Abstract
The effect of organic and inorganic amendments on the extractability and plant uptake of copper (Cu) was investigated in contaminated coffee orchard soils of the Kilimanjaro and Arusha regions of Tanzania. The soil was collected from 0–5 cm depth at a distance of 50 cm from coffee tree trunks. The total Cu concentration in the soil used was 250 mg kg−1. Beans (Phaseolus vulgaris L.) and maize (Zea mays L.) were used as indicator crops and were grown in a greenhouse. The amendments used consisted of beringite, a modified aluminosilicate residue from coal mines, farmyard manure, banana compost, Albizia litter, and phosphate fertilizer. In general, the application of all amendments resulted in increased soil organic carbon (SOC) content and soil pH, which in consequence reduced both CaCl2-extractable Cu in soils and Cu concentration in both plant species. Beringite at all rates of application reduced the CaCl2-extractable Cu in soils and increased soil pH significantly, but the effect was most pronounced at an application rate of 5%. Similarly, the application of farmyard manure and banana compost at a rate of 10% significantly reduced the CaCl2-extractable Cu in soils. The increase in soil pH caused by the amendments was negatively correlated to both CaCl2-extractable Cu in soils and Cu concentration in plant tissue. However, the increasing concentration of CaCl2-extractable Cu in soils showed a positive correlation with Cu in plant tissue. Albizia litter at 10% incorporation rate significantly reduced the CaCl2-extractable Cu in soils. The application of P to maize reduced CaCl2-extractable Cu in soils, but the reduction in plant Cu was not significant. The results suggest that beringite, FYM and banana compost may be suitable amendments to reduce plant available Cu in soils and Cu uptake by plants, but their applicability and relative efficiency under field conditions need to be investigated.
Introduction
Copper (Cu) contamination of soils by the frequent use of Cu based fungicides is reported by many investigators (Lepp & Dickinson, Citation1994; Semu & Singh, Citation1996). In a field survey, serious Cu contamination of soils in the coffee orchards in Tanzania was registered. In this survey, the mean total Cu concentration at 0–5 cm soil depth was 333 mg kg−1 on commercial estates and 397 mg kg−1 on small farms in the Kilimanjaro region of Tanzania (Loland & Singh, Citation2004). Some samples of leaves from coffee, beans and maize plants grown in the coffee orchards of this region showed Cu concentrations of up to 991, 842 and 21 mg kg−1 of dry matter, respectively. For beans and coffee plants, these concentrations were many-fold higher than the toxic level of this nutrient, and even for maize the concentration was approaching the toxic limit of 20–25 mg kg−1 of dry matter (Wrigley, Citation1988; Fageria et al., Citation1991).
In such highly contaminated soils not only plant toxicity seems to be a problem, but also soil microbial life can be seriously affected (Frostegård et al., Citation1993). However, microbial life has a higher ability to adapt to a changing environment, because when one group of organisms is halted in its decomposition activity, there may be other organisms which can withstand the contamination and gradually take over its role (Valsecchi et al., Citation1994). A specific microorganism may also be able to adapt to a changing environment by altering its metabolism.
Among the different approaches used to reduce the risk of Cu toxicity in contaminated soils, in situ immobilization of soil Cu by organic and inorganic amendments has proved successful (Mench et al., Citation1993; Ma et al., Citation1993; Narwal & Singh, Citation1998; Singh & Oste, Citation2001). In this process metals are rendered unavailable to plants. Even when total soil Cu is high, the plant available Cu fraction may be low, because the solubility of Cu in soils is affected by their physical and chemical properties. One of the important soil properties in this context is the ability of soils to adsorb Cu. In particular, soil organic matter (SOC) and pH often have a crucial function with regard to the adsorption of Cu, since the element is strongly controlled through complexification with organic matter and adsorption on colloidal fractions. The efficiency of SOC to immobilize Cu is dependent on soil pH (Yuan & Lavkulich, Citation1997).
The use of a strong metal absorbing aluminosilicate called beringite (Vangronsveld et al., Citation1993; Wessolek & Fahrenhorst, Citation1994) and organic amendments (farm yard manure (FYM) and composts) (Narwal & Singh, Citation1998) has proved effective in reducing metal solubility and plant uptake. Phosphate minerals have shown great potential to immobilize metals (especially Pb) in soil (Ma et al., Citation1993). Therefore, some of these amendments were used in the present study to explore the possibility of Cu immobilization and thus reduction of its solubility and phytoavailability in contaminated soils of the coffee orchards of the Kilimanjaro region of Tanzania. The experiment was conducted in a greenhouse by using both organic and inorganic amendments.
Materials and methods
A pot experiment was conducted in a locally constructed greenhouse covered with plastic sheets and wire netting. The soil was collected from 0–5 cm depth at a distance of 50 cm from coffee tree trunks at the Lyamungu Research Station in the Kilimanjaro region of Tanzania. The soil was dried, ground, and sieved through a 1.69 mm sieve and placed in 2 litre plastic pots. The total Cu concentration in the soil used was 250 mg kg−1. The background level of Cu in virgin soils of this region ranges from 40 to 80 mg kg−1. The soil used was of volcanic origin, and classified as Lithic Dystrandept or Lithic Eutrandept (Soil Survey Staff, Citation1998). A basal dose of N, P and K at the rates of 120, 30 and 120 mg kg−1, respectively, was applied to all pots. Nitrogen was added in the form of urea, P as triple superphosphate, and K as muriate of potash. All fertilizers were thoroughly mixed with dry soil.
Treatments
The following treatments were used for maize and beans:
| 1. | Treatment 1–3 (maize and beans) Beringite, at the rate of 1.0, 2.5 and 5.0% (on weight basis) was mixed with dry soil. | ||||
| 2. | Treatment 4 (maize and beans) Dry farm yard manure (FYM) at the rate of 10% was applied on a volume basis (v/v). The FYM was collected at the Lyamungu dairy farm, air-dried, ground and passed through a 1.69 mm sieve prior to incorporation in the soil. | ||||
| 3. | Treatment 5 (maize and beans) Dry banana leaf compost at the rate of 10% (v/v) was mixed with dry soil. The processing of compost was done in the same way as FYM. | ||||
| 4. | Treatment 6 (maize and beans) Litter from Albizia species, a common shade tree in coffee, was mixed with dry soil at 10% (v/v) in the same way as FYM or compost. | ||||
| 5. | Treatment 7 and 8 (maize) Phosphorus at the rate of 0 and 60 mg kg−1 was applied to dry soil. | ||||
| 6. | Treatment 9 and 10 These were control treatments for maize and beans | ||||
At harvesting, the plants were dried at about 70°C in an oven and the total dry matter (DM) yield was recorded for each pot. The soil from each pot was spread out in separate plastic trays, and left to dry outdoors. Upon drying the soil was mixed thoroughly in each tray, and a representative sample was collected. After drying the soil was sieved through a 1.69 mm sieve and stored for chemical analysis.
Chemical analysis
Soil analysis was carried out in the Department of Plant and Environmental Sciences at the Agricultural University of Norway. Soil pH (water) was measured with a glass electrode in a soil-water suspension of 1:10 on weight basis. The high ratio was used due to the small quantity of soil samples available. However, to obtain an approximation of pH at 1:2.5 ratios on volume basis, eight soil samples within a wide pH spectrum were analysed by both methods. The correlation between the pH values measured on v/v and w/w basis was elucidated and a correction factor was calculated. The pH values of the w/w method were multiplied by the correction factor to obtain the pH values on a volume basis. An EC 12 carbon analyser was used to determine organic carbon. Total Cu was determined after digestion with aqua regia (Jeng & Bergseth, Citation1992). The extractable Cu was determined with CaCl2 (Houba et al., Citation1996). An atomic absorption spectrophotometer (AAS) was used to determine the concentration of Cu in the digested and extracted solutions. Total Cu in plant material was analysed according to the method described by Helrich (Citation1990). All Cu concentrations and other soil parameters are reported on an oven dry weight basis.
Statistical methods
Principal Component Analysis (PCA) as described by Christensen (Citation1996) was used to determine interactions between chemical and physical parameters. Best subsets regression and stepwise regression were used to find the best predictors of variables and to eliminate predictors that are not significant. Analysis of variance was performed, and Tukey's t-test method used to determine differences between treatments. Minitab was used as a tool to run most of the statistics, while most figures were made in EXCEL.
Results and discussion
Interactions between soil chemical parameters, plant Cu and plant dry weight
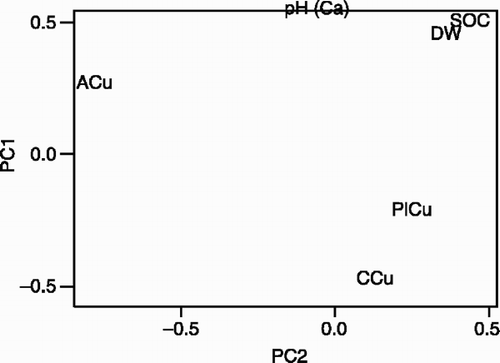
Chemical analysis results and plant dry weight (DW) were included in a PCA to determine interrelations between soil chemical parameters, plant Cu, and plant dry weight (). In , PC1 and PC2 explain 53.3% and 18.8% of the variations in the data, respectively. These two components together explain 72.1% of the variations. The data on plant dry weight and plant Cu are from two different plant species and hence it is plausible that species variations might eliminate otherwise significant correlations.
Effect of SOC on soil pH, and of pH and SOC on CaCl2-extractable Cu
There was a strong negative correlation between CaCl2-extractable Cu and pH, and between CaCl2-extractable Cu and SOC (). This implies that pH in the soils of the present experiment must have been altered by the application of different amendments, and that the increase of SOC is likely to be an important factor in this change. To discover which parameters are valid predictors of changes in CaCl2-extractable Cu concentration, all soil chemical parameters together with their transformations were included in a stepwise regression model. In this model, only H3O+ was left omitted as a valid predictor, and the regression analysis of CaCl2-extractable Cu with H3O+ gave an R 2 value of 0.88 when one extreme outlier of CaCl2-extractable Cu (4.3 mg kg−1) was excluded. The regression equation was as follows:
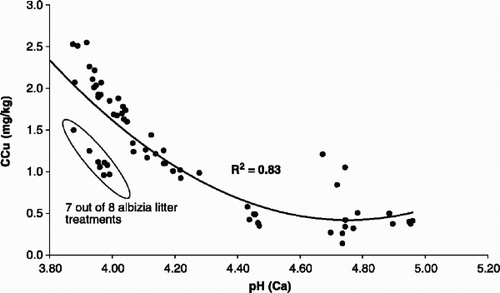
In other words, both pH and SOC influenced CaCl2-extractable Cu concentration. The increase in SOC, caused by the application of amendments, increased pH by immobilizing H3O+, but also had a direct effect by immobilizing Cu2+ ions in solution. The SOC concentration in soils is known to strongly affect the adsorption of Cu (McGrath et al., Citation1988). In general, the mobile fractions of SOC are known to increase mobility of Cu, while solid fractions reduce it (McBride et al., Citation1997). Calcium chloride is not able to extract the Cu fraction that is mobilized in soluble SOC fractions, but the Ca2+ ion in CaCl2 exchanges Cu2+ ions that are very lightly adsorbed in the soil. The influence of pH on CaCl2-extractable Cu concentration is clearly seen in . The effect of the litter treatment is likely to be an immobilization of soluble Cu through fixation in the organic matter, since it has an immobilization effect on Cu at lower concentrations than in the other treatments.
Fig. 2. Relationship between soil pH and CaCl2-extractable Cu (CCu). Treatments in the ring are those where Albizia litter was applied.
The most likely explanation of the increased levels of SOC is organic matter addition through different amendments, but there is also a possibility that growth of the plants themselves had increased SOC through excretion of root exudates. To check this hypothesis, a variance analysis was run between the beringite treatments and the control, since no organic matter was added in any of these treatments. Data from both maize and beans were amalgamated. The variance analysis showed still a significant difference between treatments. The plants in soil with 5% beringite incorporation had a significantly higher DM, and the soil had a significantly higher SOC level than the control. Tukey's t-test also confirmed that this treatment has even a higher DM and SOC than the 1% and 2.5% beringite treatments. This means that there has been SOC enrichment in the pots with strongest growth compared with those with weakest growth. Since SOC had such a strong influence on pH and CaCl2-extractable Cu, this means that throughout the life of the plants strong growth may have reduced Cu availability through excretion of root exudates. Such an excretion may be an important way of detoxifying Cu if a plant survives the seedling stage, and may explain the fact that older plants normally are more resistant to toxic elements in soil than seedlings.
Effects of different treatments on plant Cu
In there is a strong positive correlation between plant Cu and CaCl2-extractable Cu, and a negative correlation with these two parameters and pH. Both principal components also highlight a negative correlation between SOC and these two parameters (). Between pH, SOC and DM, there is a strong positive correlation. A correlation matrix was calculated between these parameters for data from beans (Eq. 2) and maize (Eq. 3), and this showed that CaCl2-extractable Cu was the best predictor for differences in plant Cu, followed by SOC and H3O+. Calcium chloride- extractable Cu was able to explain differences in plant Cu for beans, but was not good for maize. When data from beans and maize crops were combined (Eq. 4), only CaCl2-extractable Cu showed a relationship with plant Cu, but the correlation value was low. The regression equations for beans and maize, separately and combined, are shown below.
Data from beans:
The fact that plant Cu was significantly correlated with CaCl2-extractable Cu when the data from both crops are combined shows the importance of CaCl2-extractable Cu for plant Cu levels.
Effect of different amendments on pH, CaCl2-extractable Cu and plant Cu
Beringite application
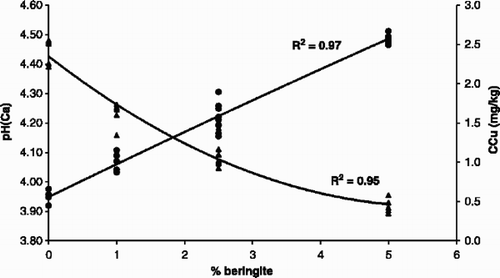
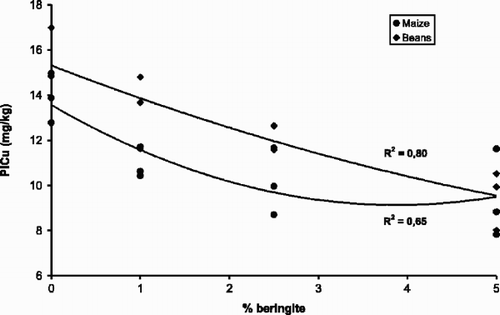
Mench et al. (Citation1993) reported a strong immobilization of Cd and Zn with the use of beringite in contaminated soils in the Netherlands, and hence the same product was tested in the present study to see if this product would also immobilize Cu. The results showed that the application of beringite reduced CaCl2-extractable Cu significantly and it increased the soil pH by about 0.5 units in the soils from beans and maize experiments (). The effect of beringite was most pronounced at the 5% application rate. The relationship between the rate of beringite application and the changes in CaCl2-extractable Cu and soil pH were generally linear as shown in . The 5% incorporation rate of beringite significantly reduced plant Cu in both crops, while the 2.5% incorporation rate reduced plant Cu in beans only (). However, there was a significant correlation between CaCl2-extractable Cu and plant Cu in the beringite treatments for maize (R 2=0.88, P<0.05) and for beans (R 2=0.42, P<0.05) ().
Fig. 5. Relationship between CaCl2-extractable Cu (CCu) and Cu concentration in bean and maize plants (PlCu).
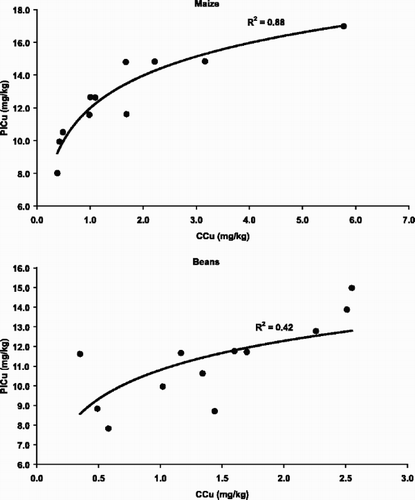
Table 1. Effects of different amendments on plant dry matter (PDM), plant Cu(PlCu), aqua regia soluble Cu(ACu), CaCl2-extractable Cu(CCu), soil pH and organic carbon
There was a significant increase in SOC with increasing beringite incorporation (). When DM was logarithmically transformed, a significant positive correlation between DM and SOC for beans (R 2=0.55, P<0.5) and for maize (R 2 =0.46, P<0.05) in the beringite treatments was observed. It is thus likely that the immobilization of CaCl2-extractable Cu was caused partly by increased SOC levels and partly by increased soil pH. Beringite is a strong metal adsorbing aluminosilicate, and thus it fixes Cu2+ and other cations, making them less available to plants. Although there was a near linear correlation between pH and beringite application, the reduction in CaCl2-extractable Cu was at its steepest when beringite application rate to soil was <3% ().
Farmyard manure and banana compost treatments
The FYM and banana compost treatments showed the strongest effects on plant DM for both maize and beans, which could be assigned to their fertilization effect (). For both crops there was a significant reduction in plant Cu with 10% banana compost, but for FYM the reduction was significant only for beans (). The application of FYM and banana compost increased pH by 1 and 0.7 units, respectively. The reduction in CaCl2-extractable Cu caused by FYM and banana compost was 86% and 69%, respectively. The strongest effect of these organic materials on CaCl2-extractable Cu seems to be caused by their effects on soil pH ().
Albizia litter
This treatment was included to determine whether litter from trees in the region could be utilized to control Cu solubility. Albizia sp. are common trees in this region (Noad & Birnie, Citation1986) and are used as shade trees in coffee orchards. Shade trees in coffee plantations are planted to improve coffee quality (Muschler, Citation2001). In , litter from Albizia trees showed lower CaCl2-extractable Cu than the other treatments at the same pH level. In spite of lower pH, although the soil pH in Albizia litter treated pots was not significantly different from the control pots, it significantly reduced the CaCl2-extractable Cu in soils from both crops, but plant Cu in beans only (). Similar to FYM and compost treatments, increased SOC levels in Albizia litter treated pots seem responsible for the reduction in CaCl2-extractable Cu in soils and consequently in plant Cu.
Phosphorus treatments
Phosphorus application also reduced the CaCl2-extractable Cu significantly but its effect on plant Cu was not significant (). Since P application did not affect either soil pH or SOC, its effect may be related to complex formation with metals as observed for Pb in the study by Ma et al. (Citation1993).
Conclusions
The CaCl2-extractable Cu is a good indicator for plant Cu levels in beans and maize in the soil from the Kilimanjaro and Arusha regions of Tanzania. CaCl2-extractable Cu is strongly dependent on pH and SOC contents in soils. Increasing amounts of SOC in the soil through amendments increased pH and reduced both CaCl2-extractable and plant Cu. The 10% FYM incorporation in the soil was the most effective treatment to increase DM and pH and to reduce CaCl2-extractable and plant Cu. Similar effects on CaCl2-extractable and plant Cu were seen in beringite treated pots, but the 5% application rate of beringite was slightly less effective than the 10% rate of FYM. The other treatments, compost and Albizia litter, were slightly inferior to FYM and beringite treatments. The application of P to maize reduced CaCl2-extractable Cu, but the reduction in plant Cu was not significant. The results suggest that beringite, FYM and banana composts may be good amendments to reduce plant available Cu in soils and its uptake by plants, but their applicability under field conditions needs to be investigated.
Acknowledgments
Thanks are expressed to I.K. Kulaya for his assistance in conducting this study at the Lyamungu Research Station, Moshi, Tanzania. The financial support from the Norwegian Centre for International Environment and Development Studies (NORAGRIC) is gratefully acknowledged.
Additional information
Notes on contributors
J. Ø. Loland
Loland, J. Ø. and Singh, B. R. (Agricultural Extension Service, The Norwegian Crop Research Institute, NO-5781 Lofthus, Norway and Department of Plant and Environmental Sciences, Agricultural University of Norway, NO-1432 Ås, Norway). Extractability and plant uptake of copper in contaminated coffee orchard soils as affected by different amendments.Notes
Loland, J. Ø. and Singh, B. R. (Agricultural Extension Service, The Norwegian Crop Research Institute, NO-5781 Lofthus, Norway and Department of Plant and Environmental Sciences, Agricultural University of Norway, NO-1432 Ås, Norway). Extractability and plant uptake of copper in contaminated coffee orchard soils as affected by different amendments.
References
References
- Christensen R 1996 Applied Statistical Methods 587 pp. Chapman and Hall London
- Fageria NK Baligar VC Jones CA 1991 Growth and Mineral Nutrition of Field Crops 476 pp. Marcel Dekker Inc New York
- Frostegård , Å. , Tunlid , A and Bååth , E . 1993 . Phospholipid fatty acid composition, biomass, activity of microbial communities from two soil types experimentally exposed to different heavy metals . Applied Environ. Microb. , 59 : 3605 – 3617 .
- Helrich K 1990 Official methods of analysis Vol. 1 Association of Analytical Chemists Inc Virginia
- Houba , VJG , Lexmond , TM , Novozamsky , I and Van der Lee , JJ . 1996 . State of the art and future development in soil analysis for bioavailability assessment . Sci. Total Environ. , 178 : 21 – 28 .
- Jeng , AS and Bergseth , H . 1992 . Chemical and mineralogical properties of Norwegian alum shale soils, with special emphasis on heavy metal content and availability . Acta Agric. Scand. Sect. B. , 42 : 88 – 93 .
- Lepp NW Dickinson NM 1994 Fungicide derived Cu in tropical plantation crops In: Ross, S. (ed.) Toxic Metals in Soil-Plant Systems pp. 367–393 John Wiley and Sons Ltd
- Loland JØ. Singh BR 2004 Copper contamination of soil and vegetation in coffee orchards after long-term use of Cu fungicides Nutr. Cyl. Agroecosystems (in press)
- Ma , OY , Logan , TJ and Traina , SJ . 1993 . In situ lead immobilization by apatite . Environ. Sci. Technol. , 27 : 1803 – 1810 .
- McBride , M , Sauvé , S and Hendershot , W . 1997 . Solubility control of Cu, Zn, Cd and Pb in contaminated soils . Europ. J. Soil. Sci. , 48 : 337 – 346 .
- McGrath , SP , Sanders , JR and Shalaby , MH . 1988 . The effects of organic matter levels on soil solution concentration and extractability of manganese, zinc and copper . Geoderma , 42 : 177 – 188 .
- Mench , MJ , Vangronsveld , J , Didier , V and Clijsters , H . 1993 . Evaluation of metal mobility, plant availability and immobilization by chemical agents in a limed silty soil . Environ. Pollut. , 86 : 279 – 286 .
- Muschler , RG . 2001 . Shade improves coffee quality in a sub-optimal coffee-zone of Costa Rica . Agrofor. Syst. , 51 : 131 – 139 .
- Narwal , RP and Singh , BR . 1998 . Effect of organic materials on partitioning, extractability and plant uptake of metals in an alum shale soil, Water, Air . Soil Pollut. , 103 : 405 – 421 .
- Noad TC Birnie A 1986 Trees of Kenya Fourth Edition Nairobi 308 pp.
- Semu , E and Singh , BR . 1996 . Accumulation of heavy metals in soils and plants after long term use of fertilizers and fungicides in Tanzania . Fert. Res. , 44 : 241 – 248 .
- Singh , BR and Oste , L . 2001 . In situ immobilization of metals in contaminated or naturally metal rich soils . Environ. Rev. , 9 : 81 – 97 .
- Soil Survey Staff 1998 Keys to Soil Taxonomy Soil Conservation Service, USDA Washington DC
- Valsecchi , G , Giglioti , C and Farini , A . 1994 . Microbial biomass, activity, and SOC accumulation in soils contaminated with heavy metals . Biol. Fertil. Soils. , 20 : 253 – 259 .
- Vangronsveld , J , Van Assche , F and Clijsters , H . 1993 . Reclamation of a bare industrial area contaminated by non-ferrous metals: in situ metal immobilization and revegetation . Env. Pol. , 87 : 51 – 59 .
- Wessolek , G and Fahrenhorst , C . 1994 . Immobilization of heavy metals in a polluted soil of a sewage farm by application of a modified aluminosilicate: a laboratory and numerical displacement study . Soil Technol. , 7 : 221 – 232 .
- Wrigley G 1988 Coffee 639 pp. John Wiley & Sons, Inc New York
- Yuan , G and Lavkulich , LM . 1997 . Adsorption behaviour of copper, zinc, and cadmium in response to simulated changes in soil properties . Commun. Soil. Sci. Anal. , 26 : 571 – 587 .