Abstract
Fungi within the phylum Glomeromycota were investigated in arable fields throughout Sweden. Sweden is located between 55° and 69° North. The fungi within this phylum form arbuscular mycorrhizal symbiosis with plant roots. Sampling of soil was carried out to a depth of 30 cm in the rhizosphere. Arbuscular mycorrhizal fungi were found at all 45 sampling sites, at densities between 3 and 44 spores per g dry weight of soil. No significant differences in spore densities were found between different agro-climatic zones or between semi-natural grassland and ploughed fields. Our study revealed that the upper half (0–15 cm) of the soil profiles had significantly more spores than the lower half (15–30 cm). Spores from eight sampling sites were identified from the indigenous soils. Almost 90% were shown to belong to the genus Glomus. The other genera found were Gigaspora and Scutellospora.
Introduction
Sweden is located between 55° and 69° North and has a temperate climate except for the mountainous, north-western parts, which are subarctic. Despite the high latitudes the climate is mild due to the Gulf Stream. A division of Sweden into eight different agro-climatic zones, based on climate and soil properties, has been used in Swedish agricultural research (, ). The most common field crops grown in Sweden are leys, cereals, oilseed rape and potatoes (Yearbook of agricultural statistics, Citation2001). Sugar beet is another common crop in the most southern part of the country, while due to the colder climate ley is by far the most common crop in the north.
Fig. 1. Classification of agro-climatic zones in Sweden, by CitationStatistics Sweden, based on both climate and soil properties. Chosen sampling localities are indicated by O for ploughed fields and by Δ for semi-natural grasslands. The average barley yield ranged from approximately 5860 kg per ha in zone 1 to 1660 kg per ha in zone 8 (Yearbook of agricultural statistics 2001).
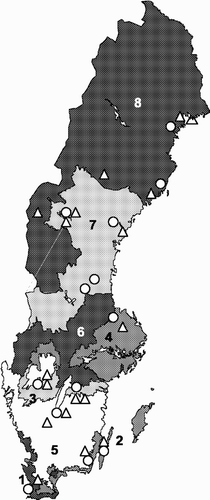
Table 1. Vegetation period and hours of daylight on 15 June for different agro-climatic zones in Sweden
The fungi within the phylum Glomeromycota are considered to be arbuscular mycorrhizal (AM) fungi, although biological knowledge is lacking for some of the described species (Schüssler et al., Citation2001). AM fungi form symbiotic associations with plants and are known to have an impact on many agricultural crops, for instance increasing their tolerance to drought (Davies et al., Citation1993), pests and diseases (Hooker et al., Citation1994; Borowicz, Citation2001). AM fungi also contribute to the uptake of phosphorus and nitrogen, both organic and inorganic, by plants (George et al., Citation1995; Hawkins et al., Citation2000). In agricultural systems the function of AM fungi is known to be reduced by systemic fungicides (Kling & Jakobsen, Citation1997), as well as disturbances such as ploughing (Kabir et al., Citation1997; McGonigle & Miller, Citation1999). Jensen & Jakobsen (Citation1980) found that high inputs of nitrogen and available phosphorus reduce the AM colonisation rate in the roots and the spore density in the soil. AM fungi are also affected by the host diversity, in that higher plant diversity results in a higher spore production (Burrows & Pfleger, Citation2002).
Since the growing period in Sweden is relatively short, the possible beneficial effects of AM fungi most probably depend on early infection, which is related to sufficient inoculum density and efficient colonisation by AM fungi in the soil. A study in Germany has shown that mycorrhizal colonisation in spring does not commence until the soil temperature has reached 5°C (Land & Schönbeck, Citation1991). At latitudes between 74–80° North in the Arctic, spores extracted from indigenous soils have been reported at the rate of 1–3 spores g−1 dry soil in 9 of 13 collected sites (Dalpé & Aiken, Citation1998). In an investigation in Finland, conducted on sites at latitudes between 61 to 68° North, AM fungi were trapped from 266 soil samples from various parts of the country using different mycorrhiza-forming plants in the greenhouse. The proportion of soil samples where AM fungi were identified decreased from close to 100% in the southern and central parts of Finland to about 50% in northern Finland (Vestberg, Citation1995). Arbuscular mycorrhizal fungi were detected more often levels under wild plants than under cultivated plants. No soil was analysed from the rhizosphere of wild plants in northern Finland (Vestberg, Citation1995). According to Miller et al. (Citation1985) the AM fungi spores detected from trap cultures are not directly comparable with those from indigenous soils. In indigenous soils in Poland, AM fungi were found in 330 out of 332 samples (Blaszkowski, Citation1993). Among roots of wild plants the spore density was 0–7 spores g−1 dry soil, whereas among roots of cultivated plants the spore density was 0–14 spores g−1 dry soil, although the average was shown to be higher for the wild plants. In three intensively managed arable fields in Germany, spore densities in the indigenous soil were estimated to be 1–6 spores g−1 dry soil (Land & Schönbeck, Citation1991). However, up to 300 spores g−1 dry soil in fields used intensively for agriculture have been reported in the United States (Kurle & Pfleger, Citation1996). Although AM fungi are believed to be among the most prevalent fungi in soils that support plant growth (Gerdemann & Nicolson, Citation1963), little is known about their distribution in agricultural soils in Sweden.
The aim of this study was to investigate the AM fungi distribution in arable fields representative of different agro-climatic zones in Sweden, with a distance of 1300 km between the most northerly and most southerly sampling sites. A second objective was to study if there was a lower incidence of spores, and lower spore densities, in intensively managed fields compared to fields with less intensive management.
Materials and methods
Sampling sites
Soil samples were collected from different agro-climatic zones throughout Sweden. Samples were taken from both ploughed and unploughed agricultural fields, i.e. semi-natural grasslands, within each zone. In total, 45 different sites were chosen for soil sampling within 31 sampling localities (, Appendix 1). Most of the ploughed fields were long-term plant nutrition trials (Carlgren & Mattsson, Citation2001), and the samples were taken from experimental plots which were cultivated using agricultural practices common for each area. The ploughed fields were divided into three groups. One group (referred to as monoculture) had a history consisting solely of various small grains. The remaining ploughed fields had varied cropping histories. These were divided according to ploughing history. Fields with a different crop each year (implying ploughing every year) were referred to as mixed. If ley was present for at least two successive years in the crop-rotation, the field was classified as ley-mixed. The semi-natural grasslands (referred to as SNG) had a high diversity of flora and were either pastures or cutting fields, and had been managed in a traditional manner for decades, although the cultivation continuity varied between the sites. Thus, the only sowing arose from self-seeding, with no disturbance by ploughing, and no external inputs of fertilizers or pesticides.
Soil sampling procedure
Soil sampling was carried out at the time when ear emergence was complete for cereals, i.e. from the end of June to the end of July, depending on the locality. At each site six subsamples were taken. For ploughed fields on farms, the soil samples were randomly collected along a transect over the fields; for experimental fields, the samples were randomly taken by reaching from the edges of the plots, avoiding disturbance of the experiment, and for semi-natural grasslands the samples were taken randomly over the field with an emphasis on not taking all the samples from soil with the same host species. For sampling a soil corer was inserted down towards the plant roots, at an angle of 70° to the soil surface, to a depth of 30 cm. The diameter of the soil core was 2.5 cm. Each sample was carefully transferred to a plastic bag of similar size, thereby avoiding disturbance of the soil texture. All samples were allowed to air dry by perforating the plastic bags with small holes before the samples were inserted. Samples from each field were kept separately. The samples were stored at 20°C for two to four months before analysis. Before further processing, the upper halves of the soil samples from each field were pooled to make one sample per field for analyses, while the same was done for the lower halves.
Spore extraction and identification
All soil samples were analysed for their AM fungi spore content, by modification of methods for wet sieving (Gerdemann & Nicolson, Citation1963) and centrifugation (Walker et al., Citation1982). Of each pooled sample, 30 g soil was suspended in 400 ml of tap water and stirred for 10–30 min, depending on the type of sample. The soil suspension was then wet-sieved through a series of sieves of three different pore sizes, 0.85 mm, 0.30 mm and 0.063 mm. Wet sieving was carried out by washing in aerated lukewarm running tap water. The 0.85 mm sieve was checked for spores adjacent to or inside roots, large spores, spore clusters, and sporocarps. The contents from the two sieves with the smallest pore sizes were pooled and centrifuged at 2000 rpm at +4°C for 2 min. The supernatant was discarded and a 70% sucrose solution was added carefully to the sediment before centrifuging again. The sucrose fraction thus containing AM fungal spores was then sieved through a 0.045 mm sieve and rinsed thoroughly with running aerated water. The spore-containing fraction was then vacuum-filtered through a filter paper of pore size 0.8 μm (Pall Corporation, USA). The spores were thus retained on the filter paper and were stored at +8°C before counting under a compound microscope. The data on quantity of spores were transformed to give the results per g dry matter of soil.
To obtain an overall view of the diversity of AM fungi in different types of arable land in Sweden, we identified the spores from eight different sampling sites (two each from SNG, monoculture, mixed and ley-mixed). The spores were mounted on microscope slides as described by Schenck & Pérez (Citation1990) and identified to the level of genus or species. Identifications were based on current species descriptions and identification manuals (Schenck & Pérez, Citation1990; CitationInternational Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi [http://invam.caf.wvu.edu/fungi/taxonomy/speciesID.htm]). The morphological variables used for identification of spores were: 1) occurrence of sporocarp, its shape, colour and size, 2) occurrence of peridium and its characteristics, 3) colour of spores, their size and shape, 4) number of spore walls, their colour, thickness and ornamentation and 5) attachment of spores, shape and type of occlusions. The measurements of wall thickness and spore- and hyphae-diameter were made using an image analyser (Image Pro-Plus 4.1, Media Cybernetics, USA) attached to a video camera (JVC colour TK-1280 E) and a microscope (Olympus BH2).
Chemical and physical analysis of the soils
The upper and lower parts of the soil samples from each field were mixed. One portion was analysed for phosphorus content (Egnér et al., Citation1960). To obtain more background information about the soils they were also analysed for carbon, nitrogen, pH and clay. Another portion was dried at 105°C for 4 h to obtain the dry matter content. The analyses for pH were performed with a soil: water extraction ratio of 1: 2. Total carbon and nitrogen were determined after dry combustion on a C, N autoanalyser (LECO, USA).
Statistical analysis
The relationships between spore numbers of AM fungi and agro-climatic zone, crop rotation, crop and physical and chemical analyses were assessed by simple correlation analysis and by multivariate statistics (Snedecor & Cochran, Citation1978), using Systat 5.2.1 (http://www.systat.com) and SAS (http://support.sas.com/rnd/app/da/stat.html). The relationship between the spore density of the upper and lower parts of the soil profiles was analysed by paired t-test (Snedecor & Cochran, 1978) using SAS.
Results
AM spore density in soil
Arbuscular mycorrhizal fungal spores were present in all soil samples. The density of spores varied between 3 and 44 spores g−1 dry soil (). The highest densities were found in semi-natural grasslands, although there was no statistical evidence for more spores in the semi-natural grassland than in ploughed fields. Statistical analyses showed a correlation between the upper and lower part of the same soil. The average number of spores in the upper horizon was estimated to be 19 spores g−1 dry soil. This is higher (P<0.001) than for the lower horizon, which had a mean of 15 spores g−1 dry soil. No correlation was found between spore densities and agro-climatic zone, crop, crop rotation, chemical or physical properties of the soils.
Table 2. Number of arbuscular mycorrhizal spores per g dry matter of soil at different sampling sites in Sweden1. At each site the density is given for two depths in the soil profile, 15–30 cm and 0–15 cm. The samples were taken when ear emergence was complete
Chemical and physical analysis of soils
Detailed information about the results of the physical and chemical analysis is presented in Appendix 1. The semi-natural grasslands had, with only few exceptions, lower soil phosphorus contents than the ploughed fields, with mean values of 0.37 mg P 100 g−1 dry matter of the soil (DM) for the easily available proportion, and 27 mg P 100 g−1 DM for the less soluble proportion. These levels can be compared with the mean values for soil phosphorus in the ploughed fields, which were 7.4 mg P 100 g−1 DM for the easily available proportion, and 67 mg P 100 g−1 DM for the less soluble proportion.
Diversity of AM fungi
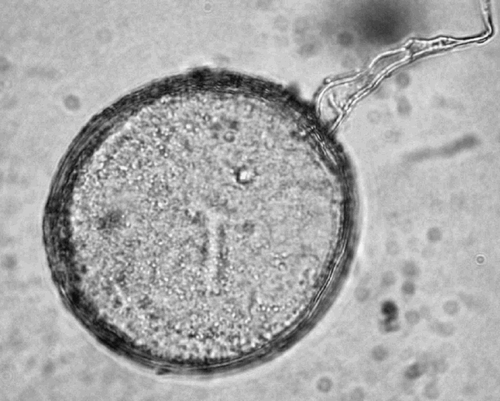
The results from the diversity study of the eight analysed soil samples are shown in . Of the identified 35 spore types, 31 belonged to the genus Glomus, two to the genus Scutellospora () and two to the genus Gigaspora. Within the genus Glomus, three spore types could be identified to species level (, –). Spores with a close resemblance to Glomus intraradices were observed inside root parts found among the spores on the filter paper at sampling site no. 18, i.e. Arbrå, which was cereals monoculture. Four soil samples each contained three spore types, while the other four contained five to seven spore types per sample.
Fig. 2. Crushed spore of Scutellospora sp. showing the bulbous suspensor, the germination shield, the spore wall and an inner wall.
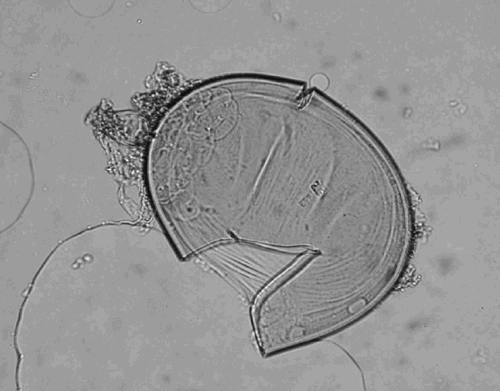
Fig. 3. A part of a sporocarp of Glomus sp. The size of the sporocarps ranged between 193 and 251 μm, and the diameter of the spores ranged between 57 and 84 μm.
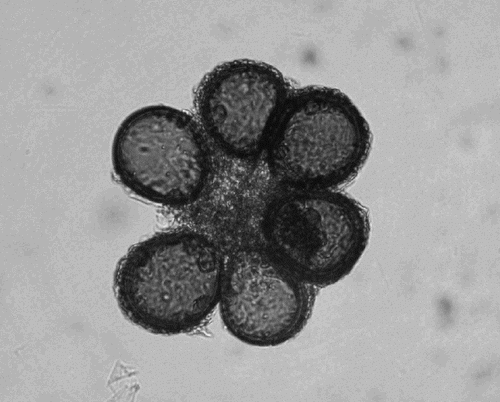
Fig. 4. Crushed spore of Glomus caledonium showing two of its four wall layers; a thin colourless outer layer and a yellow inner layer. The diameter was 307 μm. The subtending hypha was observed to have a ‘septum’ at the point of attachment, although not seen in this photograph.
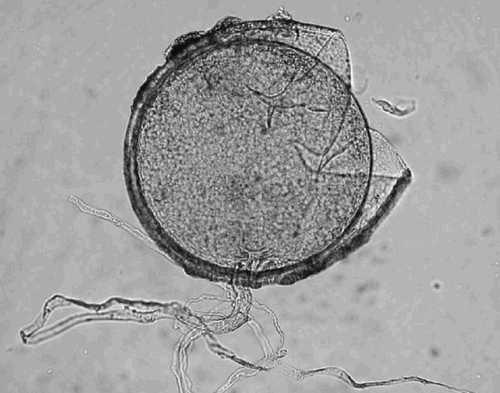
Fig. 5. An intact spore of Glomus geosporum. The diameter of the spores of this species ranged between 129 and 165 μm. None of the spores showed the construction of the subtending hypha at the spore base, which is a usual feature of the resembling species G. constrictum.
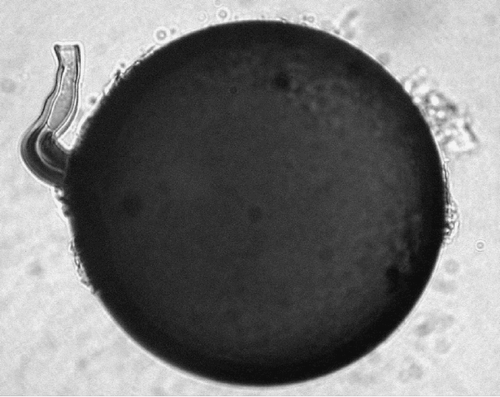
Table 3. Diversity of arbuscular mycorrhizal spores at eight different sites in Sweden
Discussion
This study reveals that AM fungi are present in a wide range of arable fields in Sweden, and were found at all sampling sites, including more intensively managed ploughed fields and semi-natural grasslands. The spore densities found in our study are relatively high, ranging from 3 to 44 spores g−1 dry soil, with more than half of the samples containing above 15 spores g−1 dry soil. Our survey further shows that there are significantly more AM spores in the upper half than the lower half of the top 30 cm of soil profiles. This relationship was not affected by ploughing.
In the neighbouring country of Finland, spores were only found in half of the indigenous soils (Vestberg, Citation1995). The reason might be that the samples in Finland were collected later in the season (25 August to 9 October), since sporulation of AM fungi differ in their seasonality (Walker et al., Citation1982). However, after trapping, AM fungi were identified in almost 100% of the soils from southern and central parts of Finland but in only 50% of the samples from the northern part of the country (Vestberg, Citation1995). The most northern sampling localities in Finland were at higher latitudes than in our study. At even higher latitudes (74–80° North), Dalpé & Aiken (Citation1998) found AM fungi at densities of 1–3 spores g−1 soil in 70% of the sampling localities. In the more southern country Poland, spores were found in almost all sampling sites at densities up to 14 spores g−1 dry soil. This suggests that the presence of AM fungi might be affected by latitude, but the lack of detection at high latitudes might also be due to the number of samples taken, along with other factors, such as edaphic differences and existing plant species (Bever et al., Citation2001; Johnson et al., Citation1991; Blaszkowski, Citation1993).
The high number of spores in our study can be compared with the study by Blaszkowski (Citation1993) in which less than one spore g−1 dry soil was found on average, among roots of wheat, barley and oat, which were among the most frequently examined plant species. In a study in three agricultural fields in Germany, AM fungi had a peak in sporulation towards the maturity of the host plant, during the two growing periods studied (Land & Schönbeck, Citation1991), which was therefore the time chosen for the sampling in our study. However, the maximum spore density in Germany was less than 6 spores g−1 dry soil. Low densities may be due to the phosphorus content, which was reported as ‘high’ in their study. Cumulative P fertilization has been shown to decrease the spore density in Northern European field conditions (Jensen & Jakobsen, Citation1980; Mårtensson & Carlgren, Citation1994; Kahiluoto et al., Citation2001). A moderate increase of phosphorus fertilizers did not affect the spore density, while an exclusion of phosphorus fertilization doubled the spore density within a few years (Mårtensson & Carlgren, Citation1994). The spore densities found in the study by Jensen & Jakobsen (Citation1980) in Denmark were up to 7 spores g−1 dry soil, and in Finland up to 2 spores g−1 dry soil were found (Kahiluoto et al., Citation2001). One of the two localities studied by Mårtensson & Carlgren (Citation1994) is the same as in our study (see Offer, Appendix 1), although the specific site is not the same. They found up to 3 spores g−1 dry soil in the sites without additional phosphorus, down to almost zero in sites with a higher level of easily available P, compared with our two sites in the same locality. For semi-natural grassland sites, the nutrient status probably affects the amount of AM fungi less than management continuity and species diversity of the vegetation. This is indicated in another study in Sweden by Eriksson (Eriksson, Citation2001) who investigated colonisation of AM fungi in roots.
Significantly higher numbers of spores in the upper half of the soil profiles does not necessarily mean that the colonisation of roots also declines down the profile. Nehl et al. (Citation1999) found that although the propagules as detected by a bioassay decreased down the profile, the mycorrhizal colonisation of roots was moderate to heavy throughout the soil profile. Other similar studies also suggest a rapid decline of spore density or whole propagule density down the profile (Jakobsen & Nielsen, Citation1983; Abbot & Robson, Citation1991). Apart from the correlation between density and depth in the profile, no other major causal determinant was identified for spore densities in our study. One reason might be that the crops vary in the degree to which they form mycorrhiza, even between cultivars among the same species (Azcon & Ocampo, Citation1981; Manske, Citation1990). In this study the crops were divided into broad groups for statistical reasons. Apart from differences in host plant, there are also considerable differences in temperature, length of growing season, crop rotation, edaphic factors, etc. Therefore, no single factor can be distinguished as the most important one. Lack of correlation between spore density and soil nutrients or other soil properties was also the outcome of a study conducted along a 355 km transect from New Jersey to Virginia, USA (Koske, Citation1987). In addition, no correlation was found either between spore density of any species and temperature parameters or distance south along the transect.
Kabir et al. (Citation1997) showed that the winter survival of AM fungi is negatively affected by disturbance. In a study at seven sites in central Europe, the density of spores decreased with increasing management intensity, with higher densities in grassland compared to fields with crop rotation or mono-cropping (Oehl et al., Citation2003). Jansa et al. (Citation2002) observed no effect of spore density between tilled and non-tilled wheat field following maize, but when it followed rape the tilling caused a decrease in spore density. No correlation was found in spore densities of AM fungi between semi-natural grassland and ploughed sites in our study. This is in line with other reports which have shown that connection of the mycelia to the host roots is not necessary for AM fungi to colonise effectively, despite a reduction in amounts of both mycelia and spores during the winter (McGonigle & Miller, Citation1999; Addy et al., Citation1997).
The number of spore types found in the eight investigated soils ranged between three and seven. The two most diverse samples originated from the semi-natural grasslands. Three spore types could be identified to species level. A total of 30 different spore types could be identified, but so far only to the genus level, as we had few spores for analysis. Most spores from field extractions often are difficult or impossible to identify unless sporulation is high and parasitism is minimal (Morton, Citation1993). We estimate that at least 30 different species are present in the subsamples, and it cannot be excluded that among these there might be as yet undescribed species. In the present study, spores closely resembling Gigaspora were found at two sampling sites. From Europe, findings of the genus Gigaspora are rare, only reported from studies in Poland and Switzerland (Blaszkowski, Citation1993; Jansa et al., Citation2002). In our study such spores were few, and therefore their identity needs to be further confirmed.
The most abundant genus found was Glomus, with a proportion of 88.6% of the specimens identified. This is approximately similar to the 87.1% that has been reported by Vestberg (Citation1995) from Finland. One common species was found in these two investigations, Glomus caledonium. The species reported from Finland were identified from trap cultures of original soils, while the species in this investigation were identified directly from the original soil, i.e. without trap culture. By detecting spores directly from natural soils there might be species that have not sporulated at the sampling time. On the other hand, some species of AM fungi do not sporulate in trap culture (Miller et al., Citation1985; Morton, Citation1993). Species such as Glomus mosseae which is well documented as occurring in arable fields in temperate regions (Blaszkowski, Citation1993; Jansa et al., Citation2002; Oehl et al., Citation2003; Vestberg, Citation1995) were not detected in our samples. One reason might be that they had not sporulated. This is indicated by the fact that we have found G. mosseae in our trap cultures from further mechanism studies. However, G. caledonium is another common species found in temperate zones (Land & Schönbeck, Citation1991; Blaszkowski, Citation1993; Jansa et al., Citation2002; Oehl et al., Citation2003), and this is also reported in our study.
Acknowledgments
The study was financially supported by the Swedish Farmers Foundation for Agricultural research (SLF), Stockholm. We thank Ms Reena Singh, Teri, New Delhi, India, for all help with identification of spores, and Dr. Stig Olsson for statistical analyses. We also extend special thanks to the personnel at the county administrative boards for their help in finding appropriate semi-natural grasslands, and to farmers and personnel at the sampling sites, and Mrs. Marianne Sjöberg for help during the sampling.
Additional information
Notes on contributors
Johanna Sjöberg
Sjöberg, J., Persson, P., Mårtensson, A., Mattsson, L., Adholeya, A. and Alström, S. (Department of Ecology and Crop Production Science, Swedish University of Agricultural Sciences, Box 7043, SE-750 07 Sweden, Department of Soil Sciences, Swedish University of Agricultural Sciences, Box 7014, SE-750 07 Sweden and Centre for Mycorrhizal Research, Teri, Darbari Seth Block, Habitat Place, Lodhi Road, New Delhi IN-110 003, India). Occurrence of Glomeromycota spores and some arbuscular mycorrhiza fungal species in arable fields in Sweden.Notes
Sjöberg, J., Persson, P., Mårtensson, A., Mattsson, L., Adholeya, A. and Alström, S. (Department of Ecology and Crop Production Science, Swedish University of Agricultural Sciences, Box 7043, SE-750 07 Sweden, Department of Soil Sciences, Swedish University of Agricultural Sciences, Box 7014, SE-750 07 Sweden and Centre for Mycorrhizal Research, Teri, Darbari Seth Block, Habitat Place, Lodhi Road, New Delhi IN-110 003, India). Occurrence of Glomeromycota spores and some arbuscular mycorrhiza fungal species in arable fields in Sweden.
References
References
- Abbot , LK and Robson , RD . 1991 . Factors influencing the occurrence of vesicular-arbuscular mycorrhizas . Agric. Ecosyst. Environ. , 35 : 121 – 150 .
- Addy , HD , Miller , MH and Peterson , RL . 1997 . Infectivity of the propagules associated with extraradical mycelia of two AM fungi following winter freezing . New Phytol. , 135 : 745 – 753 .
- Azcon , R and Ocampo , JA . 1981 . Factors affecting the vesicular-arbuscular infection and mycorrhizal dependency of 13 wheat cultivars . New Phytol. , 87 : 677 – 685 .
- Bever , JD , Schultz , PA , Pringle , A and Morton , JB . 2001 . Arbuscular mycorrhizal fungi: more diverse than meets the eye, and the ecological tale of why . Bioscience , 51 : 923 – 931 .
- Blaszkowski , J . 1993 . Comparative studies on the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland . Acta Mycol. , 28 : 93 – 140 .
- Borowicz , VA . 2001 . Do arbuscular mycorrhizal fungi alter plant-pathogen relations? . Ecology , 82 : 3057 – 3068 .
- Burrows , RL and Pfleger , FL . 2002 . Arbuscular mycorrhizal fungi respond to increasing plant diversity . Can. J. Bot. , 80 : 120 – 130 .
- Carlgren , K and Mattsson , L . 2001 . Swedish soil fertility experiments . Acta Agric. Scan. B , 51 : 49 – 76 .
- Dalpé , Y and Aiken , SG . 1998 . Arbuscular mycorrhizal fungi associated with Festuca species in Canadian High Arctic . Can. J. Bot. , 76 : 1930 – 1938 .
- Davies , FT , Porter , JR and Linderman , RG . 1993 . Drought resistance of mycorrhizal pepper plants independent of leaf P concentration-response in gas exchange and water relations . Physiol. Plant. , 87 : 45 – 53 .
- Egnér , H , Riehm , H and Domingo , WR . 1960 . Untersuschung über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden 2. Chemische Extraktionsmethoden zur Phosphor und Kaliumbestimmung . Ann. R. Agric. Coll. Swed. , 26 : 199 – 215 .
- Eriksson , Å . 2001 . Arbuscular mycorrhiza in relation to management history, soil nutrients and plant species diversity . Plant Ecol. , 155 : 129 – 137 .
- George , E , Marschner , H and Jakobsen , I . 1995 . Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil . Crit. Rev. Biotechnol. , 15 : 257 – 270 .
- Gerdemann , JW and Nicolson , TH . 1963 . Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting . Trans. Brit. Mycol. Soc. , 46 : 235 – 244 .
- Hawkins , H-J , Johansen , A and George , E . 2000 . Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi . Plant Soil , 226 : 275 – 285 .
- Hooker JE Jaizme-Vega M Atkinson D Biocontrol of plant pathogens using arbuscular mycorrhizal fungi In: Gianinazzi S., Schüepp H. (eds.), Impact of arbuscular mycorrhizas on sustainable agriculture and natural ecosystems, Birkhäuser Verlag Basel Switzerland pp. 191–200 1994
- International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi http://invam.caf.wvu.edu/fungi/taxonomy/speciesID.htm
- Jakobsen , I and Nielsen , NE . 1983 . Vesicular-arbuscular mycorrhiza in field-grown crops 1. Mycorrhizal infection in cereals and peas at various times and soil depths . New Phytol. , 93 : 401 – 413 .
- Jansa , J , Mozafar , A , Anken , T , Ruh , R , Sanders , IR and Frossard , E . 2002 . Diversity and structure of AMF communities as affected by tillage in a temperate soil . Mycorrhiza , 12 : 225 – 234 .
- Jensen , A and Jakobsen , I . 1980 . The occurrence of vesicular-arbuscular mycorrhiza in barley and wheat grown in some Danish soils with different fertilizer treatments . Plant Soil , 55 : 403 – 414 .
- Johnson , NC , Zak , DR , Tilman , D and Pfleger , FL . 1991 . Dynamics of vesicular-arbuscular mycorrhizae during old field succession . Oecologia , 86 : 349 – 358 .
- Kabir , Z , O'Halloran , IP and Hamel , C . 1997 . Overwinter survival of arbuscular mycorrhizal hyphae is favoured by attachment to roots but diminished by disturbance . Mycorrhiza , 7 : 197 – 200 .
- Kahiluoto , H , Ketoja , E , Vestberg , M and Saarela , I . 2001 . Promotion of AM utilization through reduced P fertilization 2. Field studies . Plant Soil , 231 : 65 – 79 .
- Kling , M and Jakobsen , I . 1997 . Direct application of carbendazim and propiconazole at field rates to the external mycelium of three mycorrhizal fungi species: effect on 32P transport and succinate dehydrogenase activity . Mycorrhiza , 7 : 33 – 37 .
- Koske , RE . 1987 . Distribution of VA mycorrhizal fungi along a latitudinal temperature gradient . Mycologia , 79 : 55 – 68 .
- Kurle , JE and Pfleger , FL . 1996 . Management influences on arbuscular mycorrhizal fungal species composition in a corn-soybean rotation . Agron. J. , 88 : 155 – 161 .
- Land , S and Schönbeck , F . 1991 . Influence of different soil types on abundance and seasonal dynamics of vesicular arbuscular mycorrhizal fungi in arable soils of north Germany . Mycorrhiza , 1 : 39 – 44 .
- Manske , GGB . 1990 . Genetic analysis of the efficiency of VA mycorrhiza with spring wheat . Agric. Ecosyst. Environ. , 29 : 273 – 280 .
- McGonigle , TP and Miller , MH . 1999 . Winter survival of extraradical hyphae and spores of arbuscular mycorrhizal fungi in the field . Appl. Soil Ecol. , 12 : 41 – 50 .
- Miller , DD , Domoto , PA and Walker , C . 1985 . Mycorrhizal fungi at 18 apple rootstock plantings in the United States . New Phytol. , 100 : 379 – 391 .
- Morton , JB . 1993 . Problems and solutions for the integration of glomalean taxonomy, systematic biology and the study of endomycorrhizal phenomena. . Mycorrhiza , 2 : 97 – 109 .
- Mårtensson , AM and Carlgren , K . 1994 . Impact of phosphorus fertilization on VAM diaspores in two Swedish long-term field experiments . Agric. Ecosyst. Environ. , 47 : 327 – 334 .
- National atlas of Sweden. Climate, lakes and rivers 1995 (Syrén, M., ed.) Almqvist & Wiksell International Stockholm Sweden 176 pp
- Nehl , DB , McGee , PA , Torrisi , V , Pattinson , GS and Allen , SJ . 1999 . Patterns of arbuscular mycorrhiza down the profile of a heavy textured soil do not reflect associated colonisation potential . New Phytol. , 142 : 495 – 503 .
- Oehl , F , Sieverding , E , Ineichen , K , Mäder , P , Boller , T and Wiemken , A . 2003 . Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of central Europe . Appl. Environ. Microbiol. , 69 (5) : 2816 – 2824 .
- Online photoperiod calculator http://www.nic.fi/~benefon/sun.php3
- Schenck NC Pérez Y Manual for the identification of VA mycorrhizal fungi 3rd edition Synergistic Publications Gainesville Fla 1990
- Schüssler , A , Schwartzott , D and Walker , C . 2001 . A new fungal phylum, the Glomeromycota: phylogeny and evolution . Mycol. Res. , 105 (12) : 1413 – 1421 .
- Snedecor GW Cochran G Statistical methods, Iowa State University Press Ames Iowa 593 1978
- Statistics Sweden http://www.scb.se/
- Vestberg , M . 1995 . Occurrence of some Glomales in Finland . Mycorrhiza , 5 : 329 – 336 .
- Walker C Mize CW McNabb HS Jr Populations of endogonaceous fungi at two locations in central Iowa Can. J. Bot. 60 2518 2529 1982
- Yearbook of agricultural statistics 2001 including food statistics 2001 (Hedqvist, L., ed.) Bulls tryckeriaktiebolag, Halmstad Sweden
Appendix 1
A simplified cultivation management history of the sampling sites in Sweden and physical and chemical properties of the soils
a Cereal=wheat, rye-wheat, winter wheat, barley or barley with undersown ley. SNG=semi-natural grassland.
b Monoculture=cerealsa, Mixed=cereals and other crops, Ley-mixed=at least two years of ley, i.e. at least one year without ploughing, SNG=semi-natural grassland.
c Easily available phosphorus, % of dry matter (DM). Egnér et al. (Citation1960).
d Less soluble phosphorus, % of dry matter (DM). Egnér et al. (Citation1960).
e Total carbon and nitrogen content, % of dry matter.
f Mineral particles with a diameter of less than 2 μm.
g Organically managed farm.
h Altitude 707 m above sea level. (ski slope).
i Situated on an island.