Abstract
The effects of different priming treatments and durations on germination percentage, speed, synchrony and thermal time requirement of the seeds of chickpea were investigated. Seeds were osmoprimed in four water potentials (−0.5, −1.0, −1.5 and −2.0 MPa) of polyethylene glycol (PEG) or in 4% mannitol in addition to hydropriming for 12 or 24 h at 25±1°C in darkness. Following priming, the seeds were subjected to germination tests at ten different constant temperatures ranging from 5 to 32±0.5°C. In general, there was no significant effect of priming treatments on germination percentage. However, compared to unprimed seeds, hydropriming and osmopriming treatments induced faster and more synchronous germination at all of the temperatures tested and also significantly decreased thermal time requirements. These reductions in thermal time requirements ranged between 18.1°C d and 30.3°C d for 50% germination. Seeds treated with water for 12 h generally produced the highest germination speed and the lowest thermal time requirement values. Among the osmopriming treatments, seeds treated with −0.5 MPa solution of PEG for 24 h gave the best results. Consequently, hydropriming for 12 h or osmopriming (PEG −0.5 MPa) for 24 h may be recommended for a better germination of chickpeas under cold soil conditions.
Introduction
Rapid germination and emergence are important determinants of successful stand establishment (Murungu et al., Citation2003). However, during germination, chilling range temperatures result in poor crop establishment in chickpea (Croser et al., Citation2003). Chickpea is increasingly being sown in the autumn rather than the spring. However, freezing and chilling range temperatures are considered an important problem for autumn-sown chickpea in the contries surrounding the Mediterranean Sea, the tropical highlands, and temperate growing regions (Singh, Citation1993). In these regions, low temperature stress predominantly occurs during the seed germination, seedling, and early vegetative stages of crop growth. Thus, autumn-sown chickpeas are often subjected to temperatures below the optimal range of 20 to 35°C during germination and emergence (Ellis et al., Citation1986). Chickpea seed sown early in the season in temperate areas such as parts of Turkey, Russia, and Canada are commonly exposed to chilling or even freezing temperatures during germination, which can result in a reduced stand and low seedling vigour because of low-temperature imbibitional damage (Chen et al., Citation1983). In spring planting areas where the plant growing season is short, chickpeas are drilled into cool soils to maximize the length of the production season. However, temperatures of 13°C, commonly found in spring planting, also suppress chickpea germination (Auld et al., Citation1988).
Soaking of seeds in water or an osmotic solution permits partial seed hydration so that pre-germination metabolic activities proceed but primary root protrusion is prevented. Such a treatment, which is usually followed by drying of the seeds, is known as priming (Heydecker & Gibbins, Citation1978). Priming of seeds in osmoticums such as mannitol, polyethylene glycol (PEG) and sodium chloride (osmopriming) and in water (hydropriming) has been reported to be an economical, simple and a safe technique for increasing the capacity of seeds to osmotic adjustment and enhancing seed germination, seedling establishment and crop production under stressed conditions (Kaur et al., Citation2002). There is a great deal of evidence that seed priming is an effective method for resource-poor farmers to use in order to increase yields of a range of subtropical crops growing in marginal areas, such as chickpea (Harris et al., Citation1999; Musa et al., Citation2001). It has also been reported that priming improves speed, synchrony and percentage of seed germination in many crop species particularly under sub-optimal temperatures (Yan et al., Citation1989; Zheng et al., Citation1994; McDonald, Citation1999) and yield gains in priming treatments result from earlier, faster germination and emergence (Harris, Citation1996; Harris et al., Citation1999; Musa et al., Citation2001). However, there is little information about the effects of seed priming and priming duration on germination of chickpea. Thus, in the present study, the germination response of chickpea seeds was examined at several temperatures in relation to various priming treatments and seed soaking durations in the laboratory.
Materials and methods
Seed material
This study was conducted under controlled environmental conditions at Ataturk University, Erzurum, Turkey using a nationally registered Kabuli type chickpea cultivar (Cicer arietinum L. cv. Aziziye-94) with 100-seed weight of 49.5 g obtained from Eastern Anatolia Agricultural Research Institute, Erzurum, Turkey.
Priming treatments and durations
The seeds were divided into lots. In osmopriming treatments, the seed lots were fully immersed in an aerated solution of polyethylene glycol (PEG 6000) at four water potentials (−0.5, −1.0, −1.5 and −2.0 MPa) according to Michel and Kaufmann (Citation1973) and were fully immersed in 4% mannitol. The seed lots were imbibed in distilled water in hydropriming treatments. The seeds without any treatment were termed as unprimed. Treated seed lots with PEG, mannitol and water were kept in darkness in an incubator at 25±1°C (Kaur et al., Citation2002) for 12 or 24 h. The imbibed seeds were then washed three times with tap water and dried on filter paper at 25±1°C for 24 h (Esitken et al., Citation2004).
Germination experiment and experimental design
The germination experiment consisted of a completely randomized design with four replicates in a factorial arrangement having seven different priming treatments (unprimed, PEG (−0.5, −1.0, −1.5 and −2.0 MPa), 4% mannitol and hydropriming) and two priming durations (12 and 24 h). The experiment was carried out in a darkened growth chamber at ten different (5, 8, 11, 14, 17, 20, 23, 26, 29 and 32±0.5°C) constant temperatures. Twenty seeds were placed on two sheets of filter paper in four 10-cm Petri dishes for each treatment and distilled water (20 ml) was added to each Petri dish. Benomyl (0.5 g l−1) was added into the distilled water to prevent fungal development. Germinated seeds were counted and removed at 4-h intervals to determine the germination courses.
Data collection
Total percentage germination, time required to reach 10%, 50%, and 90% germination based on the total number of germinated seeds (Garcia-Huidobro et al., Citation1982) and germination synchrony (hours between 10% and 90% germination rate) (Korkmaz & Pill, Citation2003) were calculated for each treatment and temperature. Times required to achieve 10%, 50%, and 90% germination were calculated by interpolation from the cumulative germination curve (Covell et al., Citation1986). Thermal time equation is θT (g)=(T–T b)t g where θT (g) is the thermal time (degree-days (°C d)) to primary root emergence of percentage g, T is the actual temperature at which the germination test is conducted, T b is the base temperature for germination, and t g is the actual time to germination of percentage g (Bierhuizen & Wagenvoort, Citation1974; Garcia-Huidobro et al., Citation1982; Covell et al., Citation1986; Dahal et al., Citation1990; Cheng & Bradford, Citation1999). Thermal time is equivalent to the inverse slope of the regression line (Garcia-Huidobro et al., Citation1982; Hardegree et al., Citation2002). Therefore, a linear regression equation was derived to relate germination rate (reciprocal of the time taken for 10%, 50%, and 90% of total germination to be achieved) to temperature in the sub-optimal temperature range (Hardegree et al., Citation1999) and thermal time requirements were estimated as the inverse slope of the regression line (Garcia-Huidobro et al., Citation1982; Dumur et al., Citation1990; Hardegree et al., Citation2002) for 10%, 50%, and 90% germination.
Statistical analysis
The data were subjected to analysis of variance using MSTATC Statistical Package (version 1.4, Michigan State University). Mean values were separated according to least significant differences (LSD) test at p≤0.05.
Results
Analysis of variance showed that germination percentage was significantly influenced by priming treatments at 5, 8, 14 and 32°C. Priming duration had significant effect on germination percentage only at 5, 26 and 32°C and priming treatment×priming duration interaction was insignifcant at all of the temperatures (). PEG solutions (−0.5, −1.0 and −2.0 MPa) had the higest germination percentages at 5°C. Hydropriming had the lowest germination percentages at 5, 8, and 14°C. On the other hand, except for hydropriming, all of the priming treatments significantly decreased germination percentage compared with the unprimed treatment at 32°C. Priming duration of 24 h had significantly higher germination percentage than that of 12 h at 5 and 26°C, whereas priming duration of 12 h gave a better germination percentage result at 32°C ().
Table I. Effects of seed priming and priming duration on germination percentage of chickpea seeds at different temperatures.
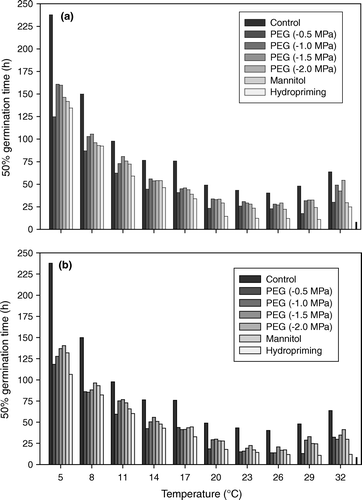
All priming treatments induced faster germination compared with the unprimed seeds ( and ). At all of the germination temperatures, hours required to reach 10%, 50%, and 90% germination were significantly reduced by priming treatments (10% and 90% germination data not shown) and were also significantly influenced by priming duration (). In general, seeds primed for 24 h significantly reduced hours required to reach 50% germination compared with the seeds primed for 12 h. Depending on the germination temperatures, reductions in the hours required to reach 50% germination under different priming treatments ranged between 14.9% (PEG −2.0 MPa at 32°C) and 77.1% (hydropriming at 29°C) for priming duration of 12 h and ranged between 21.5% (PEG −1.5 MPa at 11°C) and 81.2% (hydropriming at 32°C) for priming duration of 24 h, over the unprimed treatment. Interaction effect was also significant and, generally, seeds treated with −0.5 MPa solution of PEG and water for 12 and 24 h had the lowest 50% germination time at all of the germination temperatures ().
Figure 2. Time to 50% germination at different temperatures in relation to different priming treatments and priming durations of (a) 12 h and (b) 24 h. LSD bar is given at the end of temperature treatment 32°C and its value is 6.7.
All of the priming treatments significantly decreased hours between 10% and 90% germination (germination synchrony) at all of the tested temperatures (). Priming duration had a significant effect on germination synchrony, except for 20, 26 and 29°C. Compared with priming duration of 24 h, priming duration of 12 h significantly increased germination synchrony at 5, 8, 11, 14, 17 and 23°C, but significantly decreased synchrony at 32°C (). The interaction between priming treatment and priming duration was significant for germination synchrony at all of the tested temperatures. In terms of germination synchrony, the best combination of priming treatment and priming duration differed among germination temperatures (). Compared with the unprimed treatment, the best priming treatment×priming duration interactions decreased hours between 10% and 90% germination (germination synchrony) by 53.9, 60.6, 55.0, 62.2, 68.9, 52.2, 48.1, 55.2, 58.2 and 56.2% at 5, 8, 11, 14, 17, 20, 23, 26, 29 and 32°C, respectively.
Table II. Effects of seed priming and priming duration on germination synchrony (hours between 10% and 90% germination rate) of chickpea seeds at different temperatures and thermal time estimate for hours to 10%, 50%, and 90% germination in the sub-optimal temperature range of 5–23°C.
Compared with the unprimed treatment, priming treatments significantly decreased thermal time requirements. These reductions ranged between 13.4°C d and 22.9°C d, 18.1°C d and 30.3°C d, and 23.4°C d and 41.2°C d for 10%, 50% and 90% germination, respectively (). As an average of priming treatments, for 10%, 50% and 90% germination, seeds primed for 24 h had significantly lower thermal time requirments than that of seeds primed for 12 h. Analysis of variance also shows that thermal time requirement was significantly influenced by interaction of priming treatment×priming duration (). The best thermal time requirement results for 10%, 50% and 90% germination were obtained from seeds treated with water for 12 h.
Discussion
Primed seeds can improve germination of many crop species, particularly under adverse conditions such as low temperature (Zheng et al., Citation1994; Hardegree & Van Vactor, Citation2000). Thus, priming the seeds with water or osmotic solution before sowing is widely adopted to overcome low temperature effects on germination (Yan et al., Citation1989; McDonald, Citation1999). In our trial, germination percentage of chickpea was also enhanced slightly by priming treatments only at 5°C (), which was the lowest germination temperature. On the other hand, except for hydropriming, germination percentage was significantly decreased by all of the priming treatments at 32°C which was the highest germination temperature. This result is in agreement with that of the other researchers (Hardegree et al., Citation2002) who reported that seed priming reduced the total germination percentage of some seed lots, especially at higher germination temperatures.
All priming treatments induced faster and more synchronous germination compared with the unprimed seeds at all of the germination temperatures. Similar increases in germination speed and synchrony of chickpea (Harris et al., Citation1999; Musa et al., Citation1999), maize (Harris et al., Citation1999), soybean (Yan et al., Citation1989), grass seeds (Hardegree & Van Vactor, Citation2000), canola (Zheng et al., Citation1994), wheat and barley (Al-Karaki, Citation1998) through seed priming have been reported in previous studies. These beneficial effects of priming on seed germination are related to the repair and build-up of nucleic acid, enhanced synthesis of RNA and proteins, repair of membranes (Bray et al., Citation1989; Dell'Aquila & Bewley, Citation1989; Davison & Bray, Citation1991; Bray, Citation1995), and enhanced respiratory activity of seeds (Halpin-Ingham & Sundstrom, Citation1992).
Thermal germination models generate coefficients that integrate potential response over a wide range of temperature conditions (Garcia-Huidobro et al., Citation1982; Covell et al., Citation1986; Hardegree et al., Citation1999). These coefficients can be compared directly to rank relative potential performance of seed lots (Covell et al., Citation1986; Ellis et al., Citation1986) and can be validated by confirming the germination response under variable temperature conditions (Hardegree et al., Citation1999; Hardegree & Van Vactor, Citation1999, Citation2000). Earlier and faster germination and emergence has been associated with a lower value of thermal time requirement (Mohamed et al., Citation1988). In cold soils, low thermal time requirement is of great importance for rapid germination (Bierhuizen & Wagenvoort, Citation1974) because germination of seeds will be delayed until thermal time requirement is met. We also quantified the priming effect by calculating the thermal-response parameter from the sub-optimal temperature data. In our study, compared with the unprimed treatment, priming treatments significantly decreased thermal time requirements. Dahal et al. (Citation1990), Hardegree and Van Vactor (Citation2000) and Hardegree et al. (Citation2002) also investigated priming effects on thermal germination response. They too found that priming significantly decreased thermal time requirements.
Rate of water uptake, which is necessary to activate the physiological processes in seed, is directly related to the osmotic potential of the priming solution (Hardegree & Emmerich, Citation1992) and decreasing water potential adversely affects rate of water uptake in seeds (Al-Karaki, Citation1998). In the current study, hydropriming generally gave the highest germination speed and the lowest thermal time requirement values. Among the osmopriming treatments, −0.5 MPa solution of PEG, which had the highest water potential, also gave the best results. Compared with the hydropriming treatment, decreasing water potential in osmopriming treatments adversely affected germination speed and thermal time requirement (, ). These adverse effects may be related to the decreased water uptake in the osmopriming treatments, especially lower water potential than −0.5 MPa of PEG. Similar results have been reported by Danneberger et al. (Citation1992) and Al-Karaki (Citation1998).
In general, seeds primed for 24 h significantly reduced the hours required to reach 10%, 50%, and 90% germination compared with the seeds primed for 12 h. In addition, seeds osmoprimed for 24 h had significantly lower thermal time requirments than those of seeds osmoprimed for 12 h. These germination speed and thermal time requirement results indicated that longer priming duration than 12 h may overcome adverse effects of decreased water potential in osmopriming treatments of chickpea. Other researchers also reported that increasing priming duration in osmopriming treatments had a beneficial effect on germination of tomato (Mauromicale & Cavallaro, Citation1995) and parsley (Pill & Kilian, Citation2000). We also investigated the effect of priming duration of 48 h, but this priming duration drastically decreased the germination percentage and the other germination indices (data not shown). Similar reductions in germination with increasing priming duration were observed for carrot (Haigh et al., Citation1986) and soybean (Khalil et al., Citation2001). These results show that overpriming is detrimental. This is supported by Murray (Citation1989), who concluded that overpriming may cause oxygen deficiency and the build-up of inhibitors. As a result of our findings, we determined that safe priming duration limits ranged between 12 h and 24 h for chickpea. Similarly, soaking the seeds from overnight to 24 h has been recommended for chickpea, maize and rice (Harris et al., Citation1999).
In conclusion, seeds treated with water for 12 h generally produced the highest germination speed and the lowest thermal time requirement values. Among the osmopriming treatments, seeds treated with −0.5 MPa solution of PEG for 24 h, which had the highest water potential, gave the best results. Consequently, hydropriming for 12 h or osmopriming (PEG −0.5 MPa) for 24 h may be recommended for better germination of chickpea under cold soil conditions.
References
- Al-Karaki , G.N. 1998 . Response of wheat and barley during germination to seed osmopriming at different water potentials . Journal of Agronomy and Crop Science , 181 : 229 – 235 .
- Auld , D.L. , Bettis , B.L. , Crock , J.E. and Kephart , K.D. 1988 . Planting date and temperature effects on germination, emergence, and seed yield of chickpea . Agronomy Journal , 80 : 909 – 914 .
- Bierhuizen , F. and Wagenvoort , W.A. 1974 . Some aspects of seed germination in vegetables. I. The determination and application of heat sums and minimum temperature for germination . Scientia Horticulturae , 2 : 213 – 219 .
- Bray , C.M. 1995 . “ Biochemical processes during the osmopriming of seeds ” . In Seed development and germination , Edited by: Kigel , J and Galili , G . 767 – 789 . New York : Marcel Dekker .
- Bray , C.M. , Davison , P.A. , Ashraf , M. and Taylor , R.M. 1989 . Biochemical changes during priming of leek seeds . Annals of Botany , 63 : 185 – 193 .
- Chen , T.H.H. , Yamamoto , S.D.K. , Gusta , L.V. and Slinkard , A.E. 1983 . Imbibitional chilling injury during chickpea germination . Journal of American Society of Horticultural Science , 108 : 944 – 948 .
- Cheng , Z. and Bradford , K.J. 1999 . Hydrothermal time analysis of tomato seed germination responses to priming treatments . Journal of Experimental Botany , 50 : 89 – 99 .
- Covell , S. , Ellis , R.H. , Roberts , E.H. and Summerfield , R.J. 1986 . The influence of temperature on seed germination rate of legumes. I. A comparison of chickpea, lentil, soybean and cowpea at constant temperatures . Journal of Experimental Botany , 37 : 705 – 715 .
- Croser , J.S. , Clarke , H.J. , Siddique , K.H.M. and Khan , T.N. 2003 . Low temperature stress: implications for chickpea (Cicer arietinum L.) improvement . Critical Reviews in Plant Sciences , 22 : 185 – 219 .
- Dahal , P. , Bradford , K.J. and Jones , R.A. 1990 . Effects of priming and endosperm integrity on seed germination rates of tomato genotypes. I. Germination at suboptimal temperature . Journal of Experimental Botany , 41 : 1431 – 1439 .
- Danneberger , T.K. , McDonald , M.B. , Geron , C.A. and Kumari , P. 1992 . Rate of germination and seedling growth of perennial ryegrass seed following osmoconditioning . Hortscience , 27 : 28 – 30 .
- Davison , P.A. and Bray , C.M. 1991 . Protein synthesis during osmopriming of leek (Allium porrum L.) seeds . Seed Science Research , 1 : 29 – 35 .
- Dell'Aquila , A. and Bewley , J.D. 1989 . Protein synthesis in the axes of polyethylene glycol treated pea seeds and during subsequent germination . Journal of Experimental Botany , 40 : 1001 – 1007 .
- Dumur , D. , Pilbeam , C. and Craigon , J.J. 1990 . Use of the Weibull function to calculate cardinal temperatures in faba bean . Journal of Experimental Botany , 41 : 1423 – 1430 .
- Ellis , R.H. , Covell , S. , Roberts , E.H. and Summerfield , R.J. 1986 . The influence of temperature on seed germination rate in grain legumes. II. Intraspecific variation in chickpea (Cicer arietinum L.) at constant temperatures . Journal of Experimental Botany , 37 : 1503 – 1515 .
- Esitken , A. , Ercisli , S. , Eken , C. and Tay , D. 2004 . Seed priming effect on symbiotic germination and seedling development of Orchis palustris Jacq . Hortscience , 39 : 1700 – 1701 .
- Garcia-Huidobro , J. , Monteith , J.L. and Squire , G.R. 1982 . Time, temperature and germination of pearl millet (Pennisetum typhoides S. and H.). I. Constant temperature . Journal of Experimental Botany , 33 : 288 – 296 .
- Haigh , A.M. , Barlow , E.W.R. , Milthorpe , F.L. and Sinclair , P.J. 1986 . Field emergence of tomato, carrot and onion seeds primed in an aerated salt solution . Journal of American Society of Horticultural Science , 111 : 660 – 665 .
- Halpin-Ingham , B. and Sundstrom , F.J. 1992 . Pepper seed water content, germination response and respiration following priming treatments . Seed Science and Technology , 20 : 589 – 596 .
- Hardegree , S.P. and Emmerich , W. 1992 . Effect of matric priming duration and priming water potential on germination of four grasses . Journal of Experimental Botany , 43 : 233 – 238 .
- Hardegree , S.P. and Van Vactor , S.S. 1999 . Predicting germination response of four cool-season range grasses to field-variable temperature regimes . Environmental and Experimental Botany , 41 : 209 – 217 .
- Hardegree , S.P. and Van Vactor , S.S. 2000 . Germination and emergence of primed grass seeds under field and simulated-field temperature regimes . Annals of Botany , 85 : 379 – 390 .
- Hardegree , S.P. , Jones , T.A. and Van Vactor , S.S. 2002 . Variability in thermal response of primed and non-primed seeds of squirreltail [Elymus elymoides (Raf.) Swezey and Elymus multisetus (J.G. Smith) M.E. Jones] . Annals of Botany , 89 : 311 – 319 .
- Hardegree , S.P. , Van Vactor , S.S. , Pierson , F.B. and Palmquist , D.E. 1999 . Predicting variable-temperature response of non-dormant seeds from constant-temperature germination data . Journal of Range Management , 52 : 83 – 91 .
- Harris , D. 1996 . The effects of manure, genotype, seed priming, depth and date of sowing on the emergence and early growth of Sorghum bicolor (L.) Moench in semi-arid Botswana . Soil and Tillage Research , 40 : 73 – 88 .
- Harris , D. , Joshi , A. , Khan , P.A. , Gothkar , P. and Sodhi , P.S. 1999 . On-farm seed priming in semi-arid agriculture: development and evaluation in maize, rice and chickpea in India using participatory methods . Experimental Agriculture , 35 : 15 – 29 .
- Heydecker , W. and Gibbins , B. 1978 . The ‘priming’ of seeds . Acta Horticulturae , 83 : 213 – 215 .
- Kaur , S. , Gupta , A.K. and Kaur , N. 2002 . Effect of osmo- and hydropriming of chickpea seeds on seedling growth and carbohydrate metabolism under water deficit stress . Plant Growth Regulation , 37 : 17 – 22 .
- Khalil , S.K. , Mexal , J.G. and Murray , L.W. 2001 . Germination of soybean seed primed in aerated solution of polyethylene glycol (8000) . Online Journal of Biological Science , 1 : 105 – 107 .
- Korkmaz , A. and Pill , W.G. 2003 . The effect of different priming treatments and storage conditions on germination performance of lettuce seeds . European Journal of Horticultural Science , 68 : 260 – 265 .
- Mauromicale , G. and Cavallaro , V. 1995 . Effects of seed osmopriming on germination of tomato at different water potentials . Seed Science and Technology , 23 : 393 – 403 .
- McDonald , M.B. 1999 . Seed deterioration: physiology, repair and assessment . Seed Science and Technology , 27 : 177 – 237 .
- Michel , B.E. and Kaufmann , M.R. 1973 . The osmotic potential of polyethylene glycol 6000 . Plant Physiology , 51 : 914 – 916 .
- Mohamed , H.A. , Clark , J.A. and Ong , C.K. 1988 . Genotypic differences in the temperature responses of tropical crops. II. Seedling emergence and leaf growth of groundnut (Arachis hypogaea L.) and pearl millet (Pennisetum typhoides S & H.) . Journal of Experimental Botany , 39 : 1129 – 1135 .
- Murray , G.A. 1989 . Osmoconditioning carrot seed for improved emergence . Hortscience , 24 : 701
- Murungu , F.S. , Nyamugafata , P. , Chiduza , C. , Clark , L.J. and Whalley , W.R. 2003 . Effects of seed priming, aggregate size and soil matric potential on emergence of cotton (Gossypium hirsutum L.) and maize (Zea mays L) . Soil and Tillage Research , 74 : 161 – 168 .
- Musa , A.M. , Johansen , C. , Kumar , J. and Harris , D. 1999 . Response of chickpea to seed priming in the High Barind Tract of Bangladesh . International Chickpea and Pigeonpea Newsletter , 6 : 20 – 22 .
- Musa , A.M , Harris , D. , Johansen , C. and Kumar , J. 2001 . Short duration chickpea to replace fallow after aman rice: the role of on-farm seed priming in the High Barind Tract of Bangladesh . Experimental Agriculture , 37 : 509 – 521 .
- Pill , W.G. and Kilian , E.A. 2000 . Germination and emergence of parsley in response to osmotic or matric seed priming and treatment with gibberellin . Hortscience , 35 : 907 – 909 .
- Singh , K.B. 1993 . “ Problems and prospects of stress resistance breeding in chickpea ” . In Breeding for stress tolerance in cool-season food legumes , Edited by: Singh , KB and Saxena , MC . 17 – 37 . Chichester : John Willey & Sons .
- Yan , Y.T. , Liang , W.T. , Zheng , G.H. and Tang , P.S. 1989 . Effect of low temperature imbibition on mitochondrion respiration and phosphorylation of PEG primed soybean seed . Acta Botanica Sinica , 31 : 441 – 448 .
- Zheng , G.H. , Wilen , R.W. , Slinkard , A.E. and Gusta , L.V. 1994 . Enhancement of canola seed germination and seedling emergence at low temperature by priming . Crop Science , 34 : 1589 – 1593 .