Abstract
The uptake of micronutrient cations in relation to varying activities of Mn2+ was studied for barley (Hordeum vulgare L. var. Thule) and oat (Avena sativa L. var. Biri) grown in chelator buffered nutrient solution. Free activities of Mn2+ were calculated by using the chemical speciation programme GEOCHEM-PC. Manganese deficient conditions induced elevated concentrations of Zn and Fe in shoots of both species. The corresponding antagonistic relationship between Mn and Cu could only be seen in barley. The observed antagonistic relationships were only valid as long as the plant growth was limited by Mn deficiency. The Mn concentration in both plant species increased significantly with increasing Mn2+ activity in the nutrient solution. The concentration of Mn in the shoots of oat was higher than for barley except under severe Mn deficiency where it was found equal for both species. Manganese was accumulated in the roots of barley at high Mn2+ activity. The different shoot concentrations of Mn between barley and oat are therefore attributed to the extent of Mn translocation from roots to shoots. Manganese deficiency induced a significant increase in the shoot to root ratio in both species.
1 Introduction
Manganese in an essential micronutrient for normal growth and development of plants. Manganese deficiency in cereals is a widely reported constraint for plant growth (Hannam et al., Citation1987; Palotta et al., Citation2000), frequently occurring in crops grown on neutral and calcareous soils (Reisenauer, Citation1988). Low plant availability of Mn is mainly caused by the formation of insoluble Mn-oxides, which constitute the dominant fraction of Mn in soils (Reisenauer, Citation1988). Hydrated free Mn2+ is the dominant species in soil solution, due to the low stability between Mn and most organic and inorganic ligands (Norvell, Citation1988). Several studies have been carried out to investigate the relationships between Mn fertilization and the composition of micronutrient cations in plants, and interactions between Mn and Fe have been reported (Warden & Reisenauer, Citation1991). However, studies using chelator-buffered nutrient solutions where free activities of micronutrients can be controlled are limited. It is difficult to experimentally impose constant and controlled activity levels of Mn in soils due to the kinetics of the reactive processes in the soil solution which control Mn partitioning between the solid and aqueous phases (Gilkes & McKenzie, Citation1988). Therefore, the approach adopted here used a series of chelator-buffered nutrient solutions with increasing free activity of Mn2+ to investigate potential interactions with other micronutrient cations. Such nutrient solutions are suitable media for simulating elemental activity in the interactive relationship between plant root and soil (Parker & Norvell, Citation1999). The use of excess chelator allows the buffering of micronutrient activities, at low levels similar to those found in the soil solution, where the solid phase buffers ionic activities in the solution (Rengel, Citation2002). The present study was conducted to investigate the effect of free Mn2+ activity on yield and uptake of micronutrient cations in barley and oats.
2 Materials and methods
2.1 Nutrient solutions
Basal hydroponics solutions were prepared with double deionised water and contained 2 mM Ca(NO3)2, 1 mM KNO3, 80 μM KH2PO4, 0.5 mM MgSO4, 0.01 mM H3BO3, 0.9 mM NaOH, 75 μM Fe(NO3)3, 8.0 μM ZnCl2, 2.0 μM CuCl2, 0.1 μM NiCl2, and 0.1 μM Na2MoO4. Six different concentrations of MnCl2 applied were: 0.01875 μM (1), 0.0375 μM (2), 0.15 μM (3), 0.30 μM (4), 0.60 μM (5), and 1.20 μM (6). The nutrient solution pH was buffered at 6.0 with 1.0 mM 2-[N-Morpholino]ethanesulfonic acid (MES), and was maintained by daily adjustments with 1 M HCl. The nutrient solutions in the pots were replaced every third day throughout the experimental period. Fixed free metal activities for (Fe3+)=10−16.5, (Cu2+)=10−13.2, (Zn2+)=10−9.8, (Ni2+)=10−14.2, calculated with Geochem-PC 2.0 (Parker et al., Citation1995a), were obtained in the nutrient solutions by addition of HEDTA [N-(2-hydroxyethyl)-ethylenediamine-triacetic acid] at a concentration equal to the sum of the trace metal concentrations, plus a 25-μM excess (Parker et al., Citation1995b; Rengel, Citation1999; Pedler et al., Citation2000).
The activity of the different Mn2+ treatments, in increasing order, was calculated to (Mn2+)1=10−9.3, (Mn2+)2=10−9.0, (Mn2+)3=10−8.4, (Mn2+)4=10−8.0, (Mn2+)5=10−7.7, and (Mn2+)6=10−7.4.
2.2 Plant growth
Seeds of 6-row barley (Hordeum vulgare L. var. Thule) and oat (Avena sativa L. var. Biri) were surface-sterilised with 1% sodium hypochlorite and germinated in germination papers soaked with 0.01 M Ca(NO3)2 at 20°C. Four days after initiation of the germination process, the seedlings were transferred to nutrient solutions. Plants were grown in mesh-bottomed cups, with plastic beads covering the base of the cups. The cups were fitted onto pots containing 2.8 litres of nutrient solution. To avoid light entering the photosensitive nutrient solution, the pots were covered with black cloth bags. After establishment, seedlings were thinned, leaving 15 uniform plants in each cup. The plants were grown under environmentally controlled conditions for 22 days after the transfer to the nutrient solution. The temperature was kept at 21°C during the day and 16°C at night with a relative humidity of 55% and 65%, respectively. The photoperiod was set at 16 h day and 8 h night with an approximate photon fluence rate of 220 μE m−2 s−1. The Mn content of the seeds was 14.4 and 43.8 mg kg−1 for barley and oat, respectively. Taking seed weight into account, the seeds of barley and oat could account for a total input of 7.1 and 18.5 μg Mn pot−1, respectively.
2.3 Plant harvest and analysis
Plants were harvested 22 days after transfer to the pots. After harvest, they were divided into shoots and roots, rinsed thoroughly with double deionised water, and dried at 80°C for 48 hours. Shoot and root dry weights were recorded. The dried samples were milled, dry ashed and dissolved in 40% aqua regia (Jones & Case, Citation1990). The concentration of Cu, Fe, Mn and Zn in the digested solutions for shoots and Mn for roots was measured by atomic absorption spectrophotometry (Perkin-Elmer 2380 Atomic Absorption Spectrophotometer).
2.4 Statistical analysis and software used
The experiment included three replicate observations for each factor combination. Statistical analysis was conducted by Minitab Statistical Software 13.0, at a significance level of 95%. The analysis of variance by general linear model procedure was performed for assessing the variation in yield and elemental composition. Tukey simultaneous test and two-sample t-tests were used for comparisons of means. Graphical presentations were performed by Microsoft Excel 2000 and Minitab Statistical Software 13.0, and the speciation modelling of the nutrient solutions was processed by GEOCHEM-PC 2.0 (Parker et al., Citation1995a).
3 Results
3.1 Deficiency symptoms and biomass allocation
Deficiency symptoms in both cultivars developed only in the treatments with activity levels Mn2+ 1 and Mn2+ 2. Deficiency symptoms appeared 10 and 14 days after transfer of the seedlings to the pots for barley and oat, respectively. Based on visual observations, the first characteristic reaction to Mn deficiency was reduction in shoot elongation, followed by a general leaf chlorosis. Typical deficiency symptoms similar to those described by Bergmann & Neubert (Citation1976) appeared in both cultivars as necrotic spots with brown margins in younger leaves. The symptoms appeared to be most severe in the intermediate and youngest leaves. As the experiment progressed, the deficiency symptoms developed more severely, and showed total necrosis of the leaf tips. No symptoms of Mn toxicity were noticed in the treatments with the highest activity levels of Mn.
The dry matter yield of both crops at different levels of free Mn activity in the solution is given in . The yield of barley shoots at the activity level of Mn2+ 1, Mn2+ 2, and Mn2+ 3 was significantly lower as compared to the higher activity levels. Although no visual deficiency symptoms could be observed at the activity level of Mn2+ 3, a significant growth reduction of the shoots was observed in this treatment. For oat, only the activity levels of Mn2+ 1 and Mn2+ 2 showed significantly lower yields compared to the higher activity levels (). For the root biomass of both species, only the two lowest Mn activity levels caused a reduction in growth. Manganese deficiency induced a consistent increase in the shoot/root ratio for both species, but the increase was more pronounced in barley than oat ().
Total dry matter yield [DW] per pot (15 plants grown for 22 days) and shoot/root ratio at different Mn2+ activity levels
3.2 Concentration and uptake of Cu, Fe, Mn and Zn
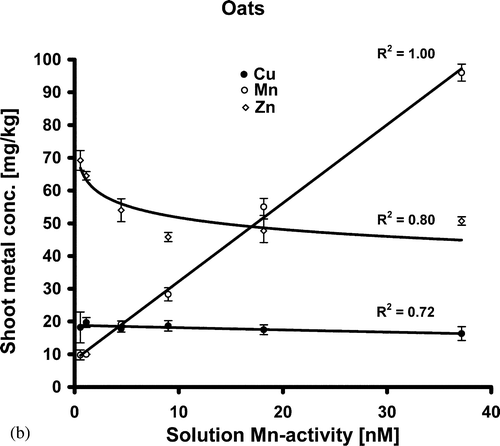
The concentrations of Cu, Mn and Zn in shoots as a function of Mn activity are given in a for barley and b for oat. The concentration of Mn in the shoot tissues of both species correlated closely with the Mn activity level in the nutrient solution (r 2=1.00). For oat, the relationship between Mn activity and its concentration in the shoot tissue was linear in the activity range used in this experiment. The corresponding relationship for barley was non-linear, showing that the Mn uptake for barley at higher Mn activities in the nutrient solution did not increase at the same rates as at lower Mn activity levels (a). At the higher (>Mn3) activity levels, oat showed significantly higher concentrations of Mn in the tissue as compared to barley. The concentration of Zn in shoot tissue of both species was reduced with increased Mn activity in the solution, only in the lower range (<Mn3) where an antagonistic relationship to the Mn supply was evident (a and 1b). The elevated concentrations of Zn in both species is attributed to the declined biomass production under Mn deficiency, since the concentration of Zn in shoots only at Mn2+ 1 and Mn2+ 2 levels was significantly higher than at the higher Mn activity levels. Thus, the antagonism between Zn and Mn is pronounced only when the Mn supply is restricted, resulting in a biomass decline. The Cu concentration in shoots of barley was higher at Mn2+ 1 than at the other Mn2+ activity levels, while Cu in oat did not show any statistically significant relationship to the Mn activity (a and 1b). Iron showed the same response to Mn supply as Zn, however a statistically significant increase in Fe concentration was observed only at the lowest Mn level (Mn1) for both species (). The total uptake of Mn increased steadily in response to Mn supply (). The total uptake of Cu and Zn by both species was significantly lower in plants suffering from Mn deficiency. The same trend was shown for Fe in barley, but the effect was less consistent in oat. Manganese concentration in roots of both species increased consistently with increased Mn activity in the solution (). Two-sample t-test showed a significantly higher Mn concentration in barley roots than in oat roots, especially at the higher Mn activity levels (>Mn4) (). At the highest Mn activity (Mn6) in the solution, the Mn concentration in barley roots was more than 5 times higher than in roots of oat.
Total uptake of micronutrient cations by shoots [μg pot−1] at different Mn activity levels
4 Discussion
The clear antagonistic relationship between Mn supply and the concentration of Fe, Mn and Zn in shoots of both species is attributed to the decline in biomass production under Mn deficiency, since no significant differences in their concentrations could be observed in the adequate range of Mn activities. Since the total uptake of Cu, Fe and Zn at the four highest activities of Mn also was not statistically different (), no significant interactions between Mn and the other micronutrient cations could be observed as long as the biomass production was not restricted by the Mn2+ supply. The antagonisms are therefore only significant in the deficient Mn2+ range. Most previous studies have focused on the antagonistic interaction between Mn and Fe. A review by Warden & Reisenauer (Citation1991) summarises 18 studies investigating Mn-Fe interactions. They concluded that the level of Mn-Fe interactions were dependent both on the media in which plants were grown, and the species studied. Solution cultures tended to give a mutually antagonistic interaction, sand no interaction, and soil a synergistic relationship between Mn and Fe. Webb et al. (Citation1993) argued later that most of the antagonistic interactions between Mn and Fe reported in previous studies of plants grown in nutrient solution involve the inhibition of Fe absorption by high levels of Mn. Since primary cell walls of plants consist of a network of glycoprotein and celluloses, free carboxylic groups will act as a cation exchanger where cations can accumulate at a non-metabolic level (Mukhopadhyay & Sharma, Citation1991). The negative electrical field found in the plasma membrane of root hair cells attracts divalent cations to enter the cells from the outside solution (Clarkson, Citation1988). Therefore, antagonistic reactions may take place if the soil or nutrient solution is highly unbalanced with respect to micronutrient cations.
Since most studies in nutrient solution were carried out prior to the introduction of computer speciation programmes, the activities of micronutrients have necessarily been difficult to control at exact levels. In well-buffered systems, i.e. soil or chelator- buffered nutrient solutions, antagonistic relationships between Mn and micronutrient cations will probably not take place as long as Mn2+ supply is sufficient at activity levels normally found in soil solution. In the study by Webb et al. (Citation1993) little or no effect could be found on shoot concentration of Cu, Fe and Zn as a response of increasing Mn-activity to barley (cv. Herta) grown in HEDTA buffered nutrient solution. When Mn2+ activity is maintained at moderate levels equal to those normally found in soil solution, our results are in agreement with those of Webb et al. (Citation1993). Since the antagonism seen in our experiment is clearly based on a general accumulation of micronutrient cations under Mn2+ deficiency, it may indicate that there is no specific physiological explanation at a metabolic level for the observed antagonism that was non-specific with regard to micronutrient cations.
The Mn accumulation in barley roots at higher supply (>Mn4) can either be attributed to a retention processes, or to a regulating mechanism retaining Mn in the root cells preventing effective xylem loading to the shoot. Oat has generally a higher Mn requirement compared to barley in terms of Mn tissue concentrations required for normal development (Aasen, Citation1997). More effective translocation mechanism of Mn may satisfy a high requirement for Mn. The translocation rate of Mn from the roots may be critical for the utilisation of available Mn. In the present study, there was a strong linear relationship (r 2=1.00) between the Mn concentration in shoots of oat and the Mn activity in the solution, suggesting that passive and unrestricted uptake took place in the whole range of Mn activity tested. However, the absence of regulated uptake can lead to Mn toxicity under conditions of high Mn availability. High tolerance to Mn toxicity is associated with restricted absorption by roots, restricted translocation of excess Mn to shoots, or tolerance to high Mn levels within the plant tissue (El-Jaoual & Cox, Citation1998). In the present experiment, the shoot tissue concentration of Mn was lower than that normally associated with Mn toxicity (Williams & Vlamis, Citation1957), and hence no detrimental effects of high Mn activity could be observed.
It is assumed that Mn transportation in the xylem occurs as free Mn2+ without the complexity of the low molecular organic acids or amino acids commonly seen as a pathway for several other micronutrients (Grusak et al., Citation1999). Compared to other nutrients, Mn has a rather low translocation rate in plants (Mengel & Kirkby, Citation1987; Pearson and Rengel, Citation1994), and older leaves usually contain a higher amount of Mn compared to younger ones (Singh & Steenberg, Citation1974). The deficiency symptoms observed in intermediate and younger leaves in this study suggest that Mn was not translocated at a sufficient degree to support normal development of the younger leaves. The reason for the low mobility of Mn from older to younger leaves is not very clear. Grusak et al. (Citation1999) suggested that immobility can be attributed to non-exchangeable incorporation of Mn into high-molecular compounds or structures within the cell, or to the requirement for chelates in the phloem loading. While the micronutrients mobilised in roots can be distributed both in the xylem and phloem tissues, their mobilisation from leaves involves only the phloem (Grusak et al., Citation1999). Phloem loading is therefore critical for the redistribution of Mn from older to younger plant organs.
Since oat is considered to be sensitive to Mn deficient conditions (Timonin, Citation1946; Graham, Citation1988) and barley fairly tolerant (Webb et al., Citation1993), a stronger growth depression for oat would have been expected in the deficient range if the hydroponics system had been able to exactly simulate rhizospheric conditions. Clearly, there are factors other than the uptake and translocation of Mn that need to be considered in assessing the capacity for growth under Mn deficient conditions. The rhizospheric influence is thought to play a major role for Mn availability. Timonin (Citation1946) subdivided roughly the variation of plant available Mn into three major groups: 1) physical and chemical aspects of Mn deficiency in soil, 2) variation among plant genotypes, and 3) microbial activity related to Mn deficiency. Hydroponics can therefore be suitable for the study of plant physiological interactions with Mn nutrition, but can not take into consideration actual rhizospheric interactions. In a study by Huang et al. (Citation1994), Mn-efficiency among barley varieties was only expressed when grown in soil, and not when the same varieties were grown in chelator-buffered nutrient solution at pH 6.0, either in terms of dry matter production or tissue Mn concentration. Mn efficiency investigated in hydroponics systems does not therefore necessarily reflect the varietal differences seen in soil-plant systems. The mechanisms responsible for cultivar differences in manganese efficiency are not known (Graham, Citation1988), and probably involve many factors. In a study of barley genotypes, Huang et al. (Citation1993) suggested that Mn concentration in roots of Mn-efficient plants may be related to Mn immobilisation in roots, and that this phenomenon may be a factor in the mechanism of Mn efficiency.
Using dry matter yield of the shoots as a single criterion, this study obtained a critical activity of Mn2+ in the range 10−8.0 (barley) to 10−8.4 (oat). The critical Mn activity required for optimal growth obtained in this experiment corresponded well with the results obtained by Webb et al. (Citation1993). They estimated a critical Mn activity of 10−8.3 for barley (cv. Herta). The difference in critical activity for barley and oats in the present short time experiment cannot state any significant difference in the Mn2+ activity requirement among these two cereal species, because the concentration of Mn in the seeds might play a significant role when determining critical activities in an extremely narrow activity range. The seed concentration of Mn was approximately three times higher in oats than in barley. Therefore, to test and compare the critical Mn activities for different species requires a set of varieties of each cereal species, and falls beyond the scope of this experiment.
The shoot to root ratio increased for both species under Mn deficiency. The detrimental effect of Mn deficiency on roots indicates a priority of Mn for the development of shoots. The development of this ratio is dependent both on species and the type of mineral stress (Marschner, Citation1995). In contrast to Mn deficiency, Zn deficiency has the opposite effect on the development of this ratio. Observations made under Zn deficiency for wheat and barley (Lombnæs & Singh, Citation2003), showed that the shoot to root ratio decreased under Zn deficiency.
To study interactions between micronutrients, the chelator buffering technique is critical with respect to obtain controlled activities of cations. However, the major limitation of this approach may be the lack of rhizospheric processes, which play an important role for Mn nutrition in plants grown under field conditions.
References
- Aasen I. Deficiency diseases in crop plants Landbruksforlaget: Oslo, Norway (in Norwegian) 1997
- Bergmann W. Neubert P. Pflanzendiagnose und Pflanzen-analyse. 1. Auflage Gustav Fischer Verlag: Jena, Germany 1976
- Clarkson D. T. The uptake and translocation of manganese by plant roots, Manganese in soils and plants Graham R. D., Hannam R. J., Uren N. C. (eds) Kluwer Academic Publishers: Dordrecht, The Netherlands 1988 101 110
- El-Jaoual , T. and Cox , D. A. 1998 . Manganese toxicity in plants . J. Plant Nutr. , 21 : 353 – 386 .
- Gilkes R. J. McKenzie R. M. Geochemistry and mineralogy of manganese in soils, Manganese in soils and plants Graham R. D., Hannam R. J., Uren N. C. (eds) Kluwer Academic Publishers: Dordrecht, The Netherlands 1988 23 35
- Graham R. D. Genotypic differences in tolerance manganese deficiency, Manganese in soils and plants Graham R. D., Hannam R. J., Uren N. C. (eds) Kluwer Academic Publishers: Dordrecht, The Netherlands 1988 261 276
- Grusak , M. A. , Pearson , J. N. and Marentes , E. 1999 . The physiology of micronutrient homeostasis in field crops . Field Crop Research , 60 : 41 – 56 .
- Hannam , R. J. , Riggs , J. L. and Graham , R. D. 1987 . The critical concentration of manganese in barley . J. Plant Nutr. , 10 : 2039 – 2048 .
- Huang , C. , Webb , M. J. and Graham , R. D. 1993 . Effect of pH on Mn absorption by barley genotypes in a chelate-buffered nutrient solution . Plant Soil , 155/156 : 437 – 440 .
- Huang , C. , Webb , M. J. and Graham , R. D. 1994 . Manganese efficiency is expressed in barley growing in soil systems but not in solution culture . J. Plant Nutr. , 17 : 83 – 95 .
- Jones J. B. Case V. W. Sampling, Handling, and Analyzing Plant Tissue Samples, Soil Testing and Plant Analysis Westerman R. L. (ed.) Soil Science Society of America: Madison, Wisc., USA 1990 389 427
- Lombnæs , P. and Singh , B. R. 2003 . Varietal tolerance to Zinc deficiency in wheat and barley grown in chelator-buffered nutrient solution and its effect on uptake of Cu, Fe, and Mn . Z. Pflanzenernärhr. Bodenk. , 166 : 76 – 83 .
- Marschner H. Mineral nutrition of higher plants 2nd edition Academic Press Limited: London, England 1995
- Mengel K. Kirkby E. A. 'Principles of plant nutrition 4th edition International Potash Institute: Bern, Switzerland 1987
- Mukhopadhyay , M. J. and Sharma , A. 1991 . Manganese in cell-metabolism of higher plants . Bot. Review , 57 : 117 – 149 .
- Norvell W. A. Inorganic reactions of manganese in soils, Manganese in soils and plants Graham R. D., Hannam R. J., Uren N. C. (eds) Kluwer Academic Publishers: Dordrecht, The Netherlands 1988 37 58
- Palotta , M. A. , Graham , R. D. , Langridge , P. , Sparrow , D. H. B. and Barker , S. J. 2000 . RFLP mapping of manganese efficiency in barley . Theor. Appl. Genet. , 101 : 1100 – 1108 .
- Parker D. R. Norvell W. A. Chaney R. L. GEOCHEM-PC: A chemical speciation programme for IMB and compatible personal computers, Chemical Equilibrium and Reaction Models, Spec. Publ. No. 42 Loeppert R. H., Schwab A. P., Goldberg S. (eds) Soil Science Society of America: Madison, Wisc., USA 1995a 253 269
- Parker D. R. Chaney R. L. Norvell W. A. Chemical equilibrium models: applications to plant nutrition research, Chemical Equilibrium and Reaction Models, Spec. Publ. No. 42 Loeppert R. H., Schwab A. P., Goldberg S. P. (eds) Soil Science Society of America: Madison, Wisc., USA 1995b 163 200
- Parker , D. R. and Norvell , W. A. 1999 . Advances in solution culture methods for plant mineral nutrition research . Adv. Agron. , 65 : 151 – 213 .
- Pearson , J. N. and Rengel , Z. 1994 . Distribution and remobilisation of Zn and Mn during grain development in wheat . J. Exp. Bot. , 45 : 1829 – 1835 .
- Pedler , J. F. , Parker , D. R. and Crowley , D. E. 2000 . Zinc deficiency-induced phytosiderophore release by the Triticaceae is not consistently expressed in solution culture . Planta , 211 : 120 – 126 .
- Reisenauer H. M. Determination of plant-available soil manganese, Manganese in soils and plants Graham R. D., Hannam R. J., Uren N. C. (eds) Kluwer Academic Publishers: Dordrecht, The Netherlands 1988 87 98
- Rengel , Z. 1999 . Physiological responses of wheat genotypes grown in chelator-buffered nutrient solutions with increasing concentrations of excess HEDTA . Plant Soil , 215 : 193 – 202 .
- Rengel , Z. 2002 . Chelator EDTA in nutrient solution decreases growth of wheat . J. Plant Nutr. , 25 : 1709 – 1725 .
- Singh , B. R. and Steenberg , K. 1974 . Plant response to micronutrients. II. Uptake, distribution and translocation of manganese in maize and barley plants . Plant Soil , 40 : 647 – 654 .
- Timonin , M. I. 1946 . Microflora of the rhizosphere in relation to the manganese deficiency disease of oats . Soil Sci. Soc. Amer. Proc. , 11 : 284 – 292 .
- Warden , B. T. and Reisenauer , H. M. 1991 . Manganese-iron interactions in the plant-soil system . J. Plant Nutr. , 14 : 7 – 30 .
- Webb , M. J. , Norvell , W. A. , Welch , R. M. and Graham , R. D. 1993 . Using a chelate-buffered nutrient solution to establish the critical solution activity of Mn2+ required by barley (Hordeum vulgare L.) . Plant Soil. , 153 : 195 – 205 .
- Williams , D. E. and Vlamis , J. 1957 . Manganese and boron toxicities in standard culture solution . Soil Sci. Soc. Amer. Proc. , 21 : 205 – 209 .