SI. 2016. Evaluation of fruit bearing habit of apple, sweet cherry, walnut and strawberry cultivars in Bulgaria. Acta Hortic 1139:177–182. doi:10.17660/ActaHortic.2016.1139.31.
- Eccher Zerbini P, Grassi M, Cubeddu R, Pifferi A, Torricelli A. 2003. Time-resolved reflectance spectroscopy can detect internal defects. Acta Hortic. 599:359–365. doi:10.17660/ActaHortic.2003.599.44.
- Endrizzi I, Torri L, Corollaro L, Demattè L, Aprea E, Charles M, Biasioli F, Gasperi F. 2015. A conjoint study on apple acceptability: sensory characteristics and nutritional information. Food Qual Prefer. 40(A):39–48. doi:10.1016/j.foodqual.2014.08.007.
- Espino-Díaz M, Sepúlveda DR, González-Aguilar G, Olivas GI. 2016. Biochemistry of apple aroma: a review. Food Technol Biotechnol. 54(4):375–397. doi:10.17113/ftb.54.04.16.4248.
- Fazio G, Lordan J, Francescatto P, Robinson TL. 2018. Breeding apple rootstocks to match cultural and nutrient requirements of scion varieties. NY Fruit Q. 20:22–28.
- Fotirić Akšić M, Dabić Zagorac D, Gašić U, Tosti T, Natić M, Meland M. 2022. Analysis of apple fruit (Malus × domestica Borkh.) quality attributes obtained from organic and integrated production systems. Sustainability. 14:5300. doi:10.3390/su14095300.
- Fotirić Akšić M, Nešović M, Ćirić I, Tešić Ž, Pezo L, Tosti T, Gašić U, Dojčinović B, Lončar B, Meland M. 2022. Polyphenolics and chemical profiles of domestic Norwegian apple (Malus × domestica Borkh.) cultivars. Front Nutr 9:941487. doi:10.3389/fnut.2022.941487.
- Frøynes O, Kviklys D, Meland M. 2020. Apple cultivar evaluation for commercial growing in Norway. NIBIO Rapport. 7(44):30.
- Frøynes O, Kviklys D, Meland M. 2021. Testing new apple cultivars for Norwegian growing conditions. NIBIO Rapport. 6(135):44.
- Garkava-Gustavsson L, Mujaju C, Sehic J, Zborowska A, Backes GM, Hietaranta T, Antonius K. 2013. Genetic diversity in Swedish and Finnish heirloom apple cultivars revealed with SSR markers. Sci Hortic 162:43–48. doi:10.1016/j.scienta.2013.07.040.
- Gasi F, Kanlić K, Kalamujić Stroil B, Pojskić N, Asdal Å, Rasmussen M, Kaiser C, Meland M. 2016. Redundancies and genetic structure among ex situ apple collections in Norway examined with microsatellite markers. HortScience. 51(12):1458–1462. doi:10.21273/HORTSCI11212-16.
- Göksen G, Keles F. 2020. Phenolic compounds and antioxidant activity of local cultivar of apple (Malus domestica Borkh) in East of Turkey. Turk J Agric Food Sci Technol. 8(9):1976–1981. doi:10.24925/turjaf.v8i9.1976-1981.3593.
- Harker FR, Kupferman EM, Marin AB, Gunson FA, Triggs CM. 2008. Eating quality standards for apples based on consumer preferences. Postharvest Biol Techn. 50:70–78. doi:10.1016/j.postharvbio.2008.03.020.
- Heinonen M, Bitz L. 2019. How to discover traditional varieties and shape in a national germplasm collection: the case of Finnish seed born apples (Malus × domestica Borkh.). Sustainability. 11(24):7000. doi:10.3390/su11247000.
- Hokanson S, Lamboy W, Szewc-McFadden A, McFerson JR. 2001. Microsatellite (SSR) variation in a collection of Malus (apple) species and hybrids. Euphytica. 118:281–294. doi:10.1023/A:1017591202215.
- Holdershaw J, Konopka R. 2018. Consumer knowledge of country of origin of fresh food at point of purchase. J Promot Manag. 24:349–362. doi:10.1080/10496491.2018.1378303.
- Iaccarino N, Varming C, Agerlin Petersen M, Viereck N, Schütz B, Toldam-Andersen TB, Randazzo A, Balling Engelsen S. 2019. Ancient Danish apple cultivars—a comprehensive metabolite and sensory profiling of apple juices. Metabolites. 9(7):139. doi:10.3390/metabo9070139.
- Iacopini P, Camangi F, Stefani A, Sebastiani L. 2010. Antiradical potential of ancient Italian apple varieties of Malus × domestica Borkh. in a peroxynitrite-induced oxidative process. J Food Comp Analys. 23(6):518–524. doi:10.1016/j.jfca.2009.05.004.
- Jakobek L, Ištuk J, Buljeta I, Voća S, Žlabur J, Babojelić M. 2020. Traditional, indigenous apple varieties, a fruit with potential for beneficial effects: their quality traits and bioactive polyphenol contents. Foods. 9(1):52. doi:10.3390/foods9010052.
- Janick J, Cummins JN, Brown SK, Mnou Hemmat M. 1996. Apples. In: Janick J, Moore JN, editors. Fruit breeding Vol. I. Tree and tropical fruits. New York: Wiley; p. 1–77.
- Kellerhals M, Bertschinger L, Gessler S. 2004. Use of genetic resources in apple breeding and for sustainable fruit production. J Fruit Ornam Plant Res. 12:53–62.
- Kiprijanovski M, Arsov T, Saraginovski N. 2020. Pomological, quality and organoleptic traits of some autochthonous apple cultivars in Prespa region, North Macedonia. Acta Hortic. 1289:35–42. doi:10.17660/ActaHortic.2020.1289.5.
- Király I, Ladányi M, Nagyistván O, Tóth M. 2015. Assessment of diversity in a Hungarian apple gene bank using morphological markers. Org Agric. 5(2):143–151. doi:10.1007/s13165-015-0100-z.
- Krogstad T, Zivanovic V, Simic A, Fotirić Akšić M, Licina V, Meland M. 2023. Nitrogen mineralization of apple orchard soils in regions of western and south-eastern Norway. Agronomy. 13:2570. doi:10.3390/agronomy13102570.
- Kschonsek J, Wolfram T, Stöckl A, Böhm V. 2018. Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants. 7:20. doi:10.3390/antiox7010020.
- Kviklys D, Robinson T. 2010. Temperature before and after application of chemical thinners affects thinning response of ‘Empire’ apple trees. Acta Hort. 884:525–530. doi:10.17660/ActaHortic.2010.884.67.
- Lacis G, Kota I, Ikase L, Rungis D. 2011. Molecular characterization of the Latvian apple (Malus) genetic resources collection based on SSR markers and scab resistance gene Vf analysis. Plant Genet Resour. 9:189–192. doi:10.1017/S1479262111000384.
- Larsen B, Toldam-Andersen TB, Pedersen C, Ørgaard M. 2017. Unravelling genetic diversity and cultivar parentage in the Danish apple gene bank collection. Tree Genet Genomes. 13:14. doi:10.1007/s11295-016-1087-7.
- Lauri PE, Lespinnasse JM. 1993. The relationship between cultivar fruiting-type and fruiting branch characteristics in apple trees. Acta Hortic 349:259–263. doi:10.17660/ActaHortic.1993.349.43.
- Lespinasse JM. 1977. La Conduite du Pommier: Types de Fructification, Incidence sur la Conduite de l’Arbre. Paris: INVUFLEC; pp. 80.
- Lončarić A, Matanović K, Ferrer P, Kovač T, Šarkanj B, Babojelić MS, Lores M. 2020. Peel of traditional apple varieties as a great source of bioactive compounds: extraction by micro-matrix solid-phase dispersion. Foods. 9(1):80. doi:10.3390/foods9010080.
- Magby J, Volk GM, Henk A, Miller S. 2019. Identification of historic homestead and orchard apple cultivars in Wyoming. HortScience. 54(1):8–16. doi:10.21273/HORTSCI13436-18.
- Marconi G, Ferradini N, Russi L, Concezzi L, Veronesi F, Albertini E. 2018. Genetic characterization of the apple germplasm collection in central Italy: the value of local varieties. Front Plant Sci. 9:1460. doi:10.3389/fpls.2018.01460.
- Meike R, Dean DL, Baird T. 2022. Understanding apple attribute preferences of US consumers. Foods. 11:166. doi:10.3390/foods11020166.
- Meland M, Fotiric Aksic M, Frøynes O, Konjic A, Lasic L, Pojskic N, Gasi F. 2022. Genetic identity and diversity of apple accessions within a candidate collection for the Norwegian National Clonal Germplasm Repository. Horticulturae. 8(7):630. doi:10.3390/horticulturae8070630.
- Mignard P, Beguería S, Gimenez R, Font i Forcada C, Reig G, Moreno MA. 2022. Effect of genetics and climate on apple sugars and organic acids profiles. Agronomy. 12:827. doi:10.3390/agronomy12040827.
- Mignard P, Beguería S, Reig G, Font i Forcada C, Moreno MA. 2021. Genetic origin and climate determine fruit quality and antioxidant traits on apple (Malus x domestica Borkh). Sci Hort. 285:110142. doi:10.1016/j.scienta.2021.110142.
- Mratinic E, Fotric Akšic M. 2011. Evaluation of phenotypic diversity of apple (Malus sp.) germplasm through the principle component analysis. Genetika. 43(2):331–340. doi:10.2298/GENSR1102331M.
- Natić M, Dabić D, Papetti A, Fotirić Akšić M, Ognjanov V, Ljubojević M, Tešić Ž. 2015. Analysis and characterization of phytochemicals in Mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 171:128–136. doi:10.1016/j.foodchem.2014.08.101.
- Nybom H, Garkava-Gustavsson L. 2009. Apple gene banks – for breeding, research or public entertainment? Acta Hortic 814:71–76. doi:10.17660/ActaHortic.2009.814.4.
- Oszmiański J, Lachowicz S, Gamsjäger H. 2020. Phytochemical analysis by liquid chromatography of ten old apple varieties grown in Austria and their antioxidative activity. Eur Food Res Technol 246:437–448. doi:10.1007/s00217-019-03411-z.
- Palmer J, Harker R, Tustin S, Johnston J. 2010. Fruit dry matter concentration: a new quality metric for apples. J Sci Food Agric 90:2586–2594. doi:10.1002/jsfa.4125.
- Pérez-Romero LF, Suárez M, Dapena E, Rallo P. 2015. Molecular and morphological characterization of local apple cultivars in Southern Spain. Gen Mol Res. 14(1):1487–1507. doi:10.4238/2015.February.20.4.
- Racskó J, Miller DD, Duarte EE, Szabó Z, Soltész M, Nyéki J, Szukics J. 2009. Is consumer preference for apple driven only by fruit quality? Acta Hortic 831:331–338. doi:10.17660/ActaHortic.2009.831.40.
- Reig G, Blanco A, Castillo AM, Gogorcena Y, Moreno MA. 2015. Phenotypic diversity of Spanish apple (Malus x domestica Borkh) accessions grown at the vulnerable climatic conditions of the Ebro Valley, Spain. Sci Hort. 185:200–210. doi:10.1016/j.scienta.2015.01.024.
- Rivero R, Sønsteby A, Heide OM, Måge F, Remberg SF. 2017. Flowering phenology and the interrelations between phenological stages in apple trees (Malus domestica Borkh.) as influenced by the Nordic climate. Acta Agric Scand B Soil Plant Sci. 67(4):292–302.
- Rufato L, da Silva PS, Kretzschmar AA, Bogo A, de Macedo TA, Welter JF, Fazio G, Petry D. 2021. Geneva® series rootstocks for apple trees under extreme replanting conditions in southern Brazil. Front Plant Sci. 12:712162. doi:10.3389/fpls.2021.712162.
- Samuolienė G, Čeidaitė A, Sirtautas R, Duchovskis P, Kviklys D. 2016. Effect of crop load on phytohormones, sugars, and biennial bearing in apple trees. Biol Plant. 60:394–400. doi:10.1007/s10535-015-0581-3.
- Shewfelt RL. 1999. What is quality? Postharvest Biol Technol 15:197–200.
- Sikorskaite S, Gelvonauskiene D, Stanys V, Baniulis D. 2012. Characterization of microsatellite loci in apple (Malus × domestica Borkh.) cultivars. Zemdirbyste-Agric. 99:131–138.
- Singleton VL, Orthofer R, Lamuela-Ravent ́os RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 299:152–178. doi:10.1016/S0076-6879(99)99017-1.
- Sæther N, Holene A, Bakkebø Fjellstad K, Frøiland C. 2021. Nøkkeltall 2020 fra Norsk genressurssenter. NIBIO-rapport. 7(107):136.
- Tancred SJ, Zeppa AG, Cooper M, Stringer JK. 1995. Heritability and patterns of inheritance of the ripening date of apples. HortScience. 30(2):325–328. doi:10.21273/HORTSCI.30.2.325.
- Testolin R, Foria S, Baccichet I, Messina R, Danuso F, Losa A, Scarbolo E, Stocco M, Cipriani G. 2019. Genotyping apple (Malus × domestica Borkh.) heirloom germplasm collected and maintained by the Regional Administration of Friuli Venezia Giulia (Italy). Sci Hortic 252:229–237. doi:10.1016/j.scienta.2019.03.062.
- Tóth M, Kása K, Szani ZS, Balikó E. 2004. Traditional old apple cultivars as new gene sources for apple breeding. Acta Hortic. 663:609–612. doi:10.17660/ActaHortic.2004.663.107.
- Tworkoski T, Miller S. 2007. Rootstock effect on growth of apple scions with different growth habits. Sci Hortic. 111(4):335–343. doi:10.1016/j.scienta.2006.10.034.
- UPOV. 2005. Apple UPOV code(s): Malus domestica Borkh. guidelines for the conduct of test for distinctness, uniformity and stability. Geneva. [accessed 2021 May 18]. https://www.upov.int/.
- Vimic AV, Mandic MV, Fotirić Akšić M, Vukicevic K, Meland M. 2023. Climate potential for apple growing in Norway—part 1: zoning of areas with heat conditions favorable for apple growing under observed climate change. Atmosphere. 14:993. doi:10.3390/atmos14060993.
- Volz RK, Oraguzie NC, Whitworth CJ, How N, Chagné D, Carlisle CM, Gardiner SE, Rikkerink EHA, Lawrence T. 2009. Breeding for red flesh colour in apple: progress and challenges. Acta Hort. 814:337–342. doi:10.17660/ActaHortic.2009.814.54.
- Wicklund T, Guyot S, Le Quéré JM. 2021. Chemical composition of apples cultivated in Norway. Crops. 1:8–19. doi:10.3390/crops1010003.